Clinical Feasibility of Reduced Field-of-View Diffusion-Weighted Magnetic Resonance Imaging with Computed Diffusion-Weighted Imaging Technique in Breast Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. MRI Acquisition
2.3. MR Image Analysis
2.3.1. Qualitative Analysis
2.3.2. Quantitative Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Qualitative Analysis
3.3. Quantitative Analysis
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Bluemke, D.A.; Gatsonis, C.; Chen, M.H.; Deangelis, G.A.; Debruhl, N.; Harms, S.E.; Heywangkobrunner, S.H.; Hylton, N.M.; Kuhl, C.K.; Lehman, C.D.; et al. Magnetic resonance imaging of the breast prior to biopsy. JAMA 2004, 292, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Sardanelli, F.; Giuseppetti, G.M.; Panizza, P.; Bazzocchi, M.; Fausto, A.; Simonetti, G.; Lattanzio, V.; Del Maschio, A. Sensitivity of MRI Versus Mammography for Detecting Foci of Multifocal, Multicentric Breast Cancer in Fatty and Dense Breasts Using the Whole-Breast Pathologic Examination as a Gold Standard. Am. J. Roentgenol. 2004, 183, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, A.M.; Prummel, M.V.; Muradali, D.; Majpruz, V.; Horgan, M.; Carroll, J.C.; Eisen, A.; Meschino, W.S.; Shumak, R.S.; Warner, E.; et al. Effectiveness of screening with annual magnetic resonance imaging and mammography: Results of the initial screen from the ontario high risk breast screening program. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 2224–2230. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.A. Diagnostic breast MR imaging: Current status and future directions. Radiol. Clin. N. Am. 2007, 45, 863–880. [Google Scholar] [CrossRef]
- Gupta, D.; Billadello, L. Breast MR Imaging in Newly Diagnosed Breast Cancer. Radiol. Clin. N. Am. 2017, 55, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Zhu, H.; Chai, W.; Zhan, Y.; Nickel, D.; Grimm, R.; Fu, C.; Yan, F. Whole-lesion histogram and texture analyses of breast lesions on inline quantitative DCE mapping with CAIPIRINHA-Dixon-TWIST-VIBE. Eur. J. Radiol. 2020, 30, 57–65. [Google Scholar] [CrossRef]
- Jiao, Z.; Gao, X.; Wang, Y.; Li, J. A deep feature based framework for breast masses classification. Neurocomputing 2016, 197, 221–231. [Google Scholar] [CrossRef]
- Hao, W.; Peng, W.; Wang, C.; Zhao, B.; Wang, G. Image quality of the CAIPIRINHA-Dixon-TWIST-VIBE technique for ultra-fast breast DCE-MRI: Comparison with the conventional GRE technique. Eur. J. Radiol. 2020, 129, 109108. [Google Scholar] [CrossRef]
- Kanda, T.; Matsuda, M.; Oba, H.; Toyoda, K.; Furui, S. Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015, 277, 924–925. [Google Scholar] [CrossRef]
- Gulani, V.; Calamante, F.; Shellock, F.G.; Kanal, E.; Reeder, S.B. Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol. 2017, 16, 564–570. [Google Scholar] [CrossRef]
- Ramalho, M.; Ramalho, J. Gadolinium-Based Contrast Agents: Associated Adverse Reactions. Magn. Reson. Imaging Clin. N. Am. 2017, 25, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Telegrafo, M.; Rella, L.; Stabile Ianora, A.A.; Angelelli, G.; Moschetta, M. Unenhanced breast MRI (STIR, T2-weighted TSE, DWIBS): An accurate and alternative strategy for detecting and differentiating breast lesions. Magn. Reson. Imaging 2015, 33, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Shin, H.J. Unenhanced magnetic resonance screening using fused diffusion-weighted imaging and maximum-intensity projection in patients with a personal history of breast cancer: Role of fused DWI for postoperative screening. Breast Cancer Res. Treat. 2017, 165, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, R.M.; Verardi, N.; Cartia, F.; Carbonaro, L.A.; Sardanelli, F. Breast Cancer Detection Using Double Reading of Unenhanced MRI Including T1-Weighted, T2-Weighted STIR, and Diffusion-Weighted Imaging: A Proof of Concept Study. Am. J. Roentgenol. 2014, 203, 674–681. [Google Scholar] [CrossRef]
- Schnall, M.D.; Blume, J.; Bluemke, D.A.; DeAngelis, G.A.; DeBruhl, N.; Harms, S.; Heywang-Kobrunner, S.H.; Hylton, N.; Kuhl, C.K.; Pisano, E.D.; et al. Diagnostic architectural and dynamic features at breast MR imaging: Multicenter study. Radiology 2006, 238, 42–53. [Google Scholar] [CrossRef]
- Pinker, K.; Stadlbauer, A.; Bogner, W.; Gruber, S.; Helbich, T.H. Molecular imaging of cancer: MR spectroscopy and beyond. Eur. J. Radiol. 2012, 81, 566–577. [Google Scholar] [CrossRef]
- Partridge, S.C.; Nissan, N.; Rahbar, H.; Kitsch, A.E.; Sigmund, E.E. Diffusion-weighted breast MRI: Clinical applications and emerging techniques. J. Magn. Reson. Imaging 2017, 45, 337–355. [Google Scholar] [CrossRef]
- Sinha, S.; Lucas-Quesada, F.A.; Sinha, U.; DeBruhl, N.; Bassett, L.W. In vivo diffusion-weighted MRI of the breast: Potential for lesion characterization. J. Magn. Reson. Imaging 2002, 15, 693–704. [Google Scholar] [CrossRef]
- Barentsz, M.W.; Taviani, V.; Chang, J.M.; Ikeda, D.M.; Miyake, K.K.; Banerjee, S.; van den Bosch, M.A.; Hargreaves, B.A.; Daniel, B.L. Assessment of tumor morphology on diffusion-weighted (DWI) breast MRI: Diagnostic value of reduced field of view DWI. J. Magn. Reson. Imaging 2015, 42, 1656–1665. [Google Scholar] [CrossRef]
- Ortendahl, D.A.; Hylton, N.; Kaufman, L.; Watts, J.C.; Crooks, L.E.; Mills, C.M.; Stark, D.D. Analytical tools for magnetic resonance imaging. Radiology 1984, 153, 479–488. [Google Scholar] [CrossRef]
- Riederer, S.J.; Suddarth, S.A.; Bobman, S.A.; Lee, J.N.; Wang, H.Z.; MacFall, J.R. Automated MR image synthesis: Feasibility studies. Radiology 1984, 153, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Higaki, T.; Nakamura, Y.; Tatsugami, F.; Kaichi, Y.; Akagi, M.; Akiyama, Y.; Baba, Y.; Iida, M.; Awai, K. Introduction to the Technical Aspects of Computed Diffusion-weighted Imaging for Radiologists. RadioGraphics 2018, 38, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Blackledge, M.D.; Leach, M.O.; Collins, D.J.; Koh, D.M. Computed diffusion-weighted MR imaging may improve tumor detection. Radiology 2011, 261, 573–581. [Google Scholar] [CrossRef] [PubMed]
- O’Flynn, E.A.; Blackledge, M.; Collins, D.; Downey, K.; Doran, S.; Patel, H.; Dumonteil, S.; Mok, W.; Leach, M.O.; Koh, D.M. Evaluating the diagnostic sensitivity of computed diffusion-weighted MR imaging in the detection of breast cancer. J. Magn. Reson. Imaging 2016, 44, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Bickel, H.; Polanec, S.H.; Wengert, G.; Pinker, K.; Bogner, W.; Helbich, T.H.; Baltzer, P.A.T. Diffusion-Weighted MRI of Breast Cancer: Improved Lesion Visibility and Image Quality Using Synthetic b-Values. J. Magn. Reson. Imaging 2019, 50, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; la Yun, B.; Jang, M.; Ahn, H.S.; Kim, S.M.; Lee, S.H.; Kang, E.; Kim, E.K.; Park, S.Y. Comparison of the Diagnostic Performance of Synthetic Versus Acquired High b-Value (1500 s/mm2) Diffusion-Weighted MRI in Women with Breast Cancers. J. Magn. Reson. Imaging 2019, 49, 857–863. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, E.; Xu, H.; Ye, Q.; Li, J.; Ye, S.; Cheng, Q.; Zhao, L.; Su, M.; Wang, M. Feasibility and Diagnostic Performance of Voxelwise Computed Diffusion-Weighted Imaging in Breast Cancer. J. Magn. Reson. Imaging 2019, 49, 1610–1616. [Google Scholar] [CrossRef]
- Von Morze, C.; Kelley, D.A.C.; Shepherd, T.M.; Banerjee, S.; Xu, D.; Hess, C.P. Reduced field-of-view diffusion-weighted imaging of the brain at 7 T. Magn. Reson. Imaging 2010, 28, 1541–1545. [Google Scholar] [CrossRef]
- Zaharchuk, G.; Saritas, E.U.; Andre, J.B.; Chin, C.T.; Rosenberg, J.; Brosnan, T.J.; Shankaranarayan, A.; Nishimura, D.G.; Fischbein, N.J. Reduced Field-of-View Diffusion Imaging of the Human Spinal Cord: Comparison with Conventional Single-Shot Echo-Planar Imaging. Am. J. Neuroradiol. 2011, 32, 813–820. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.M.; Yoon, J.H.; Jang, J.Y.; Kim, S.W.; Ryu, J.K.; Kannengiesser, S.; Han, J.K.; Choi, B.I. Reduced Field-of-View Diffusion-Weighted Magnetic Resonance Imaging of the Pancreas: Comparison with Conventional Single-Shot Echo-Planar Imaging. Korean J. Radiol. 2015, 16, 1216–1225. [Google Scholar] [CrossRef]
- Korn, N.; Kurhanewicz, J.; Banerjee, S.; Starobinets, O.; Saritas, E.; Noworolski, S. Reduced-FOV excitation decreases susceptibility artifact in diffusion-weighted MRI with endorectal coil for prostate cancer detection. Magn. Reson. Imaging 2015, 33, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Singer, L.; Wilmes, L.J.; Saritas, E.U.; Shankaranarayanan, A.; Proctor, E.; Wisner, D.J.; Chang, B.; Joe, B.N.; Nishimura, D.G.; Hylton, N.M. High-resolution Diffusion-weighted Magnetic Resonance Imaging in Patients with Locally Advanced Breast Cancer. Acad. Radiol. 2012, 19, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Wilmes, L.J.; McLaughlin, R.L.; Newitt, D.C.; Singer, L.; Sinha, S.P.; Proctor, E.; Wisner, D.J.; Saritas, E.U.; Kornak, J.; Shankaranarayanan, A.; et al. High-Resolution Diffusion-Weighted Imaging for Monitoring Breast Cancer Treatment Response. Acad. Radiol. 2013, 20, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, Y.; Li, H.; Wang, B.; Hu, B. Study of the Reduced Field-of-View Diffusion-Weighted Imaging of the Breast. Clin. Breast Cancer 2014, 14, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Shin, H.J.; Shin, K.C.; Sung, Y.S.; Choi, W.J.; Chae, E.Y.; Cha, J.H.; Kim, H.H. Comparison of readout segmented echo planar imaging (EPI) and EPI with reduced field-of-VIew diffusion-weighted imaging at 3t in patients with breast cancer. J. Magn. Reson. Imaging 2015, 42, 1679–1688. [Google Scholar] [CrossRef]
- D’Orsi, C.J.; Sickles, E.; Mendelson, E.B.; Morris, E.A.; et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Liberman, L.; Morris, E.A.; Lee, M.J.; Kaplan, J.B.; LaTrenta, L.R.; Menell, J.H.; Abramson, A.F.; Dashnaw, S.M.; Ballon, D.J.; Dershaw, D. Breast lesions detected on MR imaging: Features and positive predictive value. Am. J. Roentgenol. 2020, 179, 171–178. [Google Scholar] [CrossRef]
- Sechopoulos, I. A review of breast tomosynthesis, Part I. The image acquisition process. Med. Phys. 2013, 40, 014301. [Google Scholar] [CrossRef]
- Comstock, C.E.; Gatsonis, C.; Newstead, G.; Snyder, B.S.; Gareen, I.F.; Bergin, J.T.; Rahbar, H.; Sung, J.S.; Jacobs, C.; Harvey, J.A.; et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA 2020, 323, 746–756. [Google Scholar] [CrossRef]
- Lewin, J.M.; Issacs, P.K.; Vance, V.; Larke, F.J. Dual-energy contrast-enhanced digital subtraction mammography: Feasibility. Radiology 2003, 229, 261–268. [Google Scholar] [CrossRef]
- Dromain, C.; Thibault, F.; Muller, S.; Rimareix, F.; Delaloge, S.; Tardivon, A.; Balleyguier, C. Dual-energy contrast-enhanced digital mammography: Initial clinical results. Eur. Radiol. 2010, 21, 565–574. [Google Scholar] [CrossRef]
- Samei, E.; Saunders, R.S. Dual-energy contrast-enhanced breast tomosynthesis: Optimization of beam quality for dose and image quality. Phys. Med. Biol. 2011, 56, 6359–6378. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.P.; Lewin, J.M.; Chiang, C.L.; Hung, B.H.; Yang, T.L.; Huang, J.S.; Pan, H.B. Clinical evaluation of contrast-enhanced digital mammography and contrast enhanced tomosynthesis—Comparison to contrast-enhanced breast MRI. Eur. J. Radiol. 2015, 84, 2501–2508. [Google Scholar] [CrossRef] [PubMed]
- Seifert, P.; Conover, D.; Zhang, Y.; Morgan, R.; Arieno, A.; Destounis, S.; Somerville, P.; Murphy, P.F. Evaluation of Malignant Breast Lesions in the Diagnostic Setting with Cone Beam Breast Computed Tomography (Breast CT): Feasibility Study. Breast J. 2014, 20, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Prionas, N.D.; Lindfors, K.K.; Ray, S.; Huang, S.Y.; Beckett, L.A.; Monsky, W.L.; Boone, J.M. Contrast-enhanced Dedicated Breast CT: Initial Clinical Experience. Radiology 2010, 256, 714–723. [Google Scholar] [CrossRef]
- He, N.; Wu, Y.P.; Kong, Y.; Lv, N.; Huang, Z.M.; Li, S.; Wang, Y.; Geng, Z.; Wu, P.H.; Wei, W.D. The utility of breast cone-beam computed tomography, ultrasound, and digital mammography for detecting malignant breast tumors: A prospective study with 212 patients. Eur. J. Radiol. 2016, 85, 392–403. [Google Scholar] [CrossRef]
- Aminololama-Shakeri, S.; Abbey, C.K.; Gazi, P.; Prionas, N.D.; Nosratieh, A.; Li, C.S.; Boone, J.M.; Lindfors, K.K. Differentiation of ductal carcinoma in-situ from benign micro-calcifications by dedicated breast computed tomography. Eur. J. Radiol. 2016, 85, 297–303. [Google Scholar] [CrossRef]
- Shah, J.P.; Mann, S.D.; McKinley, R.L.; Tornai, M.P. Three dimensional dose distribution comparison of simple and complex acquisition trajectories in dedicated breast CT. Med. Phys. 2015, 42, 4497–4510. [Google Scholar] [CrossRef]
- Boone, J.M.; Nelson, T.R.; Lindfors, K.K.; Seibert, J.A. Dedicated breast CT: Radiation dose and image quality evaluation. Radiology 2001, 221, 657–667. [Google Scholar] [CrossRef]
- Moon, J.I.; Choi, B.H.; Baek, H.J.; Ryu, K.H.; Park, S.E.; Ha, J.Y.; Jung, E.J.; Lee, H.S.; An, H.J. Comprehensive analyses with radiological and biological markers of breast cancer on contrast-enhanced chest CT: A single center experience using dual-later spectal detector CT. Eur. Radiol. 2020, 30, 2782–2790. [Google Scholar] [CrossRef]
- Le Bihan, D.; Poupon, C.; Amadon, A.; Lethimonnier, F. Artifacts and pitfalls in diffusion MRI. J. Magn. Reson. Imaging 2006, 24, 478–488. [Google Scholar] [CrossRef]
- Dong, H.; Li, Y.; Yu, K.; Li, H. Comparison of image quality and application values on different field-of-view diffusion-weighted imaging of breast cancer. Acta Radiol. 2016, 57, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, R.; Inoue, Y.; Ramadan, S.; Hata, H.; Ozaki, M. Diffusion-weighted imaging of the breast: Comparison of b-values 1000 s/mm(2) and 1500 s/mm(2). Magn. Reson. Med Sci. Off. J. Jpn. Soc. Magn. Reson. Med. 2013, 12, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Takahashi, S.; Kitajima, K.; Kimura, T.; Aoki, I.; Kawakami, F.; Miyake, H.; Ohno, Y.; Sugimura, K. Computed diffusion-weighted imaging using 3-T magnetic resonance imaging for prostate cancer diagnosis. Eur. Radiol. 2013, 23, 3509–3516. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Higaki, T.; Akiyama, Y.; Fukumoto, W.; Kajiwara, K.; Kaichi, Y.; Honda, Y.; Komoto, D.; Tatsugami, F.; Iida, M.; et al. Diffusion-weighted MR imaging of non-complicated hepatic cysts: Value of 3T computed diffusion-weighted imaging. Eur. J. Radiol. Open 2016, 3, 138–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuhl, C.K.; Schrading, S.; Strobel, K.; Schild, H.H.; Hilgers, R.D.; Bieling, H.B. Abbreviated Breast Magnetic Resonance Imaging (MRI): First Postcontrast Subtracted Images and Maximum-Intensity Projection—A Novel Approach to Breast Cancer Screening with MRI. J. Clin. Oncol. 2014, 32, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
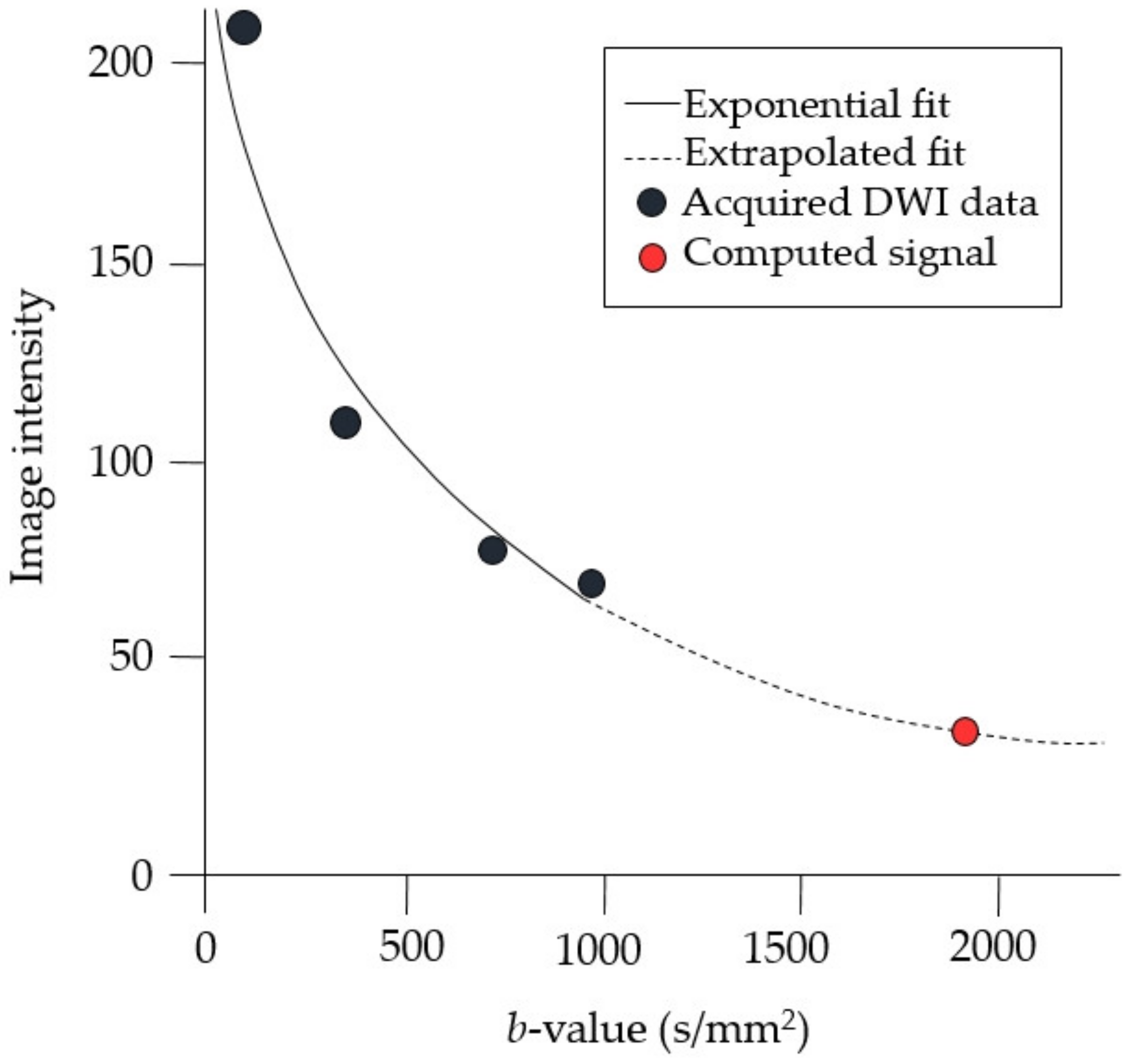
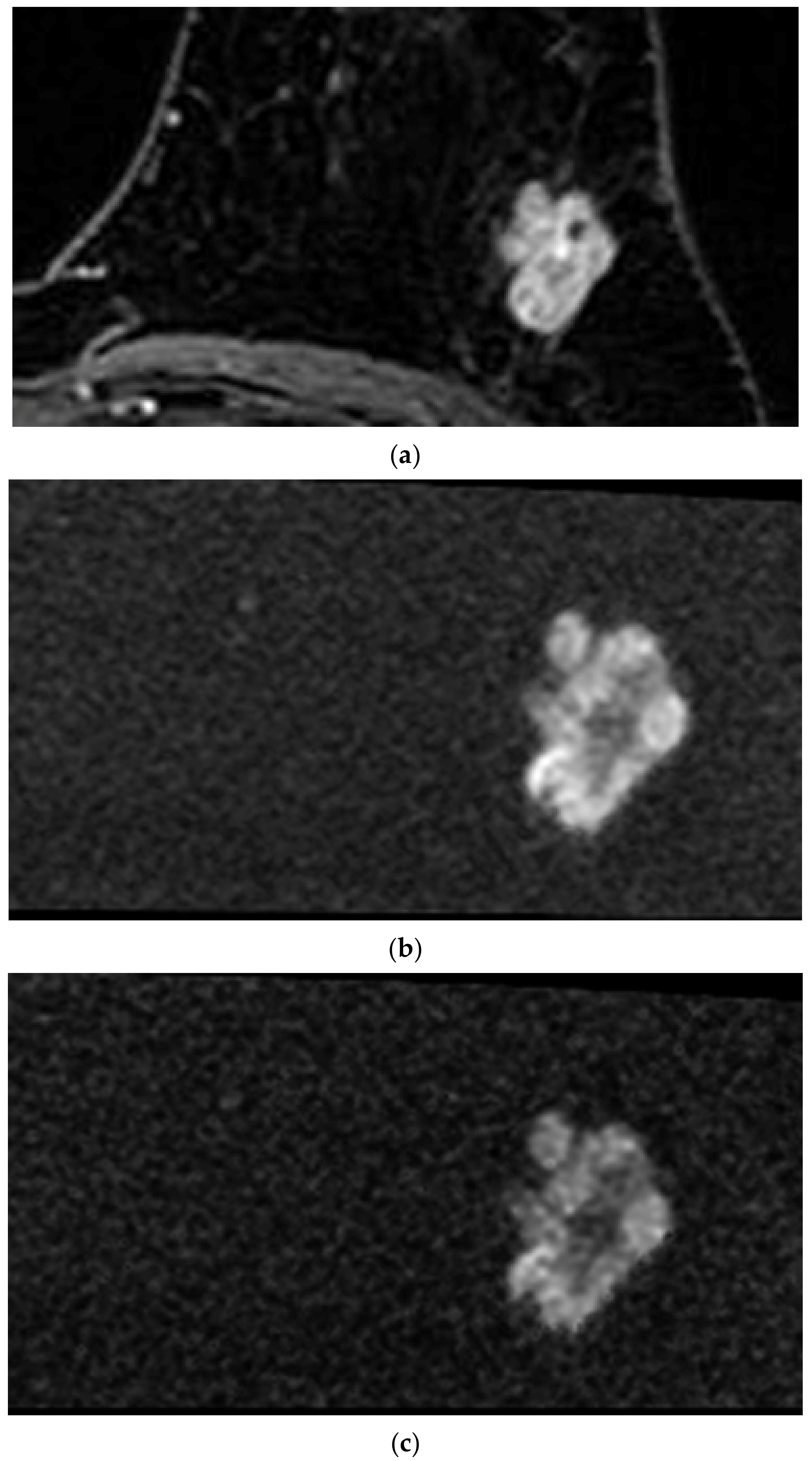
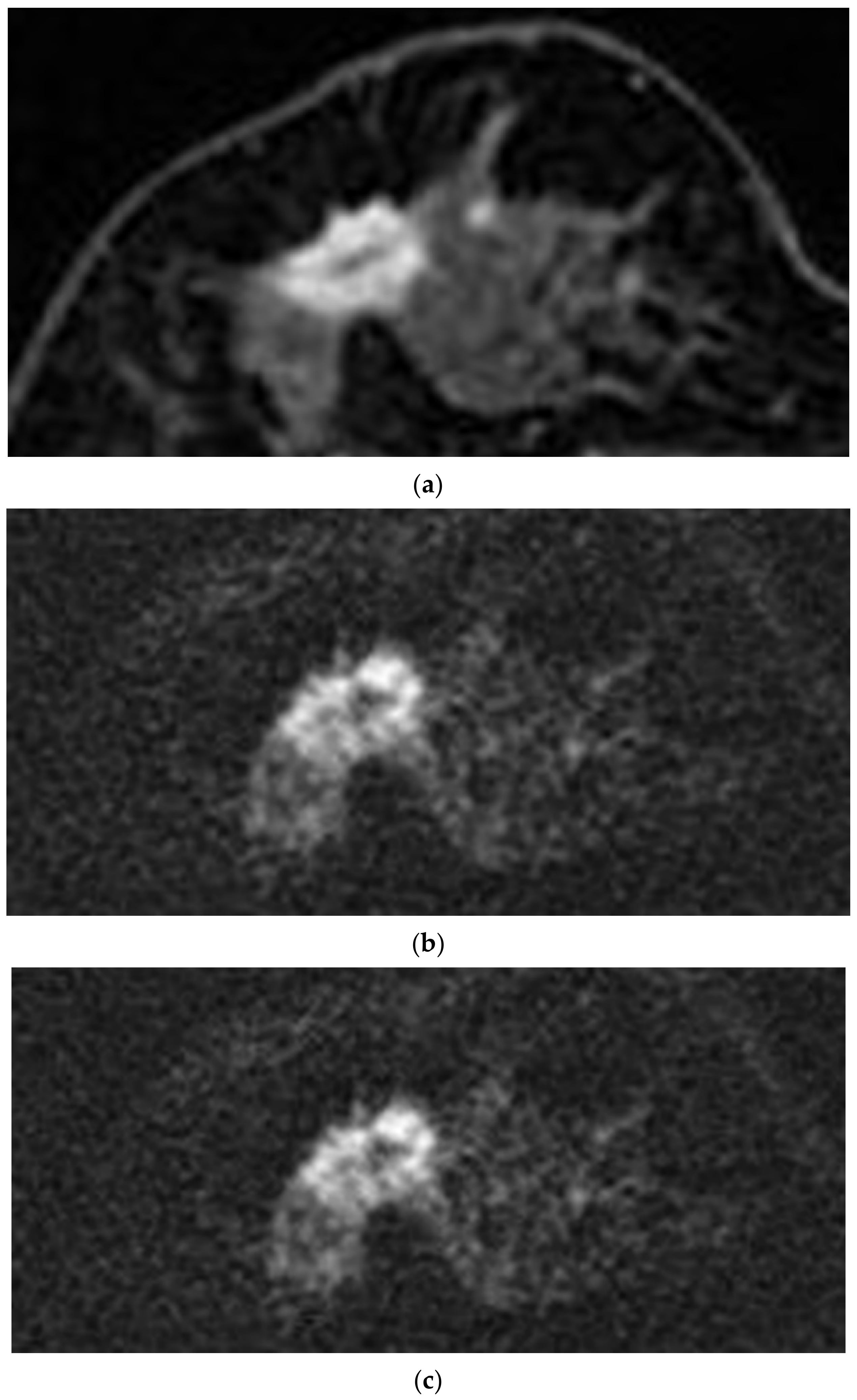
| Sequence Parameter | rFOV DWI | DCE MRI | |
|---|---|---|---|
| b = 500 s/mm2 | b = 1000 s/mm2 | ||
| b value (s/mm2) | 0, 500 | 0, 1000 | Non-applicable |
| Fat suppression | Short tau inversion recovery | Short tau inversion recovery | Chemical shift-selective fat saturation |
| Repetition time (ms) | 3000 | 3000 | 4.1 |
| Echo time (ms) | 52 | 58 | 1.7 |
| Number of excitations | 12 | 16 | 0.7 |
| Acquisition matrix | 128 × 64 | 128 × 64 | 320 × 320 |
| Field-of-view (mm2) | 100 × 50 | 100 × 50 | 320 × 320 |
| Slice thickness (mm) | 4 | 4 | 1 |
| Intersection gap (%) | 0 | 0 | 0 |
| Acquisition time (s) | 113 | 153 | 374 |
| Flip angle (°) | 90 | 90 | 10 |
| Image Quality | rFOVA-1000 a | rFOVS-1000 b | rFOVS-1500 c | rFOVS-2000 d | srFOVS e | DCE MRI f | p-Value |
|---|---|---|---|---|---|---|---|
| 1 = poor | 2 | 2 | 8 | 43 | 2 | 0 | |
| 2 = not good | 6 | 6 | 29 | 41 | 6 | 1 | |
| 3 = minor degredation | 41 | 41 | 39 | 39 | 29 | 32 | |
| 4 = no problem | 81 | 81 | 54 | 7 | 93 | 97 | |
| Average score | 3.54 ± 0.66 | 3.54 ± 0.66 | 3.07 ± 0.94 | 2.08 ± 0.92 | 3.63 ± 0.65 | 3.73 ± 0.46 | 0.357 |
| Lesion Analysis | rFOVA-1000 a | srFOVS b | DCE MRI c | p-Value |
|---|---|---|---|---|
| Shape | 0.940 | |||
| Oval | 14 | 12 | 11 | |
| Round | 7 | 8 | 8 | |
| Irregular | 79 | 80 | 81 | |
| Margin | 0.662 | |||
| Circumscribed | 9 | 9 | 17 | |
| Irregular | 55 | 56 | 49 | |
| Spiculated | 38 | 37 | 36 | |
| Non-mass enhancement | 28 | 28 | 28 | |
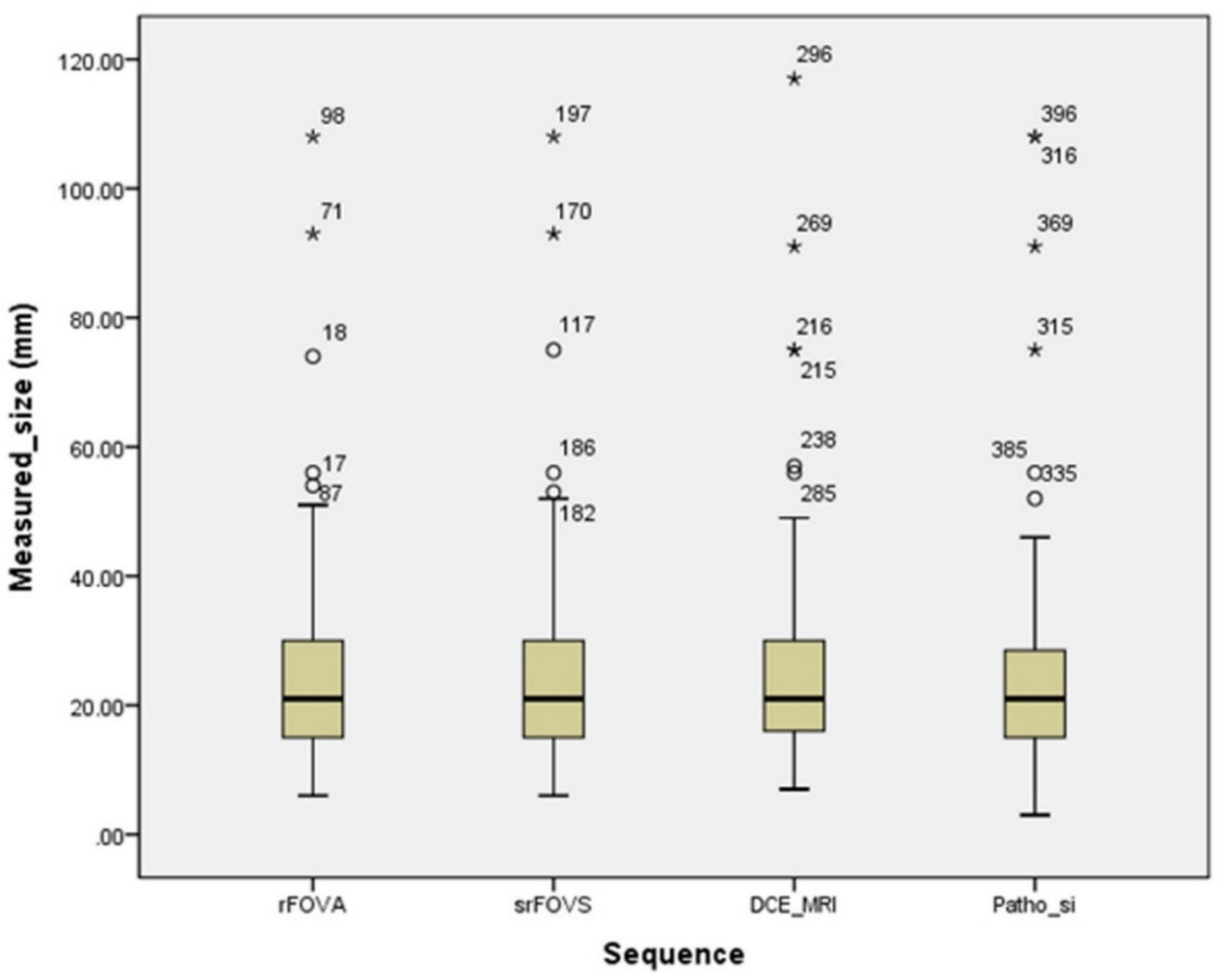
| Average tumor size (mm) | 25.5 ± 16.15 | 25.7 ± 16.17 | 26.3 ± 17.34 | 0.585 |
| rFOVA-1000 a | srFOVS b | DCE MRI c | p-Value | |
|---|---|---|---|---|
| Signal-to-noise ratio (SNR) | 16.21 ± 5.82 | 16.06 ± 5.88 | 85.03 ± 20.97 | <0.001 |
| Contrast-to-noise ratio (CNR) | 2.02 ± 1.00 | 1.98 ± 0.98 | 2.53 ± 1.01 | 0.001 |
| Tumor-to-parenchymal contrast (TPC) | 2.33 ± 0.87 | 2.35 ± 0.94 | 2.79 ± 1.66 | 0.016 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.; Lee, J.H.; Baek, H.J.; Ha, J.Y.; Ryu, K.H.; Park, S.E.; Moon, J.I.; Gho, S.-M.; Wakayama, T. Clinical Feasibility of Reduced Field-of-View Diffusion-Weighted Magnetic Resonance Imaging with Computed Diffusion-Weighted Imaging Technique in Breast Cancer Patients. Diagnostics 2020, 10, 538. https://doi.org/10.3390/diagnostics10080538
Cho E, Lee JH, Baek HJ, Ha JY, Ryu KH, Park SE, Moon JI, Gho S-M, Wakayama T. Clinical Feasibility of Reduced Field-of-View Diffusion-Weighted Magnetic Resonance Imaging with Computed Diffusion-Weighted Imaging Technique in Breast Cancer Patients. Diagnostics. 2020; 10(8):538. https://doi.org/10.3390/diagnostics10080538
Chicago/Turabian StyleCho, Eun, Jin Hwa Lee, Hye Jin Baek, Ji Young Ha, Kyeong Hwa Ryu, Sung Eun Park, Jin Il Moon, Sung-Min Gho, and Tetsuya Wakayama. 2020. "Clinical Feasibility of Reduced Field-of-View Diffusion-Weighted Magnetic Resonance Imaging with Computed Diffusion-Weighted Imaging Technique in Breast Cancer Patients" Diagnostics 10, no. 8: 538. https://doi.org/10.3390/diagnostics10080538
APA StyleCho, E., Lee, J. H., Baek, H. J., Ha, J. Y., Ryu, K. H., Park, S. E., Moon, J. I., Gho, S.-M., & Wakayama, T. (2020). Clinical Feasibility of Reduced Field-of-View Diffusion-Weighted Magnetic Resonance Imaging with Computed Diffusion-Weighted Imaging Technique in Breast Cancer Patients. Diagnostics, 10(8), 538. https://doi.org/10.3390/diagnostics10080538





