Peculiar Characteristics of Arteriovenous Malformations Arising in the Galenic Region
Abstract
1. Introduction
2. Materials and Methods
3. Results
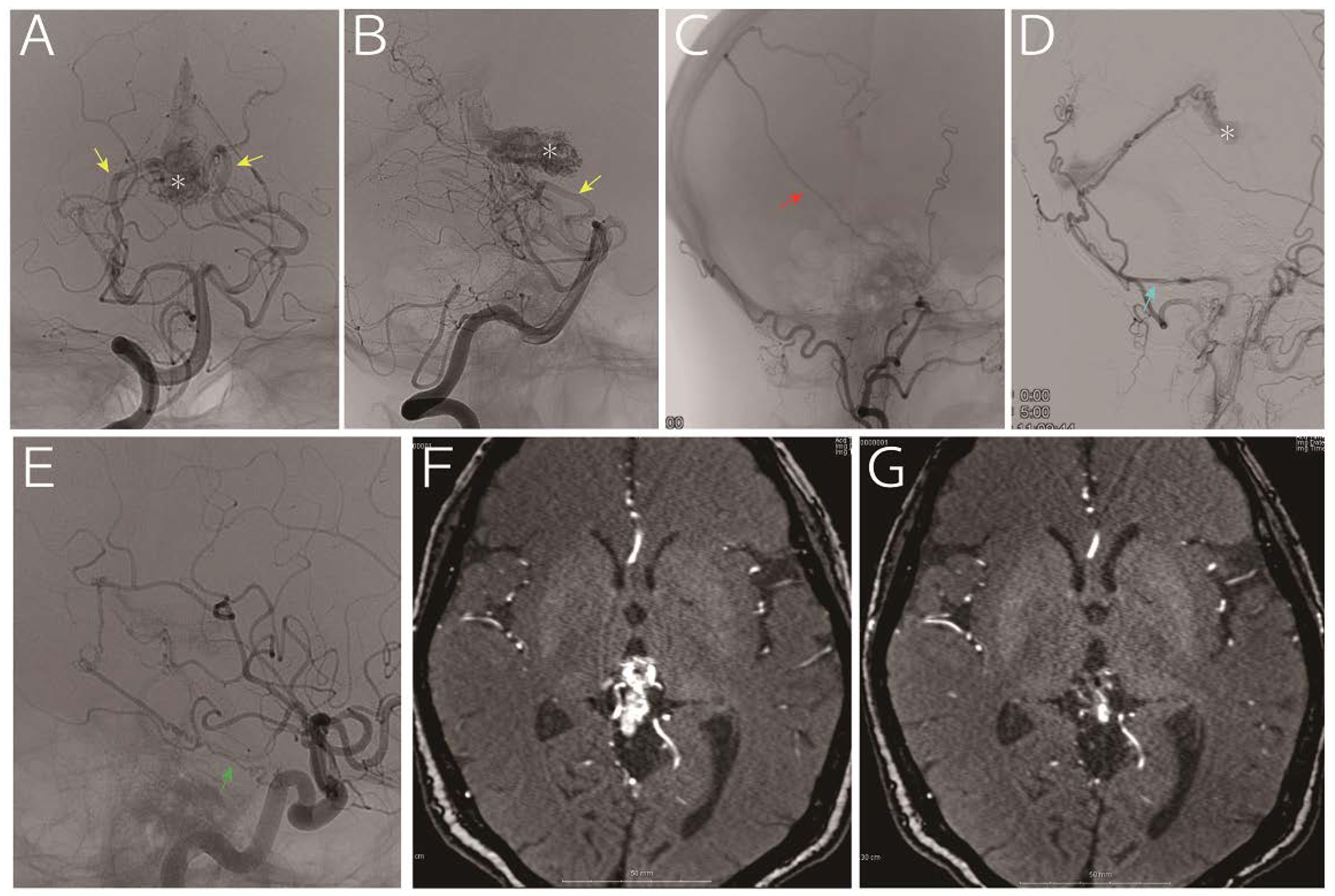
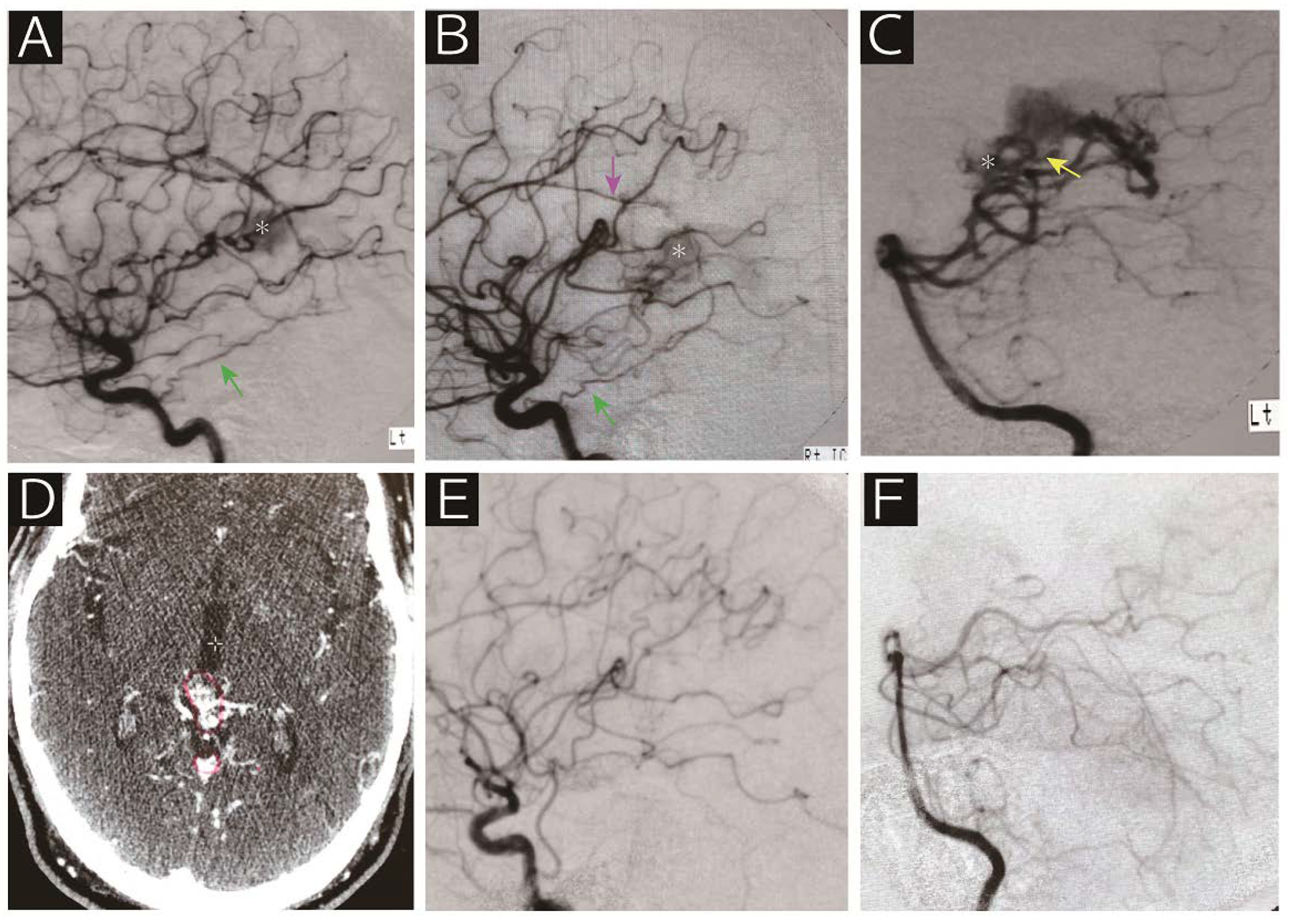
Radiographic Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choi, J.H.; Mohr, J.P. Brain arteriovenous malformations in adults. Lancet Neurol. 2005, 4, 299–308. [Google Scholar] [CrossRef]
- Fleetwood, I.G.; Steinberg, G.K. Arteriovenous malformations. Lancet 2002, 359, 863–873. [Google Scholar] [CrossRef]
- Friedlander, R.M. Clinical practice. Arteriovenous malformations of the brain. N. Engl. J. Med. 2007, 356, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.J.; Perret, G.E.; Torner, J.C. Bleeding from cerebral arteriovenous malformations as part of their natural history. J. Neurosurg. 1983, 58, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Ondra, S.L.; Troupp, H.; George, E.D.; Schwab, K. The natural history of symptomatic arteriovenous malformations of the brain: A 24-year follow-up assessment. J. Neurosurg. 1990, 73, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.P.; Lane, B.; Steinberg, G.K.; Chang, P.J. Hemorrhage in intracerebral arteriovenous malformations: Angiographic determinants. Radiology 1990, 176, 807–813. [Google Scholar] [CrossRef]
- Gross, B.A.; Du, R. Natural history of cerebral arteriovenous malformations: A meta-analysis. J. Neurosurg. 2013, 118, 437–443. [Google Scholar] [CrossRef]
- Stefani, M.A.; Porter, P.J.; terBrugge, K.G.; Montanera, W.; Willinsky, R.A.; Wallace, M.C. Angioarchitectural factors present in brain arteriovenous malformations associated with hemorrhagic presentation. Stroke 2002, 33, 920–924. [Google Scholar] [CrossRef]
- Yamamoto, I.; Kageyama, N. Microsurgical anatomy of the pineal region. J. Neurosurg. 1980, 53, 205–221. [Google Scholar] [CrossRef]
- Wilner, H.I.; Crockett, J.; Gilroy, J. The galenic venous system: A selective radiographic study. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1972, 115, 1–13. [Google Scholar] [CrossRef][Green Version]
- Weigele, J.B.; Chaloupka, J.C.; Lesley, W.S. Galenic dural arteriovenous fistula: Unusual clinical presentation and successful endovascular therapy. Case report. J. Neurosurg. 2002, 97, 467–470. [Google Scholar] [CrossRef] [PubMed]
- De Vleeschouwer, S.; Smets, C.A.; Wilms, G. Long-lasting, complete exclusion of a large galenic dural arteriovenous fistula after clipping of the central venous aneurysm of the vein of galen: Case report. Neurosurgery 2011, 68, E571–E574. [Google Scholar] [CrossRef] [PubMed]
- Choudhri, O.; Steinberg, G.K. Microsurgical treatment of a tentorial galenic dural arteriovenous fistula. Neurosurg. Focus 2016, 40. (Video Suppl. S1), (2016 2011 FocusVid 15420). [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.E.; Gomori, J.M.; Rajz, G.; Paldor, I.; Moscovici, S.; Itshayek, E. Clinical and angioarchitectural factors influencing the endovascular approach to galenic dural arteriovenous fistulas in adults: Case series and review of the literature. Acta Neurochir. 2017, 159, 845–853. [Google Scholar] [CrossRef]
- Gupta, A.K.; Varma, D.R. Vein of Galen malformations: Review. Neurol. India 2004, 52, 43–53. [Google Scholar]
- Hoang, S.; Choudhri, O.; Edwards, M.; Guzman, R. Vein of Galen malformation. Neurosurg. Focus 2009, 27, E8. [Google Scholar] [CrossRef]
- Solomon, R.A.; Connolly, E.S., Jr. Arteriovenous Malformations of the Brain. N. Engl. J. Med. 2017, 376, 1859–1866. [Google Scholar] [CrossRef]
- Tomsick, T.A.; Ernst, R.J.; Tew, J.M.; Brott, T.G.; Breneman, J.C. Adult choroidal vein of Galen malformation. AJNR Am. J. Neuroradiol. 1995, 16, 861–865. [Google Scholar]
- Black, K.L.; Farhat, S.M. Giant arteriovenous malformation of the vein of Galen in an adult. Surg. Neurol. 1984, 22, 382–386. [Google Scholar] [CrossRef][Green Version]
- Kerolus, M.G.; Tan, L.A.; Lopes, D.K. Giant vein of Galen malformation in an adult. Radiol. Case Rep. 2017, 12, 585–589. [Google Scholar] [CrossRef]
- Xu, D.S.; Usman, A.A.; Hurley, M.C.; Eddleman, C.S.; Bendok, B.R. Adult presentation of a familial-associated vein of Galen aneurysmal malformation: Case report. Neurosurgery 2010, 67, E1845–E1851. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.D.; Kortman, H.; Wälchli, T.; Radovanovic, I.; Pereira, V.M.; Krings, T. Artery of Davidoff and Schechter Supply in Dural Arteriovenous Fistulas. AJNR Am. J. Neuroradiol. 2020, 41, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Kurita, H.; Tago, M.; Kirino, T. Stereotactic radiosurgery for tentorial dural arteriovenous fistulae draining into the vein of Galen: Report of two cases. Neurosurgery 2000, 46, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Hacein-Bey, L.; Konstas, A.A.; Pile-Spellman, J. Natural history, current concepts, classification, factors impacting endovascular therapy, and pathophysiology of cerebral and spinal dural arteriovenous fistulas. Clin. Neurol. Neurosurg. 2014, 121, 64–75. [Google Scholar] [CrossRef]
- Graeb, D.A.; Dolman, C.L. Radiological and pathological aspects of dural arteriovenous fistulas. Case report. J. Neurosurg. 1986, 64, 962–967. [Google Scholar] [CrossRef]
- Bulters, D.O.; Mathad, N.; Culliford, D.; Millar, J.; Sparrow, O.C. The natural history of cranial dural arteriovenous fistulae with cortical venous reflux--the significance of venous ectasia. Neurosurgery 2012, 70, 312–319. [Google Scholar] [CrossRef]
- Gross, B.A.; Du, R. The natural history of cerebral dural arteriovenous fistulae. Neurosurgery 2012, 71, 594–602. [Google Scholar] [CrossRef]
- Gandhi, D.; Chen, J.; Pearl, M.; Huang, J.; Gemmete, J.J.; Kathuria, S. Intracranial dural arteriovenous fistulas: Classification, imaging findings, and treatment. AJNR Am. J. Neuroradiol. 2012, 33, 1007–1013. [Google Scholar] [CrossRef]
- Starke, R.M.; Kano, H.; Ding, D.; Lee, J.Y.; Mathieu, D.; Whitesell, J.; Pierce, J.T.; Huang, P.P.; Kondziolka, D.; Yen, C.P.; et al. Stereotactic radiosurgery for cerebral arteriovenous malformations: Evaluation of long-term outcomes in a multicenter cohort. J. Neurosurg. 2017, 126, 36–44. [Google Scholar] [CrossRef]
- Friedman, W.A.; Blatt, D.L.; Bova, F.J.; Buatti, J.M.; Mendenhall, W.M.; Kubilis, P.S. The risk of hemorrhage after radiosurgery for arteriovenous malformations. J. Neurosurg. 1996, 84, 912–919. [Google Scholar] [CrossRef]
- Pollock, B.E.; Flickinger, J.C.; Lunsford, L.D.; Bissonette, D.J.; Kondziolka, D. Hemorrhage risk after stereotactic radiosurgery of cerebral arteriovenous malformations. Neurosurgery 1996, 38, 652–661. [Google Scholar] [CrossRef] [PubMed]

| No. | Age *, Sex | Initial Presentation | Volume | Cerebral Artery Feeders | Dural Artery Feeders | Drainers | Follow-Up Period | Current Status |
|---|---|---|---|---|---|---|---|---|
| 1 | 65, M | Hydrocephalus | 9.4 mL | MPChA, BA perforators | Tentorial a., MMA, PMA | VG | 153 mo | Died due to hemorrhage |
| 2 | 62, M | Seizure | 1.4 mL | MPChA, PCA perforators, pericallosal a. | Tentorial a. | VG | 255 mo | Alive, nidus obliteration |
| 3 | 68, M | Hydrocephalus | 0.9 mL | MPChA, PCA perforators | N/A | VG | 137 mo | Alive, nidus shrinkage |
| 4 | 31, F | Hydrocephalus and hemorrhage † | 12.2 mL | MPChA, PCA perforators | Tentorial a (N/A for the other ECA feeders) | VG | 38 mo | Alive, nidus shrinkage |
| 5 | 63, F | Headache | 2.3 mL | MPChA, PCA perforators, BA perforators | Tentorial a, MMA, PMA | VG | 47 mo | Alive, nidus shrinkage |
| 6 | 62, M | Hemorrhage | 1.2 mL | MPChA, BA perforators | Tentorial a, ILT | VG, superior vermian vein | 23 mo | Alive, nidus shrinkage |
| 7 | 55, M | Hemorrhage from flow-related AN | 1.6 mL | MPChA, pericallosal a. | ILT, PMA | VG, superior vermian vein | 12 mo | Alive, nidus shrinkage |
| 8 | 76, M | Hemorrhage | 1.7 mL | MPChA, PCA perforators | Tentorial a. | VG | 4 mo | Alive |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yajima, H.; Shinya, Y.; Hasegawa, H.; Shin, M.; Ueki, K.; Kawashima, M.; Ishikawa, O.; Saito, N. Peculiar Characteristics of Arteriovenous Malformations Arising in the Galenic Region. Diagnostics 2020, 10, 481. https://doi.org/10.3390/diagnostics10070481
Yajima H, Shinya Y, Hasegawa H, Shin M, Ueki K, Kawashima M, Ishikawa O, Saito N. Peculiar Characteristics of Arteriovenous Malformations Arising in the Galenic Region. Diagnostics. 2020; 10(7):481. https://doi.org/10.3390/diagnostics10070481
Chicago/Turabian StyleYajima, Hirohisa, Yuki Shinya, Hirotaka Hasegawa, Masahiro Shin, Keisuke Ueki, Mariko Kawashima, Osamu Ishikawa, and Nobuhito Saito. 2020. "Peculiar Characteristics of Arteriovenous Malformations Arising in the Galenic Region" Diagnostics 10, no. 7: 481. https://doi.org/10.3390/diagnostics10070481
APA StyleYajima, H., Shinya, Y., Hasegawa, H., Shin, M., Ueki, K., Kawashima, M., Ishikawa, O., & Saito, N. (2020). Peculiar Characteristics of Arteriovenous Malformations Arising in the Galenic Region. Diagnostics, 10(7), 481. https://doi.org/10.3390/diagnostics10070481






