Evaluation of Commercial qPCR Kits for Detection of SARS-CoV-2 in Pooled Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. RNA Extraction
2.3. Real-Time PCR Analysis
2.4. Pooling Validation
2.5. Ethical Considerations
3. Results
3.1. Evaluation of Commercial SARS-CoV-2 qPCR Kits
3.2. Evaluation of Different Clinical Specimens Collected from COVID-19-Infected Patients
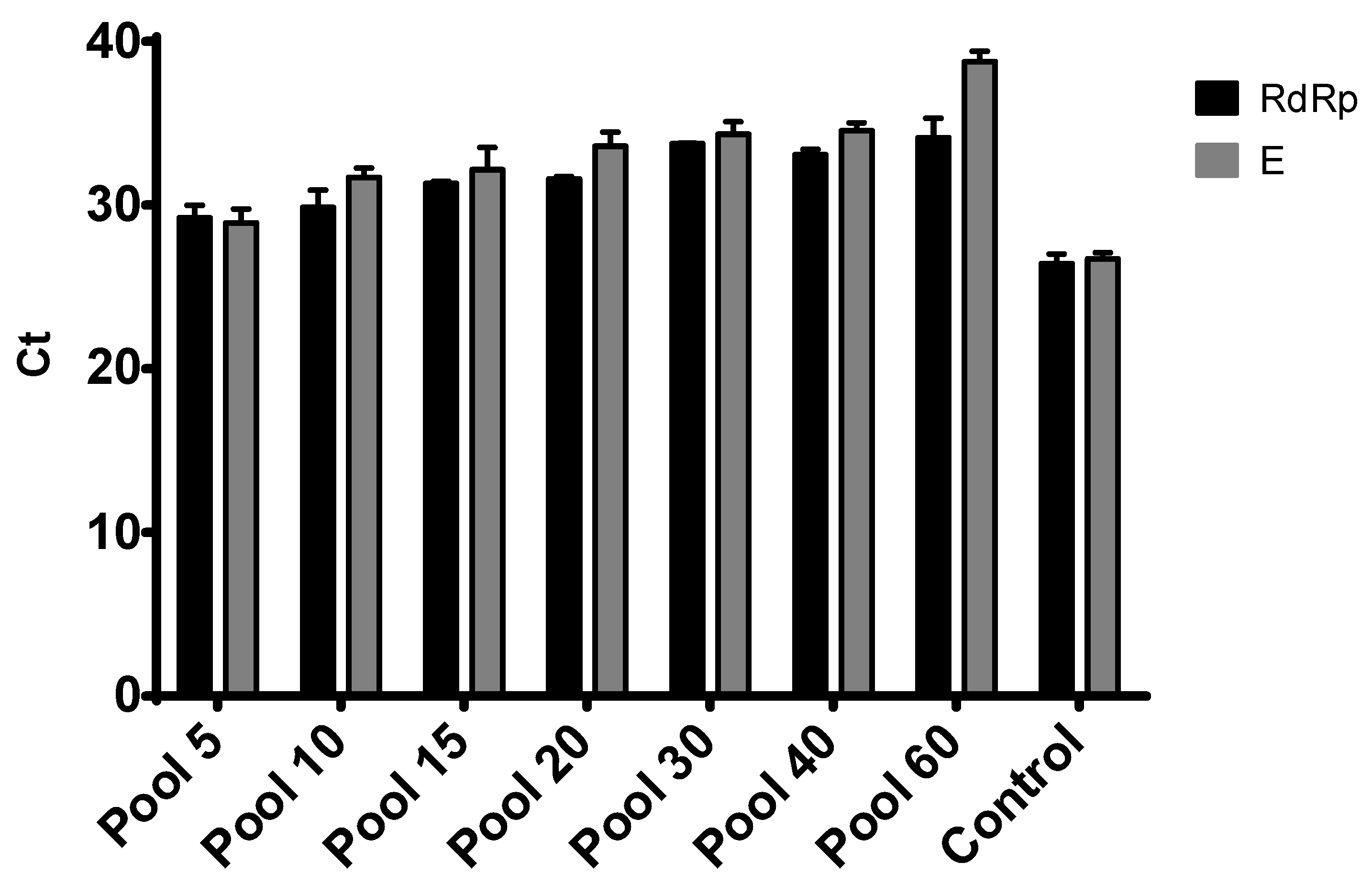
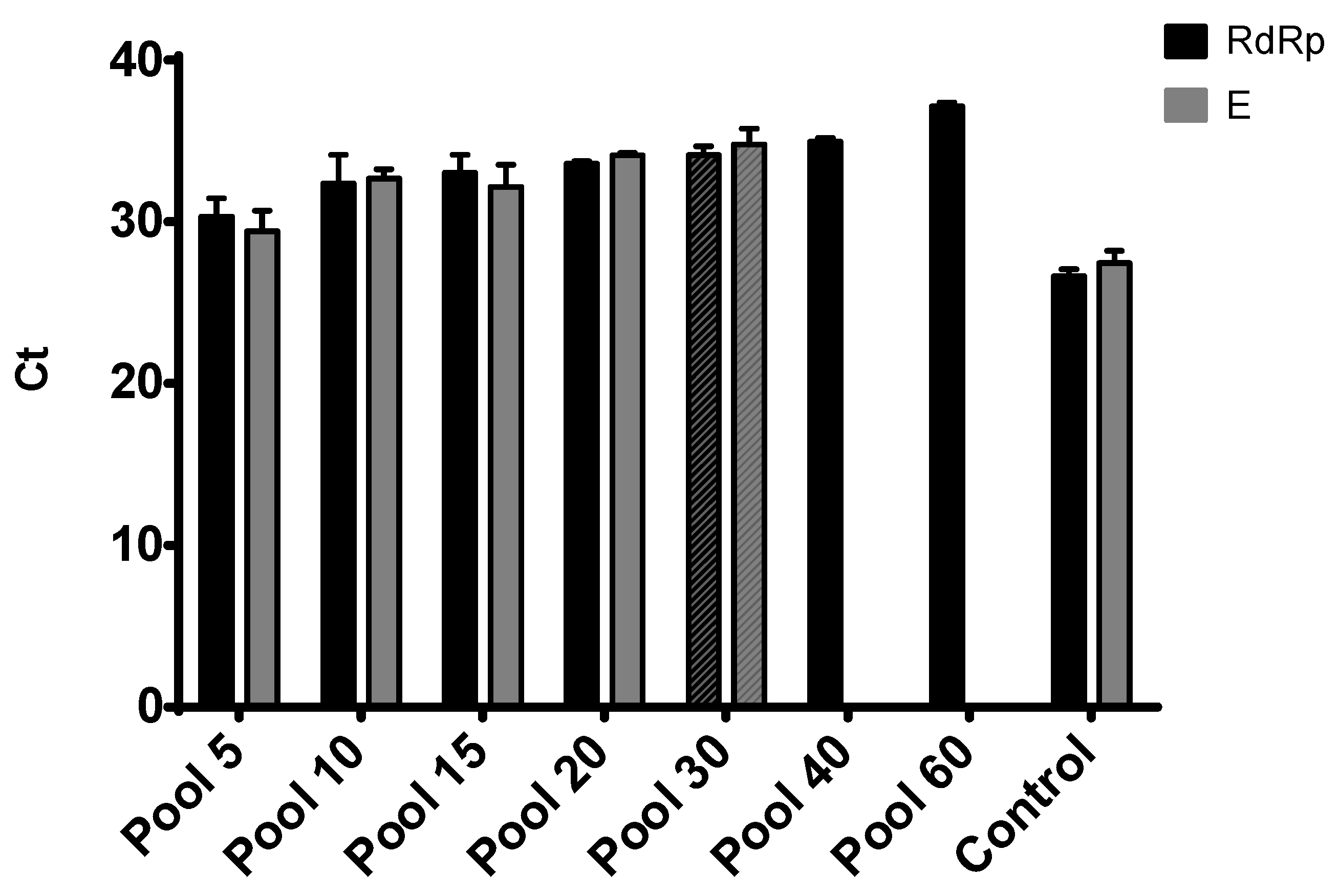
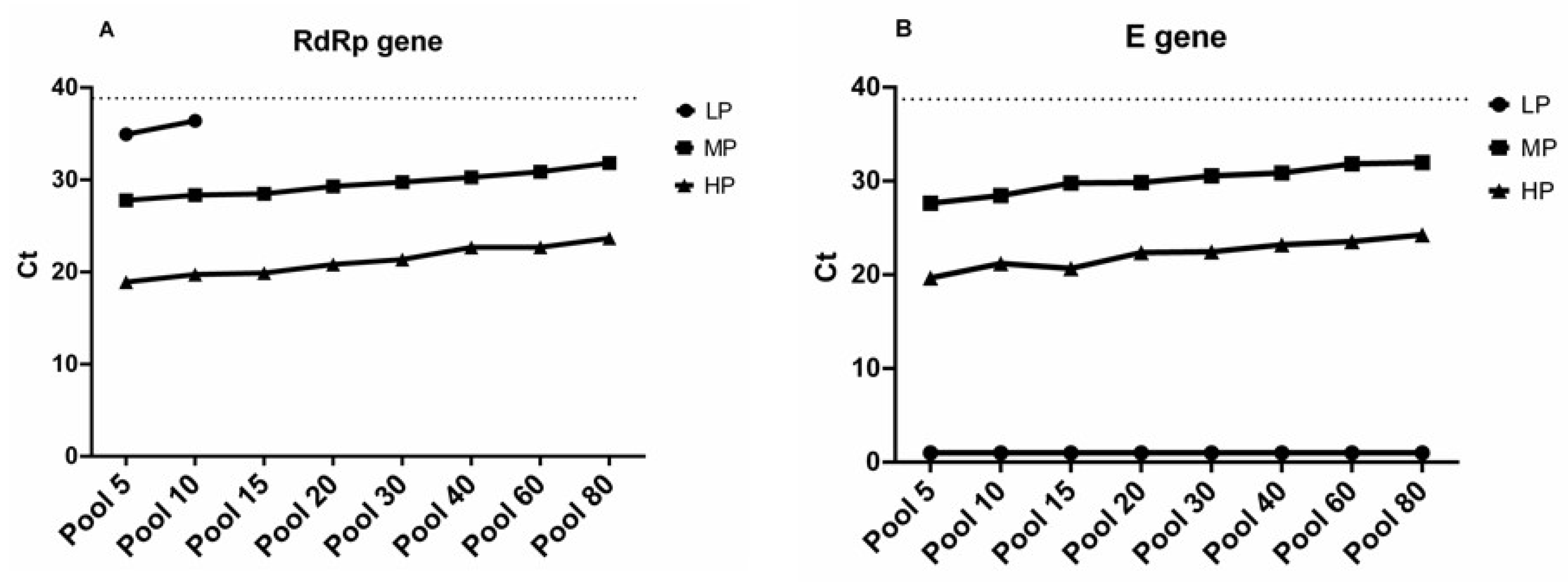
3.3. Sample Pooling and Comparative Performance of Targets
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Coronavirus. Geneva: WHO. 2020. Available online: https://www.who.int/health-topics/coronavirus (accessed on 20 June 2020).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Esbin, M.N.; Whitney, O.N.; Chong, S.; Maurer, A.; Darzacq, X.; Tjian, R. Overcoming the bottleneck to widespread testing: A rapid review of nucleic acid testing approaches for COVID-19 detection. RNA 2020. In press. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Pan, Y.; Cheng, S.M.; Hui, K.P.; Krishnan, P.; Liu, Y.; Ng, D.Y.; Wan, C.K.; Yang, P.; Wang, Q.; et al. Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clin. Chem. 2020, 66, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Detmer, S.E.; Patnayak, D.P.; Jiang, Y.; Gramer, M.R.; Goyal, S.M. Detection of Influenza A virus in porcine oral fluid samples. J. Vet. Diag. Investig. 2011, 23, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Prickett, J.R.; Zimmerman, J.J. The development of oral fluid-based diagnostics and applications in veterinary medicine. Anim. Health. Res. Rev. 2010, 11, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Grau, F.R.; Schroeder, M.E.; Mulhern, E.L.; McIntosh, M.T.; Bounpheng, M.A. Detection of African swine fever, classical swine fever, and foot-and-mouth disease viruses in swine oral fluids by multiplex reverse transcription real-time polymerase chain reaction. J. Vet. Diagn. Investig. 2015, 27, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.J.; Patel, P.; Hutchinson, A.; Ethridge, S.F.; Parker, M.M. Evaluation of pooling strategies for acute HIV-1 infection screening using nucleic acid amplification testing. J. Clin. Microbiol. 2011, 49, 3667–3668. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Aprahamian, H.; Bish, E.K.; Bish, D.R. A methodology for deriving the sensitivity of pooled testing, based on viral load progression and pooling dilution. J. Transl. Med. 2019, 17, 252. [Google Scholar] [CrossRef] [PubMed]
- Yelin, I.; Aharony, N.; Shaer-Tamar, E.; Argoetti, A.; Messer, E.; Berenbaum, D.; Shafran, E.; Kuzli, A.; Gandali, N.; Hashimshony, T.; et al. Evaluation of COVID-19 RT-qPCR test in multi-sample pools. Clin. Infect Dis. 2020, In press. [CrossRef] [PubMed]
- Chan, J.F.W.; Yip, C.C.Y.; To, K.K.W.; Tang, T.H.C.; Wong, S.C.Y.; Leung, K.H.; Fung, A.Y.F.; Ng, A.C.K.; Zou, Z.; Tsoi, H.W.; et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J. Clin. Microb. 2020, 58, 5. [Google Scholar] [CrossRef] [PubMed]
- European Economies to Open before the U.S. Available online: https://www.forbes.com/sites/kenrapoza/2020/04/20/european-economies-to-open-before-the-us/ (accessed on 20 June 2020).
- Torres, I.; Albert, E.; Navarro, D. Pooling of nasopharyngeal swab specimens for SARS-CoV-2 detection by RT-PCR. J. Med. Virol. 2020. In press. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.X.; Liu, B.Z.; Deng, H.J.; Wu, G.C.; Deng, K.; Chen, Y.K.; Liao, P.; Qiu, J.F.; Lin, Y.; Cai, X.F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Xu, H.; Shen, Q.; Zhang, X.; Fan, B.; Wang, C.; Zeng, B.; Li, Z.; Li, X.; Li, H.; et al. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur. J. Radio. 2020, 126, 108961. [Google Scholar] [CrossRef] [PubMed]
| Kit | Kogene2019-nCoV | COVID-19 Diatheva | 2019-nCoV CDC EUA | |||
|---|---|---|---|---|---|---|
| Gene | RdRp | E | RdRp | E | N1 | N2 |
| Undil. | 20.25 ± 0.25 | 21.61 ± 0.31 | 24.68 ± 0.47 | 26.63 ± 0.22 | 24.63 ± 0.25 | 25.05 ± 0.46 |
| 10−1 | 22.12 ± 0.45 | 23.45 ± 0.62 | 26.02 ± 0.42 | 28.65 ± 0.74 | 26.22 ± 0.44 | 28.02 ± 0.13 |
| 10−2 | 25.45 ± 0.78 | 25.78 ± 0.46 | 29.35 ± 0.78 | 30.45 ± 0.54 | 30.21 ± 0.66 | 31.44 ± 0.54 |
| 10−3 | 27.88 ± 0.23 | 29.95 ± 0.22 | 31.44 ± 0.38 | 31.86 ± 0.33 | 33 ± 0.72 | - |
| 10−4 | 30.66 ± 0.77 | 32.12 ± 1.13 | 33.78 ± 0.12 | 34.74 ± 0.71 | - | - |
| 10−5 | 33.35 ± 0.66 | 34.13 ± 1.52 | 36.25 ± 0.46 | 36.67 ± 0.39 | - | - |
| 10−6 | 35.85 ± 0.89 | 35.94 * | 38.34 ± 1.25 | 38.63 ± 1.19 | - | - |
| 10−7 | 38.21 ± 0.95 | 39.10 ± 0.95 | - | - | ||
| 10−8 | - | - | ||||
| Gender | Age | Classification Status | Matrix | Comorbidities | Ct | |
|---|---|---|---|---|---|---|
| E | RdRp | |||||
| Male * | 35 | asymptomatic | swab | - | 26.21 ± 1.12 | 26.72 ± 0.88 |
| Male * | 70 | severe | sputum | hypertension, diabetes | 25.95 ± 0.81 | 23.67 ± 0.45 |
| Female | 55 | moderate | sputum | hypertension, obesity | 26.18 ± 0.65 | 26.92 ± 0.75 |
| Female | 42 | mild | swab a | - | 25.95 ± 0.35 | 26.26 ± 0.45 |
| Male | 66 | asymptomatic | swab a | - | 26.32 ± 0.78 | 26.14 ± 0.23 |
| Female * | 48 | moderate | swab | hypertension, asthma | 25.87 ± 0.45 | 26.38 ± 0.44 |
| Male | 65 | moderate | swab | pneumonia | 25.75 ± 0.56 | 26.54 ± 0.57 |
| Male | 71 | severe | sputum | hearth disease | 20.43 ± 0.87 | 21.56 ± 0.98 |
| Female | 23 | moderate | sputum | diabetes, kidney disease | 25.91 ± 0.93 | 26.37 ± 0.65 |
| Female | 38 | asymptomatic | swab | - | 26.13 ± 0.21 | 26.98 ± 0.74 |
| Male | 61 | severe | swab | obesity, heart disease | 25.87 ± 0.08 | 26.56 ± 0.44 |
| Male | 45 | asymptomatic | swab | - | 25.89 ± 0.65 | 26.48 ± 0.36 |
| Male * | 47 | asymptomatic | swab a | - | 26.51 ± 0.47 | 26.56 ± 0.34 |
| Female | 38 | mild | swab a | - | 26.27 ± 0.24 | 25.93 ± 0.22 |
| Female | 50 | severe | swab | immunocompromised | 25.87 ± 0.89 | 26.13 ± 0.71 |
| Male | 22 | mild | swab | - | 26.01 ± 1.12 | 26.48 ± 0.52 |
| Male * | 80 | moderate | swab | dementia | 25.65 ± 1.23 | 26.38 ± 1.3 |
| Male * | 77 | moderate | sputum | diabetes | 26.29 ± 0.69 | 24.67 ± 0.44 |
| Female | 62 | mild | swab b | - | 26.37 ± 0.77 | 26.52 ± 0.29 |
| Male * | 66 | mild | swab b | - | 26.01 ± 0.35 | 26.64 ± 0.86 |
| Female | 50 | mild | swab | - | 25.87 ± 0.62 | 26.59 ± 0.41 |
| Male | 53 | asymptomatic | swab | - | 26.03 ± 0.21 | 26.48 ± 0.36 |
| Male | 58 | mild | swab b | - | 25.73 ± 0.15 | 26.12 ± 0.55 |
| Female | 48 | asymptomatic | swab | - | 25.87 ± 0.44 | 26.48 ± 0.22 |
| ref. sample 1 | 16.58 ± 0.33 | 16.78 ± 0.51 | ||||
| ref. sample 2 | 25.27 ± 0.70 | 26.50 ± 0.38 | ||||
| ref. sample 3 | 33.98 ± 0.97 | 34.50 ± 0.85 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovan, V.; Vrajmasu, V.; Bucur, A.C.; Soare, D.S.; Radu, E.; Dimon, P.; Zaulet, M. Evaluation of Commercial qPCR Kits for Detection of SARS-CoV-2 in Pooled Samples. Diagnostics 2020, 10, 472. https://doi.org/10.3390/diagnostics10070472
Petrovan V, Vrajmasu V, Bucur AC, Soare DS, Radu E, Dimon P, Zaulet M. Evaluation of Commercial qPCR Kits for Detection of SARS-CoV-2 in Pooled Samples. Diagnostics. 2020; 10(7):472. https://doi.org/10.3390/diagnostics10070472
Chicago/Turabian StylePetrovan, Vlad, Virgil Vrajmasu, Ana Cristina Bucur, Dan Sebastian Soare, Eugen Radu, Paula Dimon, and Mihaela Zaulet. 2020. "Evaluation of Commercial qPCR Kits for Detection of SARS-CoV-2 in Pooled Samples" Diagnostics 10, no. 7: 472. https://doi.org/10.3390/diagnostics10070472
APA StylePetrovan, V., Vrajmasu, V., Bucur, A. C., Soare, D. S., Radu, E., Dimon, P., & Zaulet, M. (2020). Evaluation of Commercial qPCR Kits for Detection of SARS-CoV-2 in Pooled Samples. Diagnostics, 10(7), 472. https://doi.org/10.3390/diagnostics10070472





