Multifocal Amelanotic Melanoma of the Hard Palate: A Challenging Case
Abstract
:1. Introduction
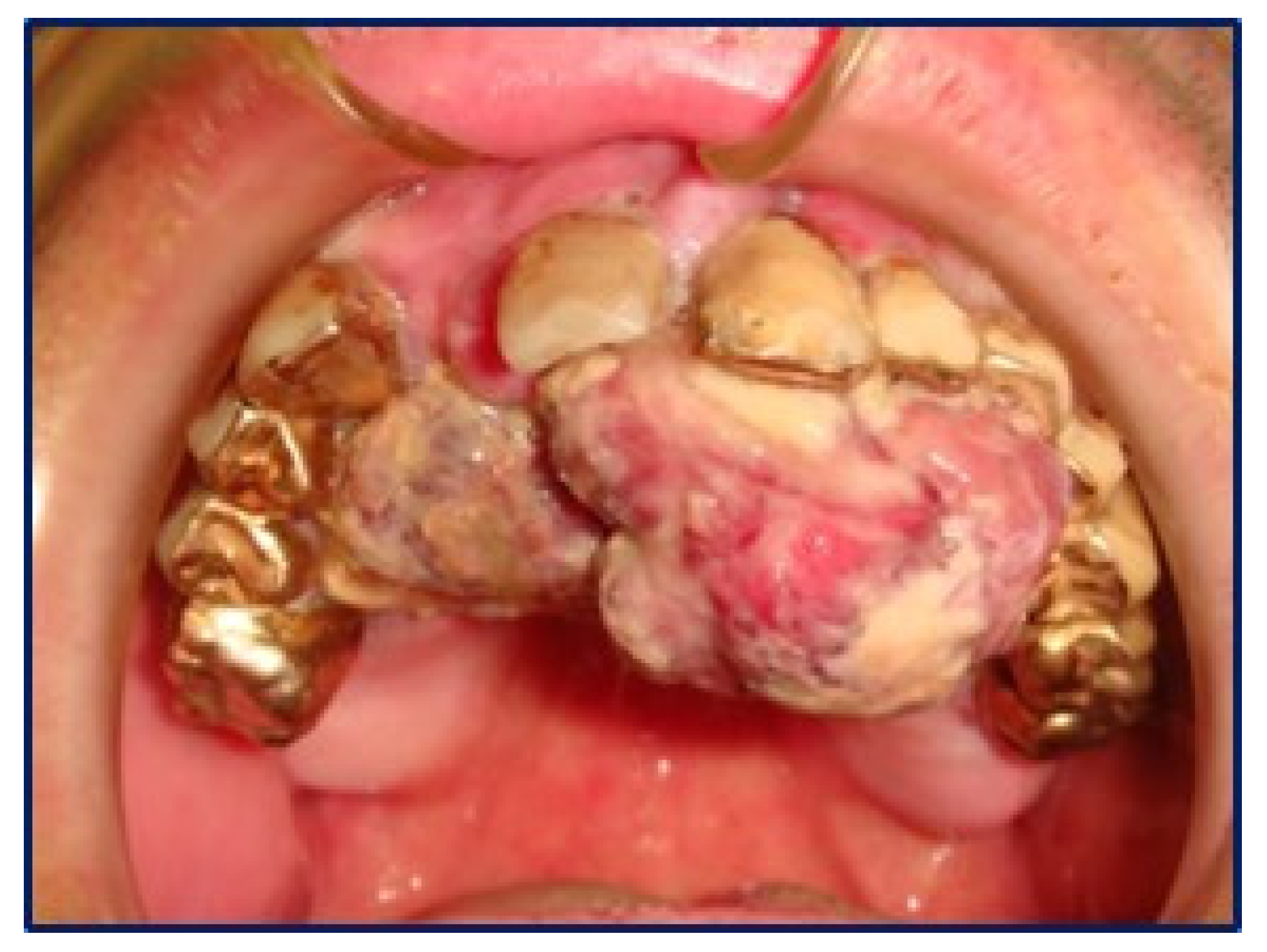
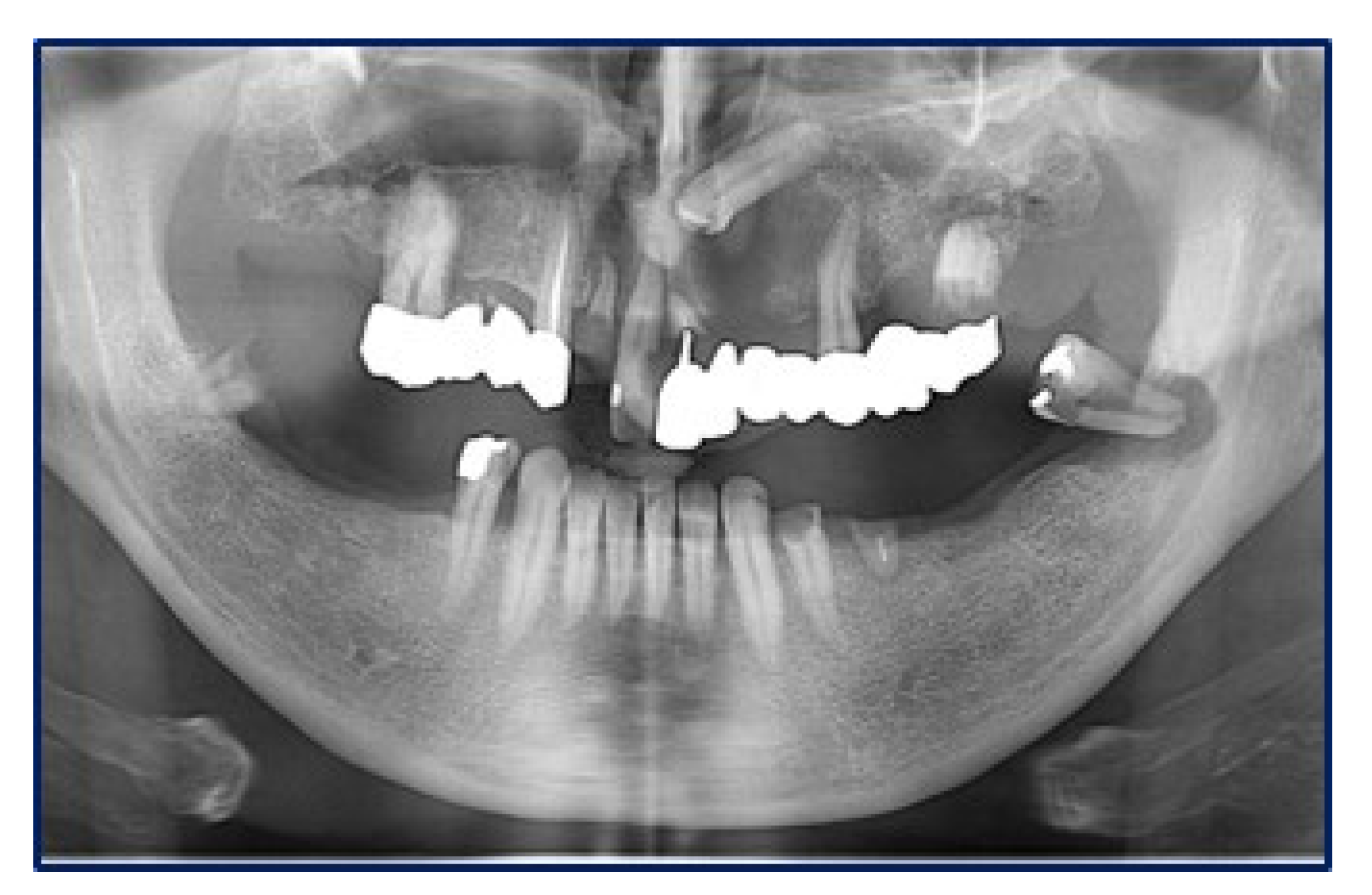
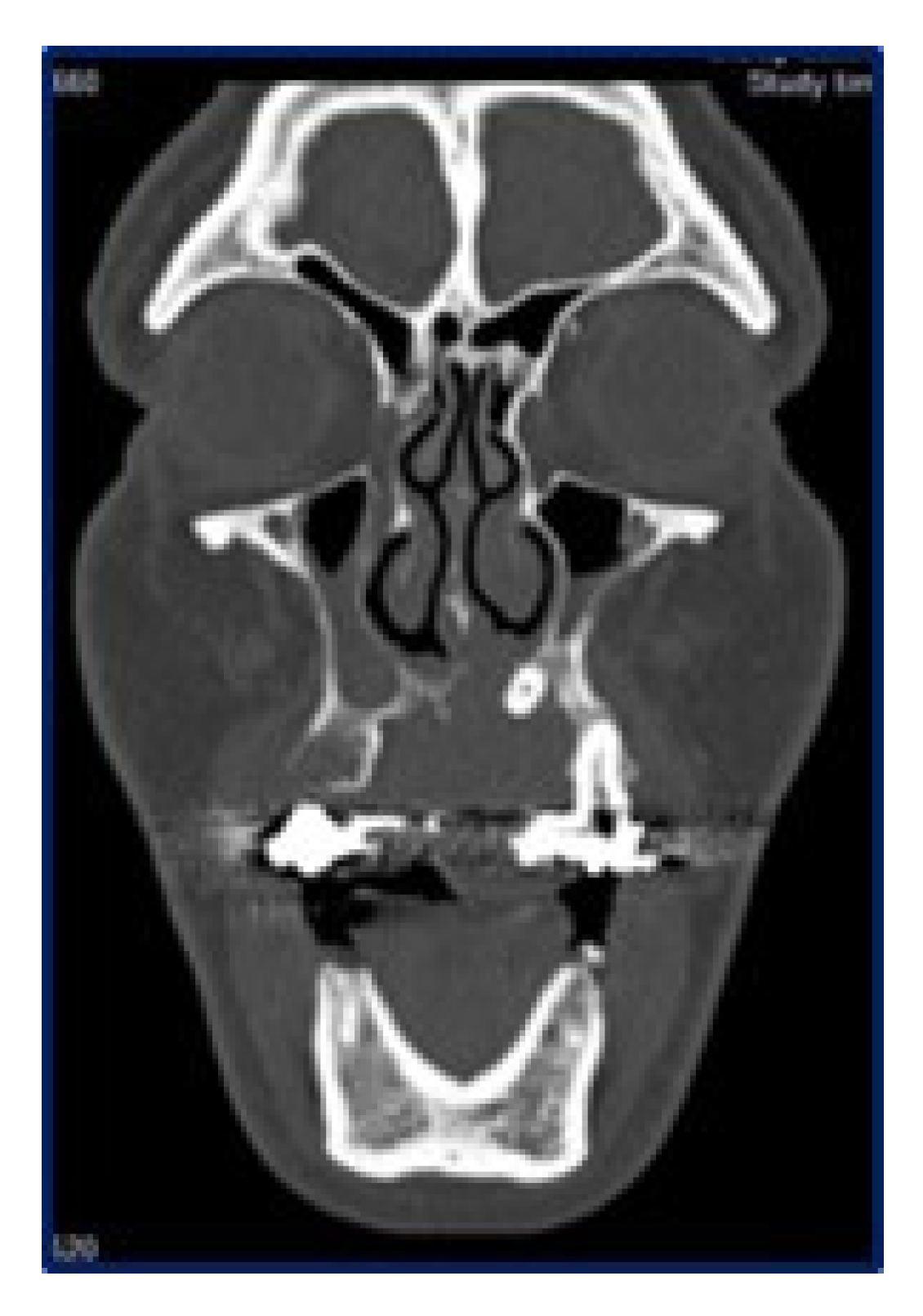
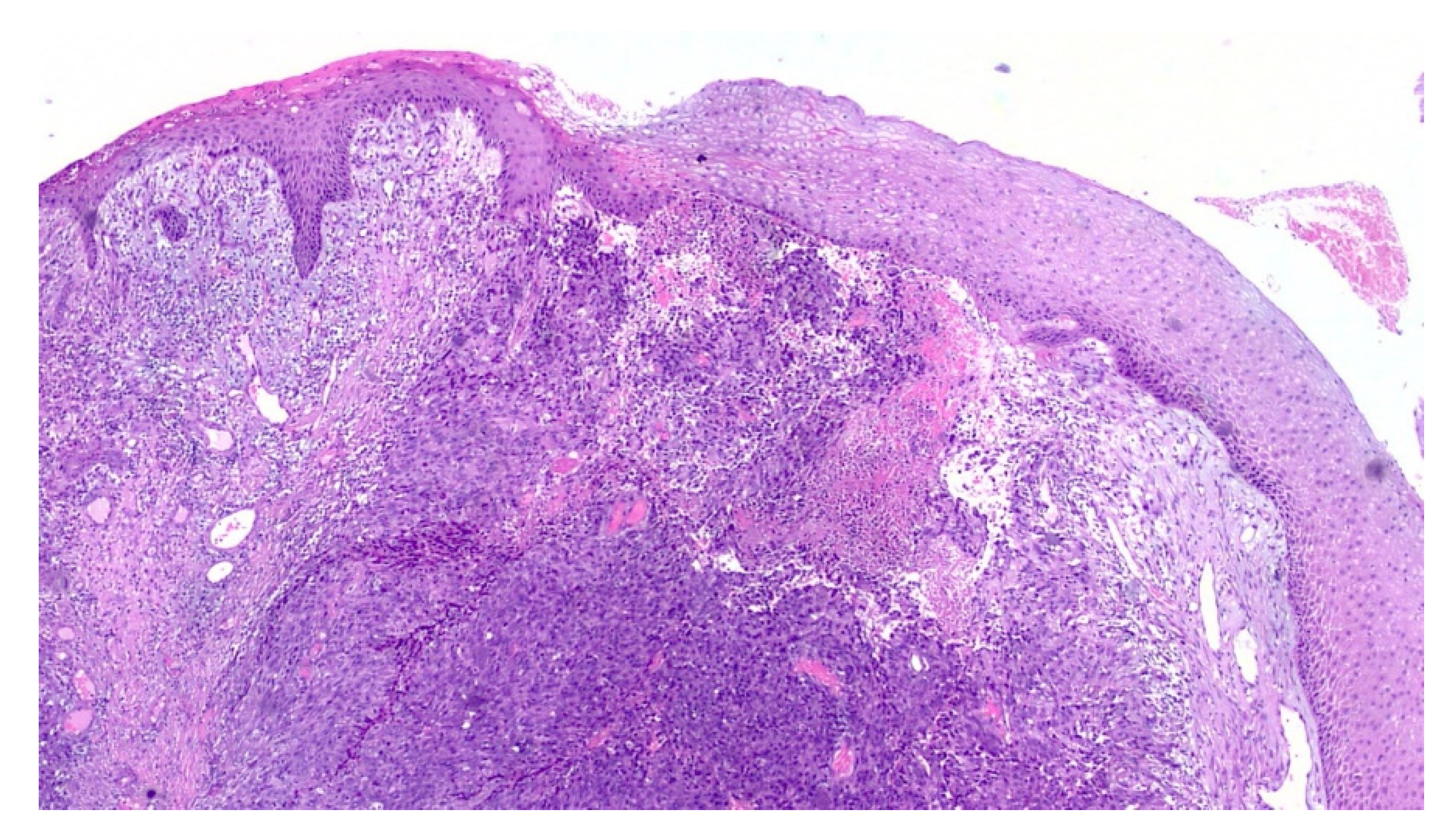
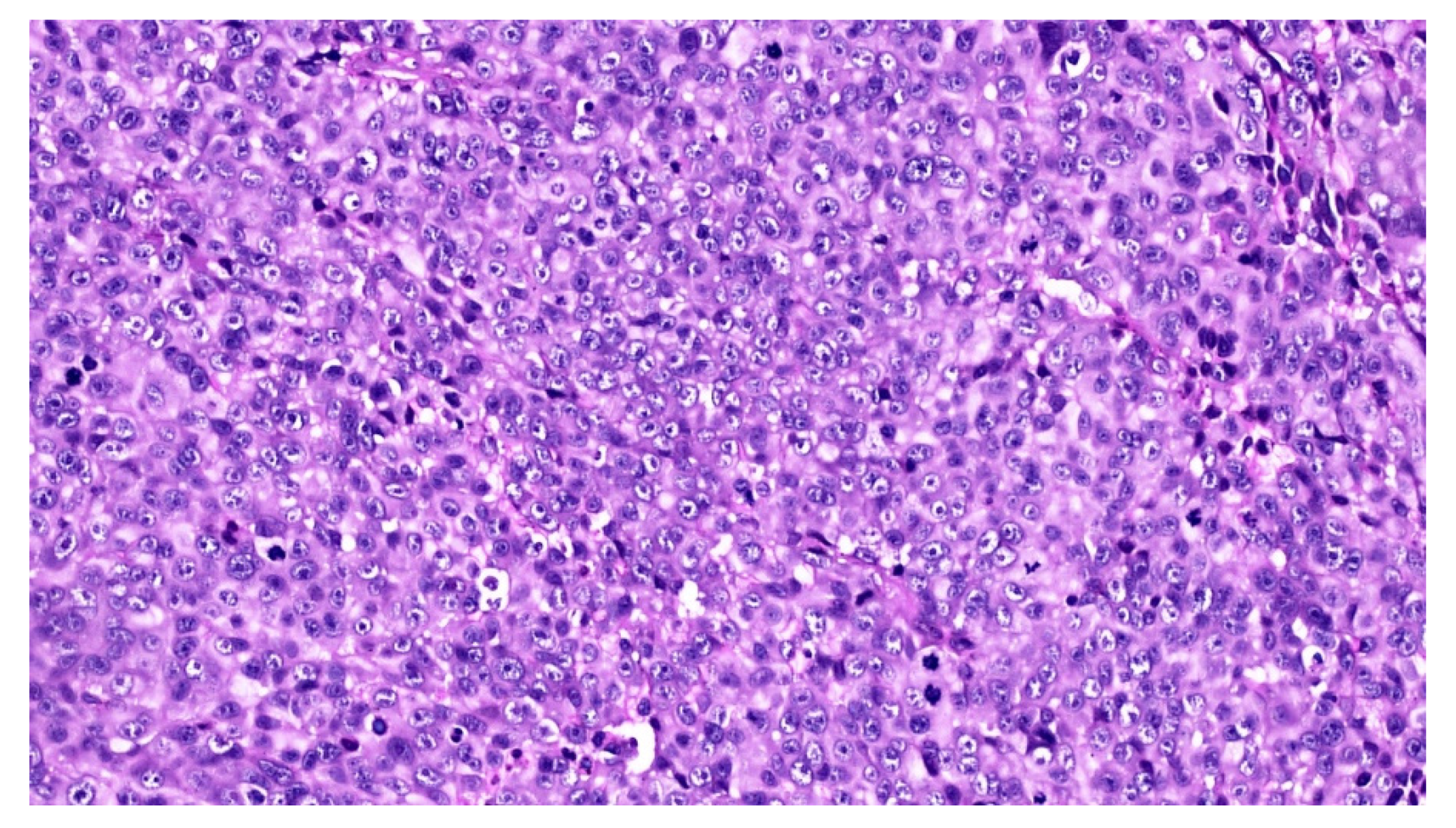
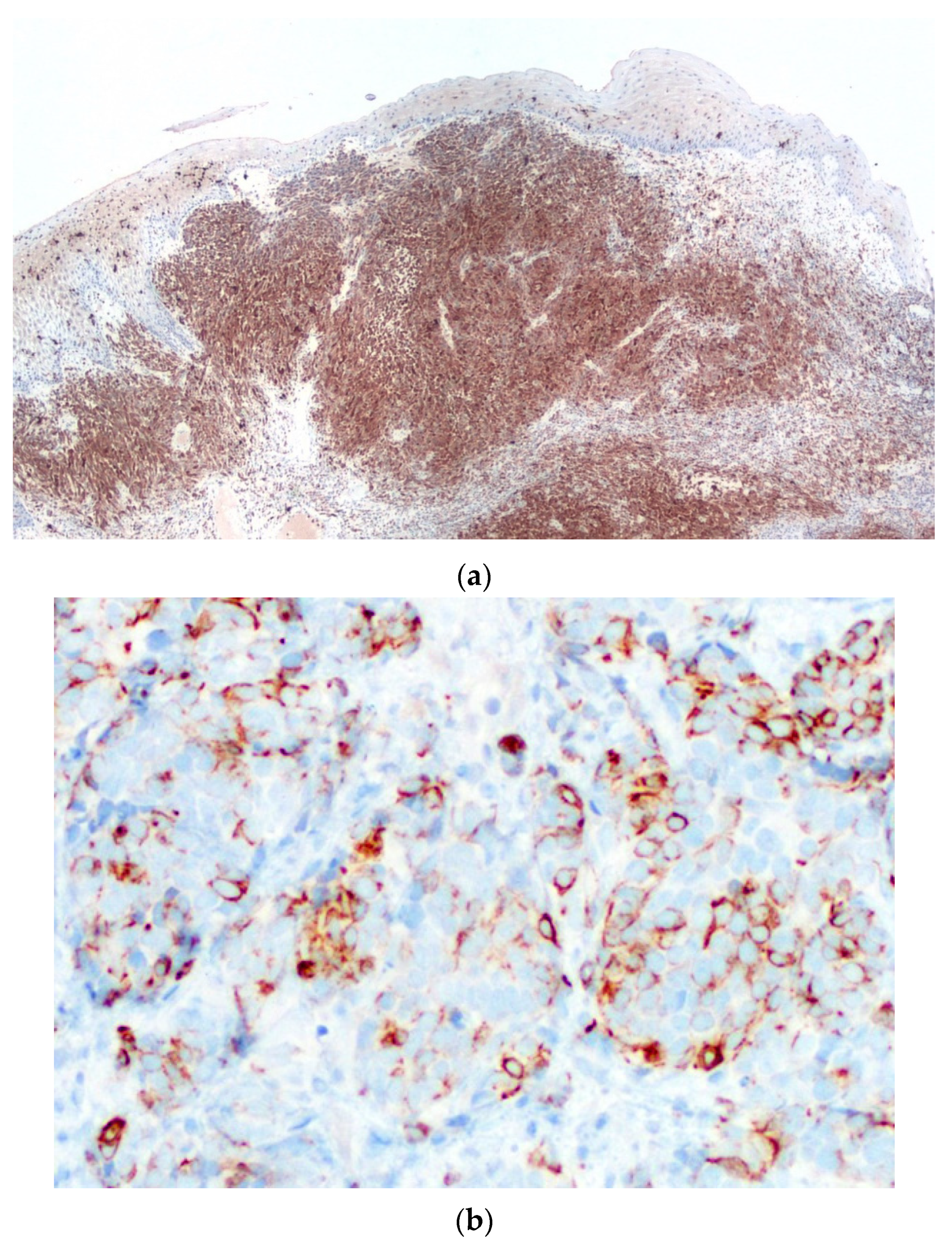
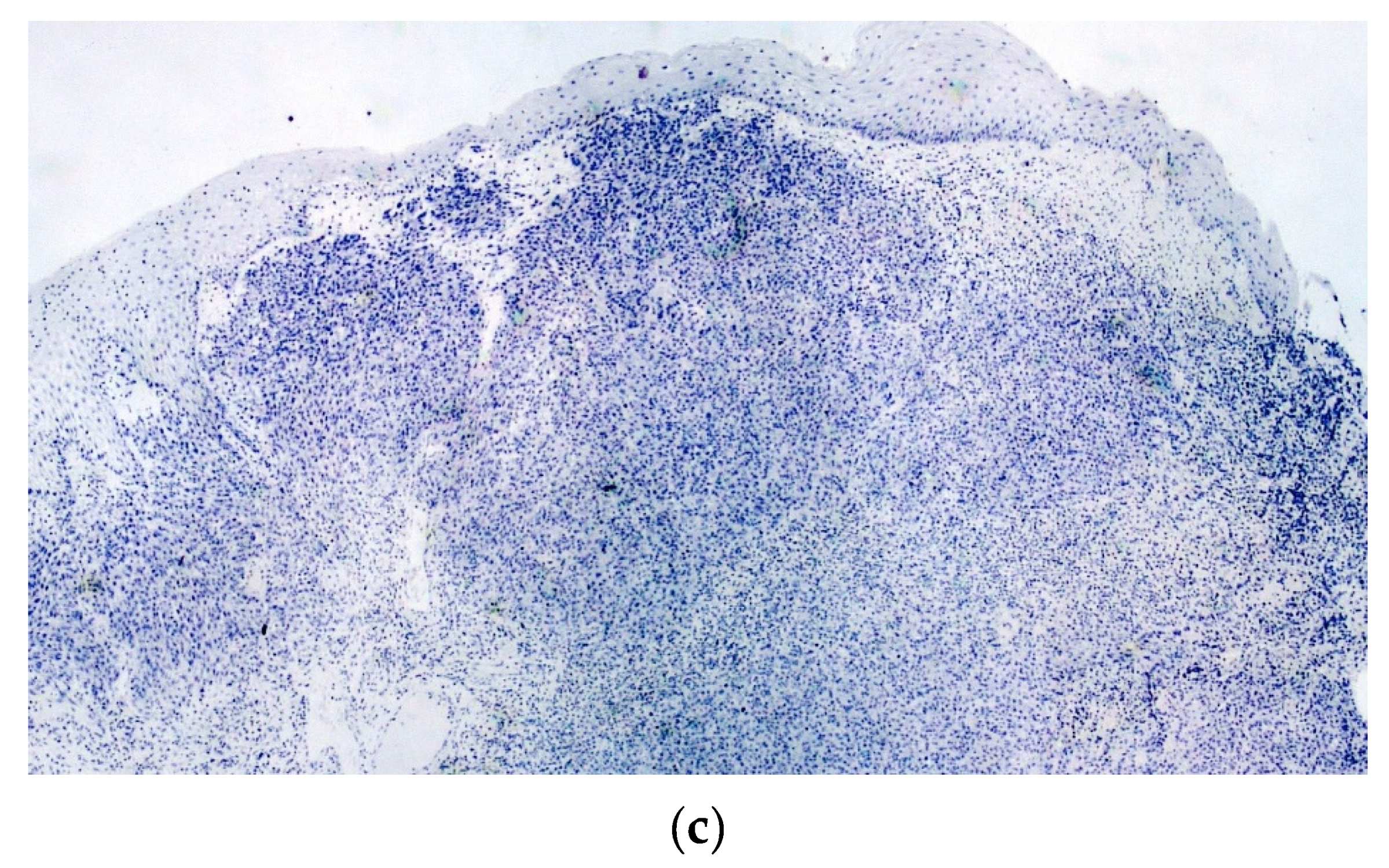
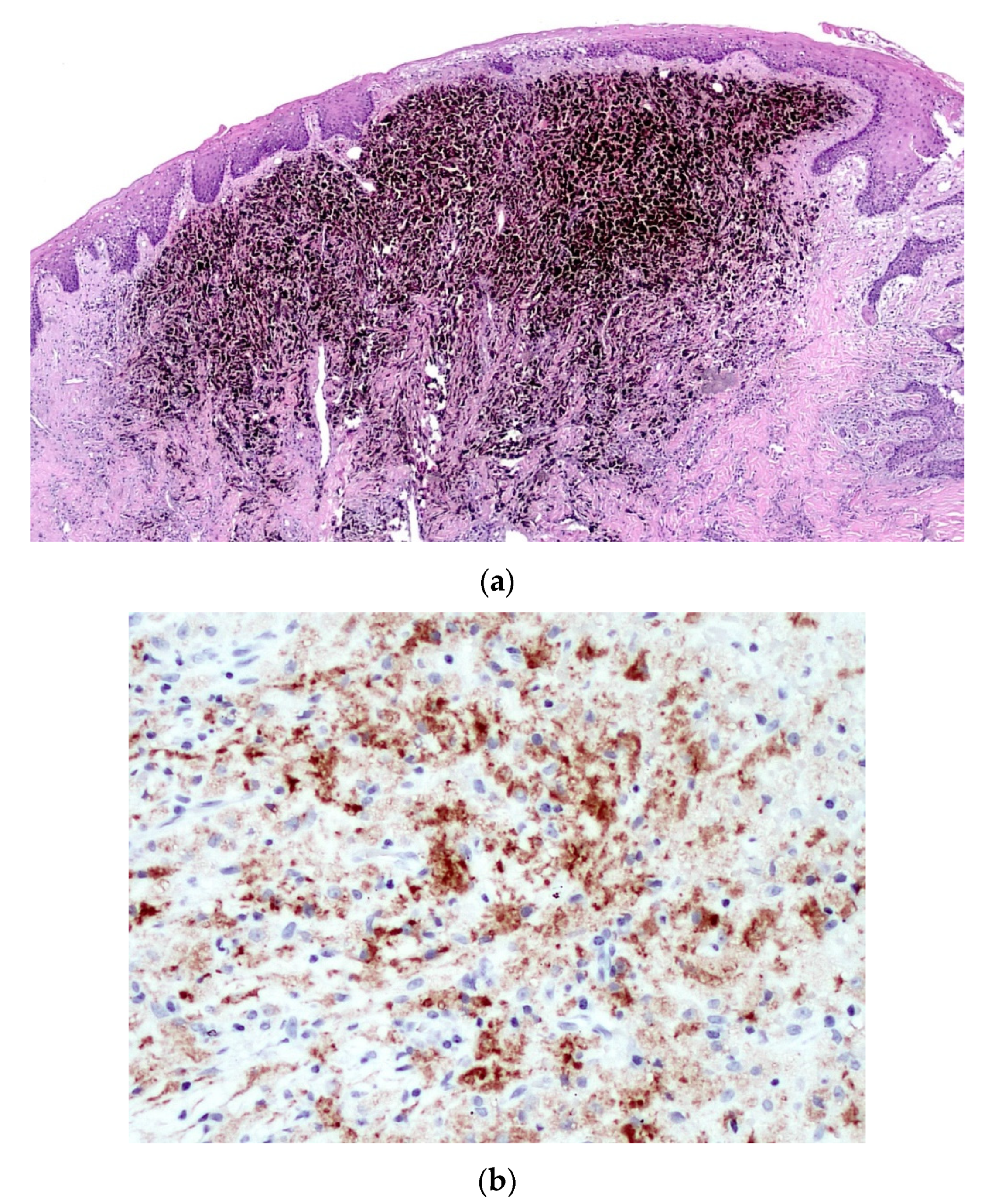
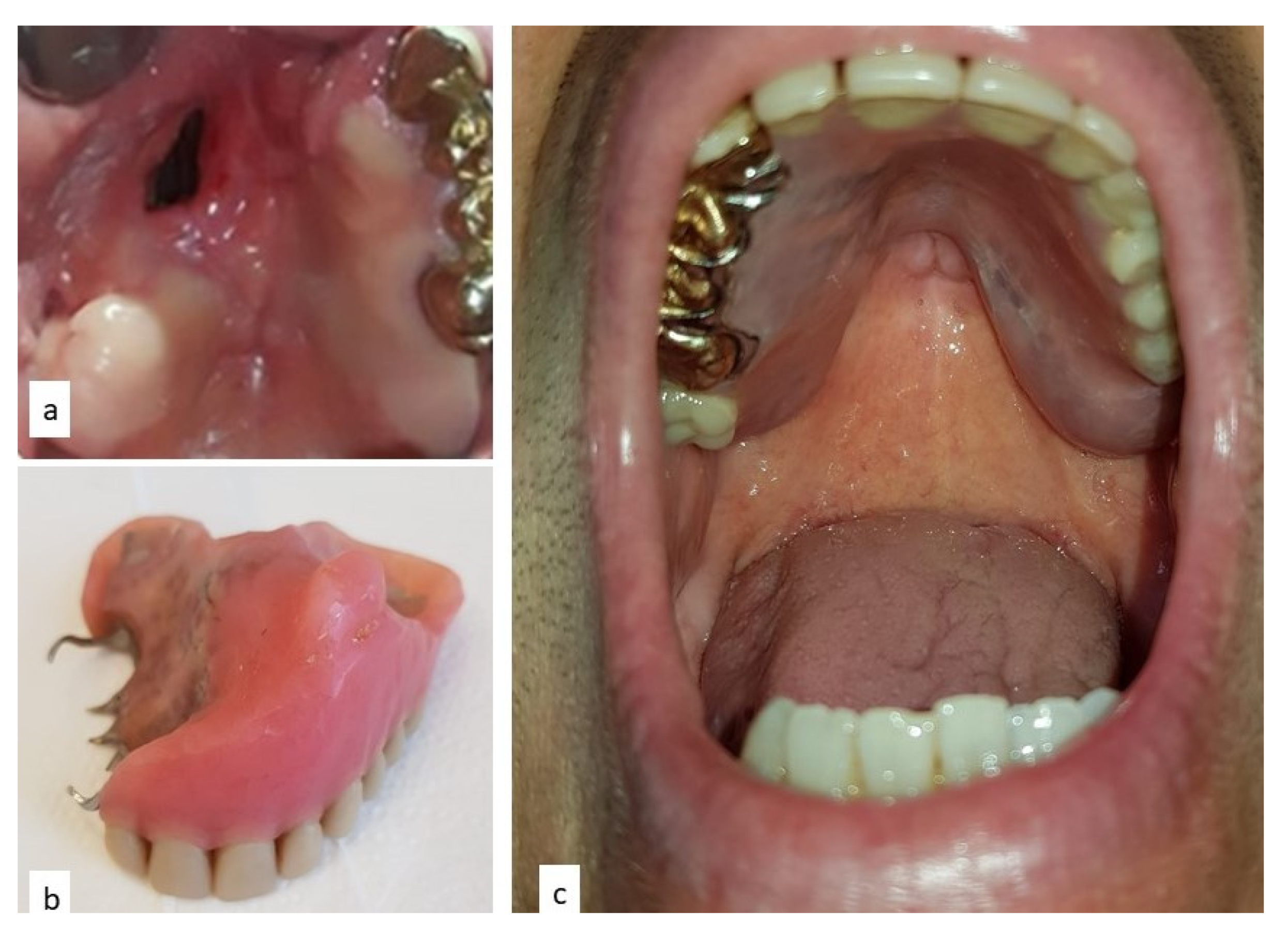
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yde, S.S.; Sjoegren, P.; Heje, M.; Stolle, L.B. Mucosal melanoma: A literature review. Curr. Oncol. Rep. 2018, 20, 28. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Accorona, R.; Botti, G.; Farina, D.; Fossati, P.; Gatta, G.; Gogas, H.; Lombardi, D.; Maroldi, R.; Nicolai, P. Mucosal melanoma of the head and neck. Crit. Rev. Oncol. Hematol. 2017, 112, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Crowson, A.N.; Magro, C.M.; Sanchez-Carpintero, I.; Mihm, M.C. The precursors of malignant melanoma. In Cancers of the Skin; Springer: Berlin/Heidelberg, Germany, 2002; pp. 75–84. [Google Scholar]
- Patel, S.G.; Prasad, M.L.; Escrig, M.; Singh, B.; Shaha, A.R.; Kraus, D.H.; Boyle, J.O.; Huvos, A.G.; Busam, K.; Shah, J.P. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002, 24, 247–257. [Google Scholar] [CrossRef]
- Merkel, E.A.; Gerami, P. Malignant melanoma of sun-protected sites: A review of clinical, histological, and molecular features. Lab. Investig. 2017, 97, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons: Oxford, UK, 2017; pp. 45–46. [Google Scholar]
- Rapini, R.P.; Golitz, L.E.; Greer, R.O.J.; Krekorian, E.A.; Poulson, T. Primary malignant melanoma of the oral cavity. A review of 177 cases. Cancer 1985, 55, 1543–1551. [Google Scholar] [CrossRef]
- González-García, R.; Naval-Gías, L.; Martos, P.L.; Nam-Cha, S.H.; Rodríguez-Campo, F.J.; Muñoz-Guerra, M.F.; Sastre-Pérez, J. Melanoma of the oral mucosa. Clinical cases and review of the literature. Med. Oral. Patol. Oral. Cir. Bucal. 2005, 10, 264–271. [Google Scholar]
- De Piano, E.; Cinotti, E.; Tognetti, L.; Rubegni, P. Commentary on ‘Oral melanoma and other pigmentations: When to biopsy’? J. Eur. Acad. Dermatol. Venereol. 2018, 32, e398–e399. [Google Scholar] [CrossRef]
- Tognetti, L.; Cinotti, E.; Moscarella, E.; Farnetani, F.; Malvehy, J.; Lallas, A.; Pellacani, G.; Argenziano, G.; Cevenini, G.; Rubegni, P. Impact of clinical and personal data in the dermoscopic differentiation between early melanoma and atypical nevi. Dermatol. Pract. Concept. 2018, 8, 324–327. [Google Scholar] [CrossRef]
- Freedman, D.M.; Sigurdson, A.; Doody, M.M.; Rao, R.S.; Linet, M.S. Risk of melanoma in relation to smoking, alcohol intake, and other factors in a large occupational cohort. Cancer Causes Control 2003, 14, 847–857. [Google Scholar] [CrossRef]
- Holmstrom, M.; Lund, V.J. Malignant melanomas of the nasal cavity after occupational exposure to formaldehyde. Br. J. Ind. Med. 1991, 48, 9–11. [Google Scholar] [CrossRef]
- Lopez-Graniel, C.M.; Ochoa-Carrillo, F.J.; Meneses-García, A. Malignant melanoma of the oral cavity: Diagnosis and treatment experience in a Mexican population. Oral. Oncol. 1999, 35, 425–430. [Google Scholar] [CrossRef]
- Doval, D.C.; Rao, C.R.; Saitha, K.S.; Vigayakumar, M.; Misra, S.; Manie, K.; Bapsy, P.P.; Kumaraswamy, S.V. Magliant melanoma of the oral cavity: Report of 14 cases from a regional cancer centre. Eur. J. Surg. Oncol. 1996, 22, 245–249. [Google Scholar] [CrossRef]
- Safadi, R.A.; Bader, D.H.; Abdullah, N.I.; Sughayer, M.A. Immunohistochemical expression of keratins 6, 7, 8, 14, 16, 18, 19, and MNF-116 pancytokeratin in primary and metastatic melanoma of the head and neck. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2016, 121, 510–519. [Google Scholar] [CrossRef]
- De-Andrade, B.A.; Toral-Rizo, V.H.; León, J.E.; Contreras, E.; Carlos, R.; Delgado-Azañero, W.; Mosqueda-Taylor, A.; de-Almeida, O.P. Primary oral melanoma: A histopathological and immunohistochemical study of 22 cases of Latin America. Med. Oral. Patol. Oral. Cir. Bucal. 2012, 17, e383–e388. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.L.; Jungbluth, A.A.; Iversen, K.; Huvos, A.G.; Busam, K.J. Expression of melanocytic differentiation markers in malignant melanomas of the oral and sinonasal mucosa. Am. J. Surg. Pathol. 2001, 25, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Prieto, V.G.; Shea, C.R. Use of immunohistochemistry in melanocytic lesions. J. Cutan. Pathol. 2008, 35, 1–10. [Google Scholar] [CrossRef]
- Ohsie, S.J.; Sarantopoulos, G.P.; Cochran, A.J.; Binder, S.W. Immunohistochemical characteristics of melanoma. J. Cutan. Pathol. 2008, 35, 433–444. [Google Scholar] [CrossRef]
- Eisen, D.; Voorhees, J.J. Oral melanoma and other pigmented lesions of the oral cavity. J. Am. Acad. Dermatol. 1991, 24, 527–537. [Google Scholar] [CrossRef]
- Wick, M.R.; Stanley, S.J.; Swanson, P.E. Immunohistochemical diagnosis of sinonasal melanoma, carcinoma, and neuroblastoma with monoclonal antibodies HMB-45 and anti-synaptophysin. Arch. Pathol. Lab. Med. 1988, 112, 616–620. [Google Scholar]
- Bekers, E.M.; van Engen-van Grunsven, A.C.; Groenen, P.J.; Westdorp, H.; Koornstra, R.H.T.; Bonenkamp, J.J.; Flucke, U.; Blokx, W.A.M. Metastatic melanoma mimicking solitary fibrous tumor: ReSport of two cases. Virchows Arch. 2014, 464, 247–251. [Google Scholar] [CrossRef]
- Tavares, T.S.; Meirelles, D.P.; de Aguiar, M.C.F.; Caldeira, P.C. Pigmented lesions of the oral mucosa: A cross-sectional study of 458 histopathological specimens. Oral. Dis. 2018, 24, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Patrizi, A.; Fanti, P.A.; Melotti, B.; Caliceti, U.; Magnoni, C.; Misciali, C.; Baraldi, C.; Ravaioli, G.M.; Dika, E. Oral melanoma and other pigmentations: When to biopsy? J. Eur. Acad. Dermatol. Venereol. 2018, 32, 209–214. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limongelli, L.; Cascardi, E.; Capodiferro, S.; Favia, G.; Corsalini, M.; Tempesta, A.; Maiorano, E. Multifocal Amelanotic Melanoma of the Hard Palate: A Challenging Case. Diagnostics 2020, 10, 424. https://doi.org/10.3390/diagnostics10060424
Limongelli L, Cascardi E, Capodiferro S, Favia G, Corsalini M, Tempesta A, Maiorano E. Multifocal Amelanotic Melanoma of the Hard Palate: A Challenging Case. Diagnostics. 2020; 10(6):424. https://doi.org/10.3390/diagnostics10060424
Chicago/Turabian StyleLimongelli, Luisa, Eliano Cascardi, Saverio Capodiferro, Gianfranco Favia, Massimo Corsalini, Angela Tempesta, and Eugenio Maiorano. 2020. "Multifocal Amelanotic Melanoma of the Hard Palate: A Challenging Case" Diagnostics 10, no. 6: 424. https://doi.org/10.3390/diagnostics10060424
APA StyleLimongelli, L., Cascardi, E., Capodiferro, S., Favia, G., Corsalini, M., Tempesta, A., & Maiorano, E. (2020). Multifocal Amelanotic Melanoma of the Hard Palate: A Challenging Case. Diagnostics, 10(6), 424. https://doi.org/10.3390/diagnostics10060424









