Carotid Stenosis Assessment with Vector Concentration before and after Stenting
Abstract
1. Introduction
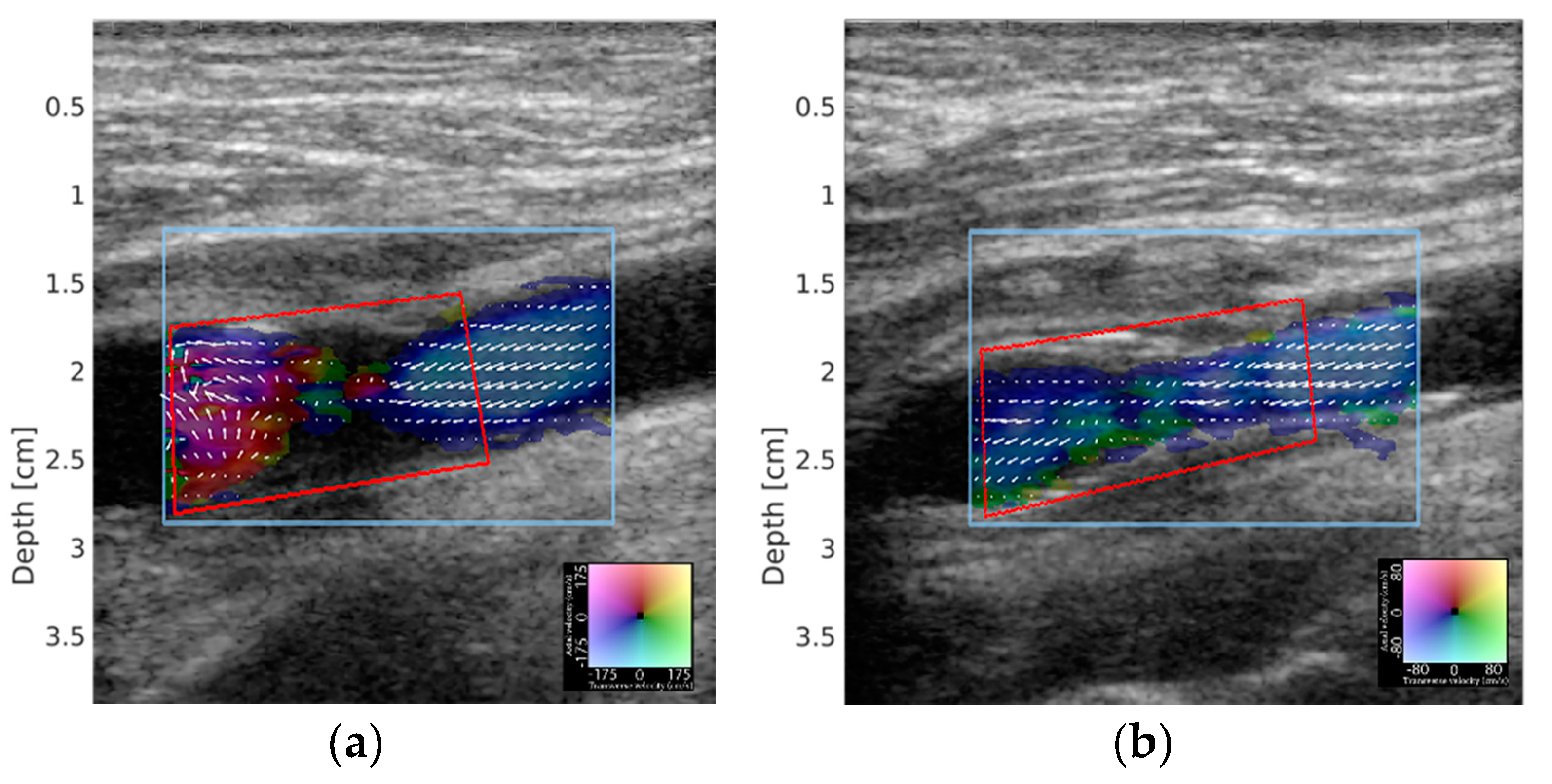
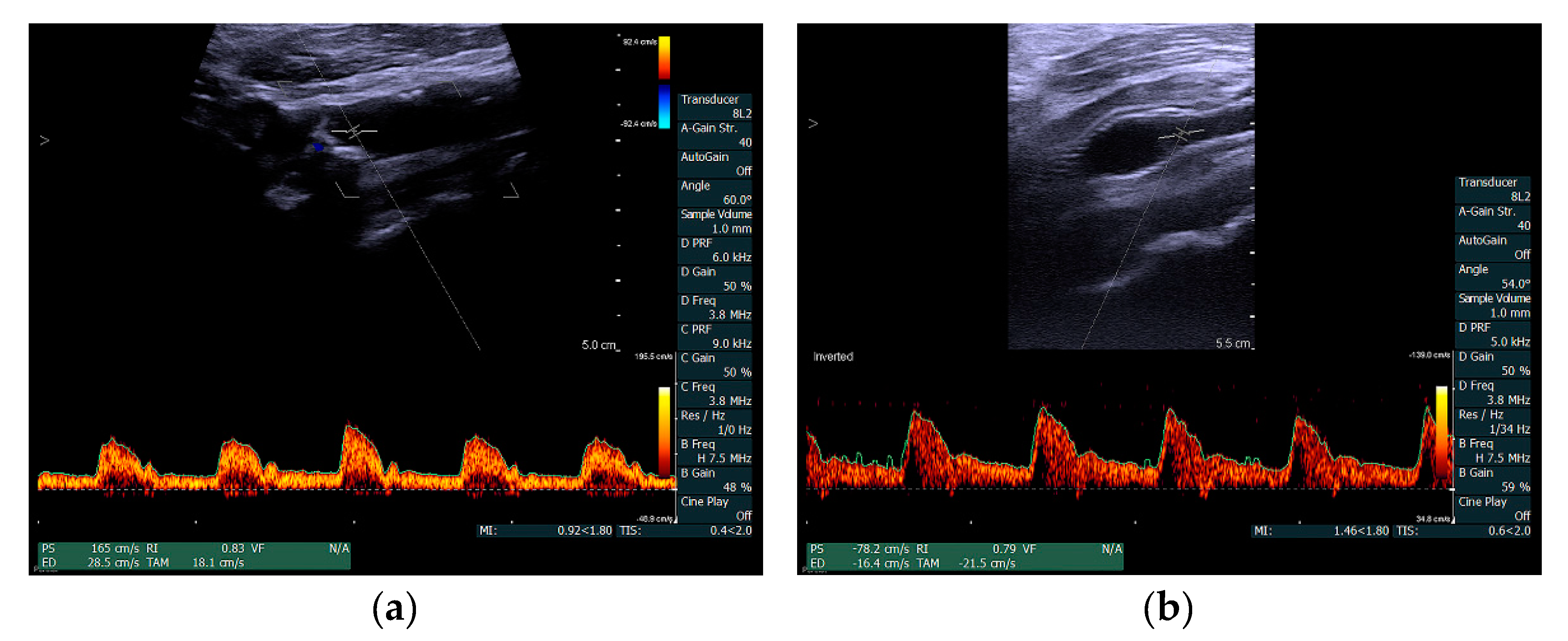
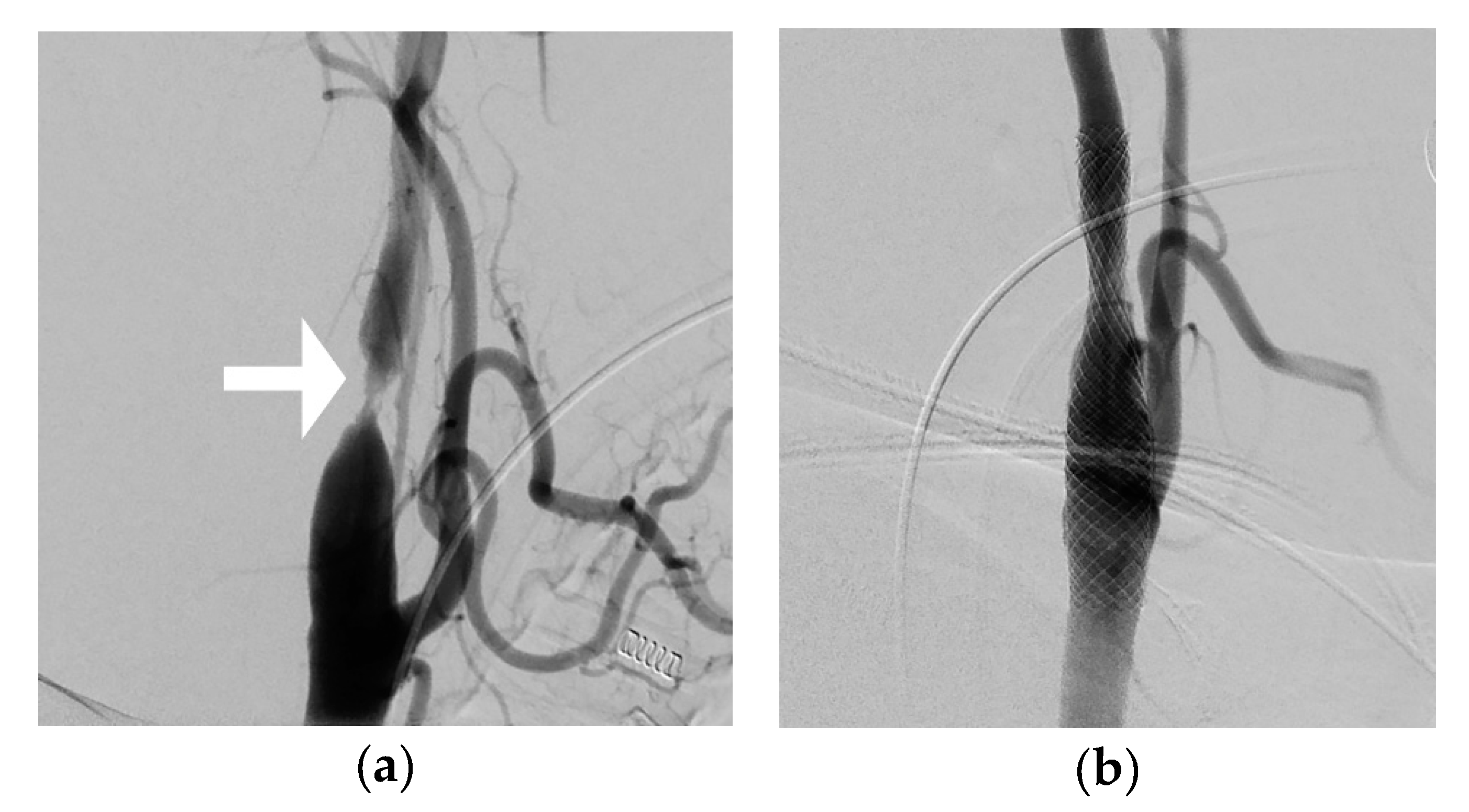
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Inzitari, D.; Eliasziw, M.; Gates, P.; Sharpe, B.L.; Chan, R.K.; Meldrum, H.E.; Barnett, H.J. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N. Engl. J. Med. 2000, 342, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Meschia, J.F.; Klaas, J.P.; Brown, R.D., Jr.; Brott, T.G. Evaluation and Management of Atherosclerotic Carotid Stenosis. Mayo Clin. Proc. 2017, 92, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.J.; Stolker, J.M.; Wang, K.; Magnuson, E.A.; Clark, W.M.; Demaerschalk, B.M.; Sam, A.D., Jr.; Elmore, J.R.; Weaver, F.A.; Aronow, H.D.; et al. Health-related quality of life after carotid stenting versus carotid endarterectomy: Results from CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial). J. Am. Coll. Cardiol. 2011, 58, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Adla, T.; Adlova, R. Multimodality Imaging of Carotid Stenosis. Int. J. Angiol. 2015, 24, 179–184. [Google Scholar] [CrossRef]
- Chilcote, W.A.; Modic, M.T.; Pavlicek, W.A.; Little, J.R.; Furlan, A.J.; Duchesneau, P.M.; Weinstein, M.A. Digital subtraction angiography of the carotid arteries: A comparative study in 100 patients. Radiology 1981, 139, 287–295. [Google Scholar] [CrossRef]
- Grant, E.G.; Benson, C.B.; Moneta, G.L.; Alexandrov, A.V.; Baker, J.D.; Bluth, E.I.; Carroll, B.A.; Eliasziw, M.; Gocke, J.; Hertzberg, B.S.; et al. Carotid artery stenosis: Gray-scale and Doppler US diagnosis—Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003, 229, 340–346. [Google Scholar] [CrossRef]
- Hoskins, P.R. A comparison of single- and dual-beam methods for maximum velocity estimation. Ultrasound Med. Biol. 1999, 25, 583–592. [Google Scholar] [CrossRef]
- Corriveau, M.M.; Johnston, K.W. Interobserver variability of carotid Doppler peak velocity measurements among technologists in an ICAVL-accredited vascular laboratory. J. Vasc. Surg. 2004, 39, 735–741. [Google Scholar] [CrossRef]
- Beach, K.W.; Leotta, D.F.; Zierler, R.E. Carotid Doppler velocity measurements and anatomic stenosis: Correlation is futile. Vasc. Endovasc. Surg. 2012, 46, 466–474. [Google Scholar] [CrossRef]
- Jensen, J.A.; Munk, P. A new method for estimation of velocity vectors. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1998, 45, 837–851. [Google Scholar] [CrossRef]
- Pedersen, M.M.; Pihl, M.J.; Haugaard, P.; Hansen, J.M.; Hansen, K.L.; Nielsen, M.B.; Jensen, J.A. Comparison of real-time in vivo spectral and vector velocity estimation. Ultrasound Med. Biol. 2012, 38, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.H.; Hansen, K.L.; Ewertsen, C.; Holbek, S.; Olesen, J.B.; Moshavegh, R.; Thomsen, C.; Jensen, J.A.; Nielsen, M.B. A Comparison Study of Vector Velocity, Spectral Doppler and Magnetic Resonance of Blood Flow in the Common Carotid Artery. Ultrasound Med. Biol. 2018, 44, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.H.; Moshavegh, R.; Hansen, K.L.; Bechsgaard, T.; Lonn, L.; Jensen, J.A.; Nielsen, M.B. Vector Flow Imaging Compared with Pulse Wave Doppler for Estimation of Peak Velocity in the Portal Vein. Ultrasound Med. Biol. 2018, 44, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Udesen, J.; Jensen, J.A. Investigation of transverse oscillation method. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.A. A new estimator for vector velocity estimation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2001, 48, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.M.; Pihl, M.J.; Haugaard, P.; Hansen, K.L.; Lange, T.; Lonn, L.; Nielsen, M.B.; Jensen, J.A. Novel flow quantification of the carotid bulb and the common carotid artery with vector flow ultrasound. Ultrasound Med. Biol. 2014, 40, 2700–2706. [Google Scholar] [CrossRef]
- Hansen, K.L.; Hansen, P.M.; Ewertsen, C.; Lonn, L.; Jensen, J.A.; Nielsen, M.B. Vector Flow Imaging Compared with Digital Subtraction Angiography for Stenosis Assessment in the Superficial Femoral Artery—A Study of Vector Concentration, Velocity Ratio and Stenosis Degree Percentage. Ultrasound Int. Open 2019, 5, E53–E59. [Google Scholar] [CrossRef]
- Hansen, K.L.; Moller-Sorensen, H.; Kjaergaard, J.; Jensen, M.B.; Jensen, J.A.; Nielsen, M.B. Aortic Valve Stenosis Increases Helical Flow and Flow Complexity: A Study of Intra-Operative Cardiac Vector Flow Imaging. Ultrasound Med. Biol. 2017, 43, 1607–1617. [Google Scholar] [CrossRef]
- Hansen, K.L.; Moller-Sorensen, H.; Kjaergaard, J.; Jensen, M.B.; Lund, J.T.; Pedersen, M.M.; Lange, T.; Jensen, J.A.; Nielsen, M.B. Intra-operative Vector Flow Imaging Using Ultrasound of the Ascending Aorta among 40 Patients with Normal, Stenotic and Replaced Aortic Valves. Ultrasound Med. Biol. 2016, 42, 2414–2422. [Google Scholar] [CrossRef]
- Hansen, K. Cardiac Vector Flow Imaging. Ph.D. Thesis, Copenhagen Univsersity Hospital, Copenhagen, Denmark, 12 September 2019. [Google Scholar]
- Saris, A.; Hansen, H.H.G.; Fekkes, S.; Menssen, J.; Nillesen, M.M.; de Korte, C.L. In Vivo Blood Velocity Vector Imaging Using Adaptive Velocity Compounding in the Carotid Artery Bifurcation. Ultrasound Med. Biol. 2019, 45, 1691–1707. [Google Scholar] [CrossRef]
- Brandt, A.H.; Nguyen, T.; Gutte, H.; Holtmannspötter, M.; Moshavegh, R.; Jensen, J.A.; Nielsen, M.B.; Hansen, K.L. Vector Concentration used for Stenosis Assessment in the Carotid Artery before and after Carotid Stenting. In Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; pp. 348–351. [Google Scholar]
- Moshavegh, R.; Martins, B.; Hansen, K.L.; Bechsgaard, T.; Nielsen, M.B.; Jensen, J.A. Hybrid segmentation of vessels and automated flow measures in in-vivo ultrasound imaging. In Proceedings of the 2016 IEEE International Ultrasonics Symposium (IUS), Tours, France, 18–21 September 2016; pp. 1–4. [Google Scholar]
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998, 351, 1379–1387. [CrossRef]
- Jahromi, A.S.; Cina, C.S.; Liu, Y.; Clase, C.M. Sensitivity and specificity of color duplex ultrasound measurement in the estimation of internal carotid artery stenosis: A systematic review and meta-analysis. J. Vasc. Surg. 2005, 41, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Nederkoorn, P.J.; van der Graaf, Y.; Hunink, M.G. Duplex ultrasound and magnetic resonance angiography compared with digital subtraction angiography in carotid artery stenosis: A systematic review. Stroke 2003, 34, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Slovut, D.P.; Romero, J.M.; Hannon, K.M.; Dick, J.; Jaff, M.R. Detection of common carotid artery stenosis using duplex ultrasonography: A validation study with computed tomographic angiography. J. Vasc. Surg. 2010, 51, 65–70. [Google Scholar] [CrossRef]
- Stringer, D.A.; O’Halpin, D.; Daneman, A.; Liu, P.; Geary, D.F. Duplex Doppler sonography for renal artery stenosis in the post-transplant pediatric patient. Pediatr. Radiol. 1989, 19, 187–192. [Google Scholar] [CrossRef]
- Hutchison, S.J.; Rosin, B.L.; Curry, S.; Chandraratna, P.A. Transesophageal Echocardiographic Assessment of Lesions of the Right Ventricular Outflow Tract and Pulmonic Valve. Echocardiography 1996, 13, 21–34. [Google Scholar] [CrossRef]
- Goddi, A.; Bortolotto, C.; Raciti, M.V.; Fiorina, I.; Aiani, L.; Magistretti, G.; Sacchi, A.; Tinelli, C.; Calliada, F. High-Frame Rate Vector Flow Imaging of the Carotid Bifurcation in Healthy Adults: Comparison With Color Doppler Imaging. J. Ultrasound Med. 2018, 37, 2263–2275. [Google Scholar] [CrossRef]
- Hansen, K.L.; Udesen, J.; Thomsen, C.; Jensen, J.A.; Nielsen, M.B. In vivo validation of a blood vector velocity estimator with MR angiography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 91–100. [Google Scholar] [CrossRef]
- Hoskins, P.R. Peak velocity estimation in arterial stenosis models using colour vector Doppler. Ultrasound Med. Biol. 1997, 23, 889–897. [Google Scholar] [CrossRef]
- Park, M.Y.; Jung, S.E.; Byun, J.Y.; Kim, J.H.; Joo, G.E. Effect of beam-flow angle on velocity measurements in modern Doppler ultrasound systems. AJR Am. J. Roentgenol. 2012, 198, 1139–1143. [Google Scholar] [CrossRef]
- Tortoli, P.; Lenge, M.; Righi, D.; Ciuti, G.; Liebgott, H.; Ricci, S. Comparison of carotid artery blood velocity measurements by vector and standard Doppler approaches. Ultrasound Med. Biol. 2015, 41, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, J.; Patel, V.I.; Romero, J.M.; Hannon, K.M.; Jaff, M.R.; Cambria, R.P.; LaMuraglia, G.M. Acoustic shadowing impairs accurate characterization of stenosis in carotid ultrasound examinations. J. Vasc. Surg. 2015, 62, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K. Vector concentration: A new ultrasound parameter for stenosis assessment using Vector Flow Imaging. Diagn. Imaging 2019, 35, 19–21. [Google Scholar]
- Nguyen, T.; Traberg, M.S.; Olesen, J.B.; Heerwagen, S.T.; Brandt, A.H.; Bechgaard, T.; Pedersen, B.L.; Moshavegh, R.; Lönn, L.; Jensen, J.A.; et al. Flow complexity estimantion in dysfynctional arteriovenous diaslysis fistulas using vector flow imaging. Ultrasound Med. Biol. 2020, in press. [Google Scholar]
- Meyer, J.I.; Khalil, R.M.; Obuchowski, N.A.; Baus, L.K. Common carotid artery: Variability of Doppler US velocity measurements. Radiology 1997, 204, 339–341. [Google Scholar] [CrossRef]

| Patient Number | Stenosis Site | Sex | Age (Year) |
|---|---|---|---|
| 1 | Right ICA | Male | 66 |
| 2 | Left CCA | Male | 81 |
| 3 | Left ICA | Female | 68 |
| 4 | Right CCA | Male | 47 |
| 5 | Left CCA | Female | 52 |
| 6 | Right ICA | Male | 58 |
| 7 | Right ICA | Female | 71 |
| 8 | Right ICA | Male | 67 |
| 9 | Left ICA | Male | 48 |
| 10 | Left ICA | Male | 77 |
| Before Stenting (SD) | After Stenting (SD) | Mean Difference [CI95] | p-Value | |
|---|---|---|---|---|
| Mean VC | 0.56 (10.7) | 0.92 (5.3) | +0.39 [0.32; 0.46] | <0.001 |
| Mean PSV (cm/s) | 149.9 (79.6) | 68.9 (20.5) | −81.2 [−104.38; −58.36] | 0.005 |
| Mean EDV (cm/s) | 58.0 (69.8) | 23.9 (13.2) | −34.1 [−70.8; 2.52] | 0.102 |
| Mean stenosis degree (%) | 72.4 (8.2) | 13.3 (7.0) | −59% [−52%; −67%] | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandt, A.H.; Nguyen, T.-Q.; Gutte, H.; Frederik Carlsen, J.; Moshavegh, R.; Jensen, J.A.; Bachmann Nielsen, M.; Hansen, K.L. Carotid Stenosis Assessment with Vector Concentration before and after Stenting. Diagnostics 2020, 10, 420. https://doi.org/10.3390/diagnostics10060420
Brandt AH, Nguyen T-Q, Gutte H, Frederik Carlsen J, Moshavegh R, Jensen JA, Bachmann Nielsen M, Hansen KL. Carotid Stenosis Assessment with Vector Concentration before and after Stenting. Diagnostics. 2020; 10(6):420. https://doi.org/10.3390/diagnostics10060420
Chicago/Turabian StyleBrandt, Andreas Hjelm, Tin-Quoc Nguyen, Henrik Gutte, Jonathan Frederik Carlsen, Ramin Moshavegh, Jørgen Arendt Jensen, Michael Bachmann Nielsen, and Kristoffer Lindskov Hansen. 2020. "Carotid Stenosis Assessment with Vector Concentration before and after Stenting" Diagnostics 10, no. 6: 420. https://doi.org/10.3390/diagnostics10060420
APA StyleBrandt, A. H., Nguyen, T.-Q., Gutte, H., Frederik Carlsen, J., Moshavegh, R., Jensen, J. A., Bachmann Nielsen, M., & Hansen, K. L. (2020). Carotid Stenosis Assessment with Vector Concentration before and after Stenting. Diagnostics, 10(6), 420. https://doi.org/10.3390/diagnostics10060420









