False-Positive Malignant Diagnosis of Nodule Mimicking Lesions by Computer-Aided Thyroid Nodule Analysis in Clinical Ultrasonography Practice
Abstract
1. Introduction
2. Materials and Methods
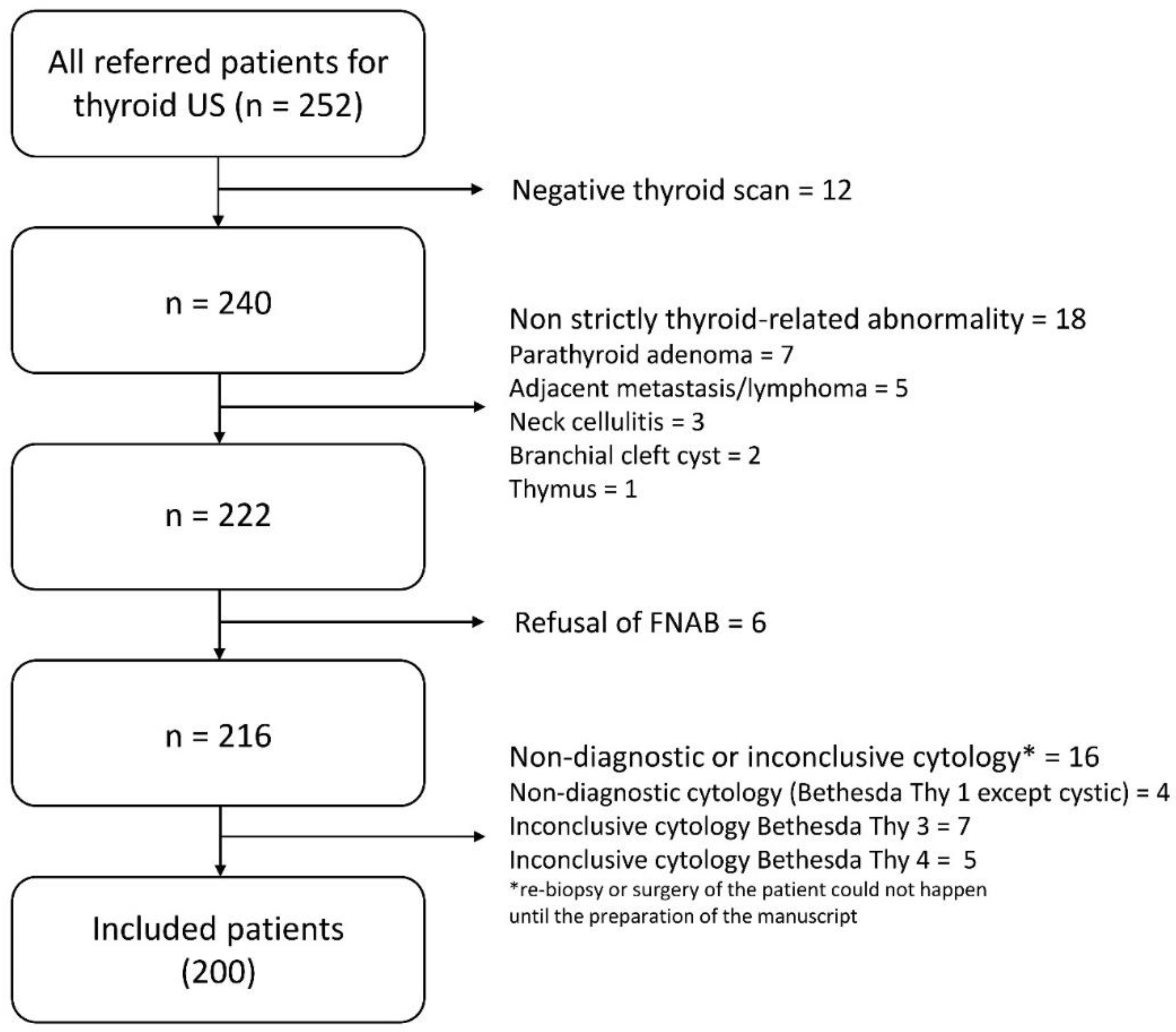
2.1. Subjects
2.2. Ultrasonography (US) Examination and Fine Needle Aspiration Biopsy (FNAB), Diagnosis Definitions
2.3. Computer-Aided Diagnosis (CAD) Analysis
2.4. Statistical Analysis
3. Results
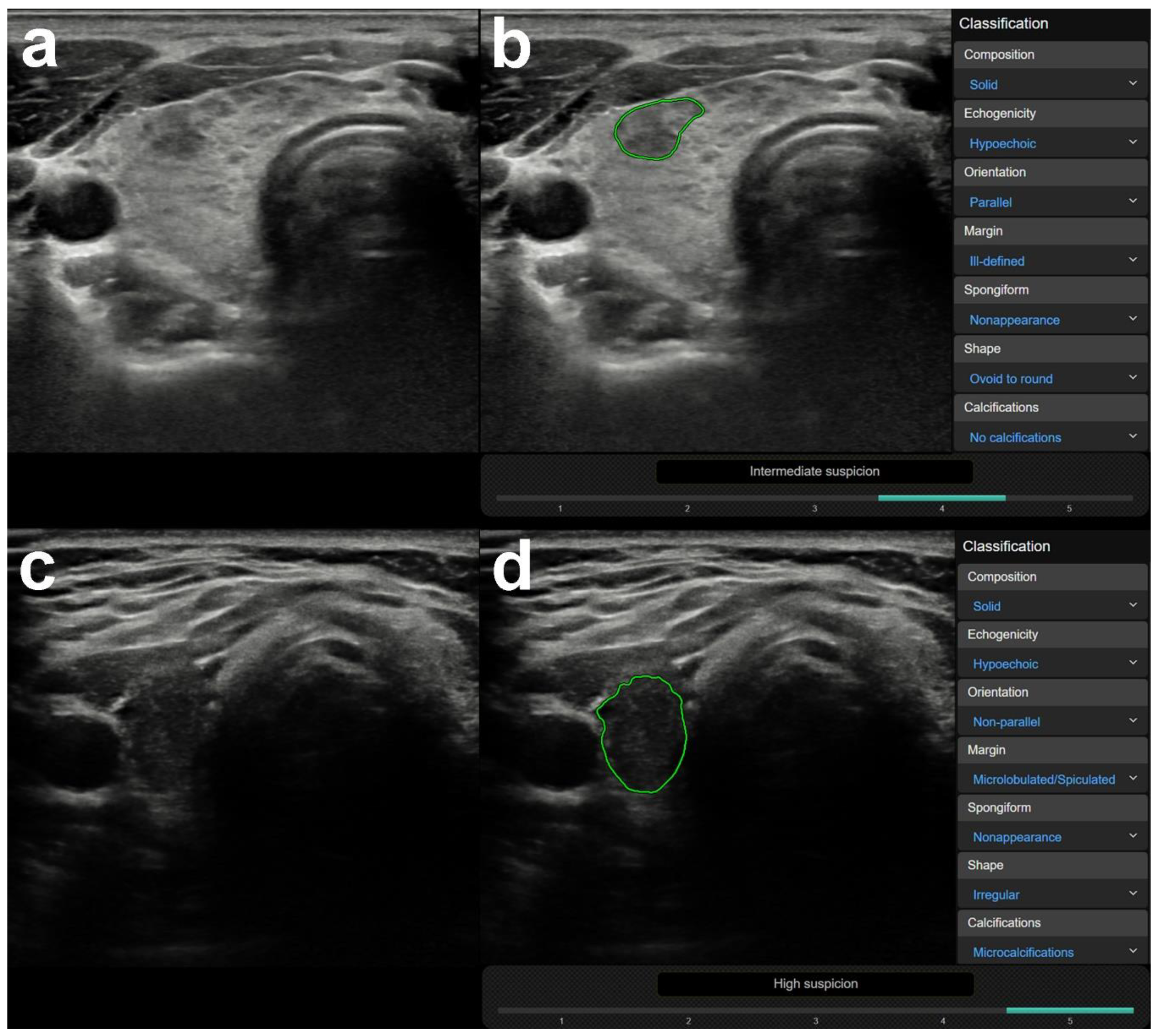
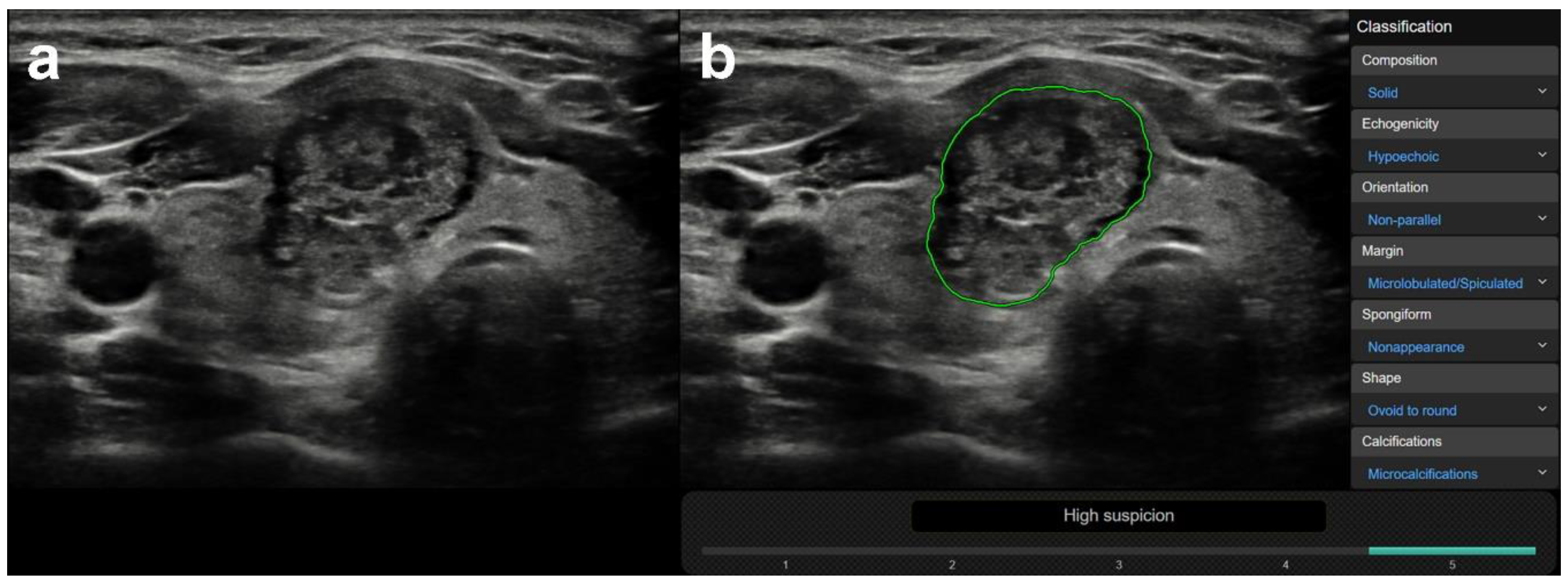
3.1. US Features or Entities Associated with CAD System Misdiagnosis Including Mimicking Lesions
3.2. US Features or Entities Associated with CAD System Misdiagnosis Excluding Mimicking Lesions
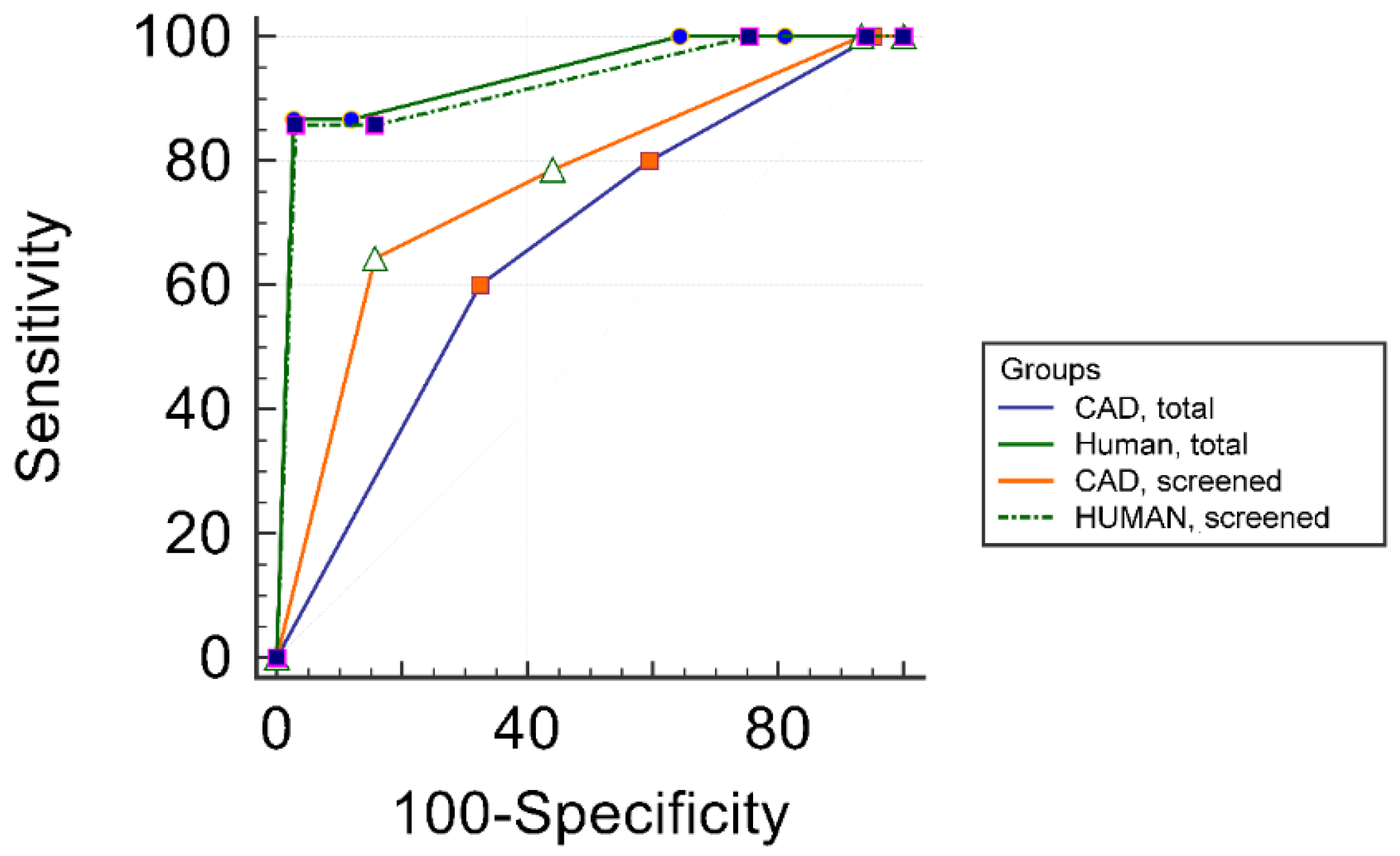
3.3. Comparison of Human and CAD System Diagnostic Performance in the Total and in the Screened Subgroup
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brander, A.; Viikinkoski, P.; Nickels, J.; Kivisaari, L. Thyroid gland: US screening in a random adult population. Radiology 1991, 181, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.A.; Cooper, D.S.; Daniels, G.H.; Ladenson, P.W.; Greenspan, F.S.; Levy, E.G.; Braverman, L.E.; Clark, O.H.; McDougall, I.R.; Ain, K.V.; et al. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. American Thyroid Association. Arch. Intern. Med. 1996, 156, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Pellegriti, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J. Cancer Epidemiol. 2013, 2013, 965212. [Google Scholar] [CrossRef] [PubMed]
- Arem, R.; Padayatty, S.J.; Saliby, A.H.; Sherman, S.I. Thyroid microcarcinoma: Prevalence, prognosis, and management. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 1999, 5, 148–156. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Tamarkin, S.W.; McHenry, C.R. The results of ultrasound-guided fine-needle aspiration biopsy for evaluation of nodular thyroid disease. Surgery 2002, 132, 648–653; discussion 653–644. [Google Scholar] [CrossRef]
- Hegedus, L. Clinical practice. The thyroid nodule. N. Engl. J. Med. 2004, 351, 1764–1771. [Google Scholar] [CrossRef]
- Haugen, B.R. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: What is new and what has changed? Cancer 2017, 123, 372–381. [Google Scholar] [CrossRef]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.H.; Lee, Y.H.; Lim, H.K.; Moon, W.J.; Na, D.G.; Park, J.S.; et al. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, H.J.; Jang, H.W.; Kim, H.K.; Yi, J.H.; Lee, W.; Kim, S.H. A proposal for a thyroid imaging reporting and data system for ultrasound features of thyroid carcinoma. Thyroid Off. J. Am. Thyroid Assoc. 2009, 19, 1257–1264. [Google Scholar] [CrossRef]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, E.K.; Kwak, J.Y.; Kim, M.J.; Son, E.J. Interobserver and intraobserver variations in ultrasound assessment of thyroid nodules. Thyroid Off. J. Am. Thyroid Assoc. 2010, 20, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Kim, S.H.; Jung, S.L.; Kang, B.J.; Kim, J.Y.; Choi, J.J.; Sung, M.S.; Yim, H.W.; Jeong, S.H. Observer variability in the sonographic evaluation of thyroid nodules. J. Clin. Ultrasound 2010, 38, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Hoang, J.K.; Middleton, W.D.; Farjat, A.E.; Teefey, S.A.; Abinanti, N.; Boschini, F.J.; Bronner, A.J.; Dahiya, N.; Hertzberg, B.S.; Newman, J.R.; et al. Interobserver Variability of Sonographic Features Used in the American College of Radiology Thyroid Imaging Reporting and Data System. Am. J. Roentgenol. 2018, 211, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Kwak, J.Y.; Kim, E.K.; Choi, S.H.; Moon, H.J. Man to man training: Can it help improve the diagnostic performances and interobserver variabilities of thyroid ultrasonography in residents? Eur. J. Radiol. 2012, 81, e352–e356. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, C.S.; Jung, S.L.; Kang, B.J.; Kim, J.Y.; Choi, J.J.; Kim, Y.I.; Oh, J.K.; Oh, J.S.; Kim, H.; et al. Observer variability and the performance between faculties and residents: US criteria for benign and malignant thyroid nodules. Korean J. Radiol. 2010, 11, 149–155. [Google Scholar] [CrossRef]
- Ko, S.Y.; Kim, E.K.; Sung, J.M.; Moon, H.J.; Kwak, J.Y. Diagnostic performance of ultrasound and ultrasound elastography with respect to physician experience. Ultrasound Med. Biol. 2014, 40, 854–863. [Google Scholar] [CrossRef]
- Park, S.J.; Park, S.H.; Choi, Y.J.; Kim, D.W.; Son, E.J.; Lee, H.S.; Yoon, J.H.; Kim, E.K.; Moon, H.J.; Kwak, J.Y. Interobserver variability and diagnostic performance in US assessment of thyroid nodule according to size. Ultraschall Med. 2012, 33, E186–E190. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.; Yang, S.; Zhao, C.; Tian, G.; Gao, Y.; Chen, Y.; Lu, Y. Automatic thyroid nodule recognition and diagnosis in ultrasound imaging with the YOLOv2 neural network. World J. Surg. Oncol. 2019, 17, 12. [Google Scholar] [CrossRef]
- Song, J.; Chai, Y.J.; Masuoka, H.; Park, S.W.; Kim, S.J.; Choi, J.Y.; Kong, H.J.; Lee, K.E.; Lee, J.; Kwak, N.; et al. Ultrasound image analysis using deep learning algorithm for the diagnosis of thyroid nodules. Medicine 2019, 98, e15133. [Google Scholar] [CrossRef]
- Sollini, M.; Cozzi, L.; Chiti, A.; Kirienko, M. Texture analysis and machine learning to characterize suspected thyroid nodules and differentiated thyroid cancer: Where do we stand? Eur. J. Radiol. 2018, 99, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Savelonas, M.; Maroulis, D.; Sangriotis, M. A computer-aided system for malignancy risk assessment of nodules in thyroid US images based on boundary features. Comput. Methods Programs Biomed. 2009, 96, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Prochazka, A.; Gulati, S.; Holinka, S.; Smutek, D. Patch-based classification of thyroid nodules in ultrasound images using direction independent features extracted by two-threshold binary decomposition. Computerized medical imaging and graphics. Off. J. Comput. Med. Imaging Soc. 2019, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.J.; Choi, C.S.; Yoon, D.Y.; Chang, S.K.; Kim, K.K.; Han, H.; Kim, S.S.; Lee, J.; Jeon, Y.H. Computer-aided diagnosis for the differentiation of malignant from benign thyroid nodules on ultrasonography. Acad. Radiol. 2008, 15, 853–858. [Google Scholar] [CrossRef]
- Li, L.N.; Ouyang, J.H.; Chen, H.L.; Liu, D.Y. A computer aided diagnosis system for thyroid disease using extreme learning machine. J. Med. Syst. 2012, 36, 3327–3337. [Google Scholar] [CrossRef]
- Chi, J.; Walia, E.; Babyn, P.; Wang, J.; Groot, G.; Eramian, M. Thyroid Nodule Classification in Ultrasound Images by Fine-Tuning Deep Convolutional Neural Network. J. Digit. Imaging 2017, 30, 477–486. [Google Scholar] [CrossRef]
- Ardakani, A.A.; Gharbali, A.; Mohammadi, A. Classification of Benign and Malignant Thyroid Nodules Using Wavelet Texture Analysis of Sonograms. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2015, 34, 1983–1989. [Google Scholar] [CrossRef]
- Acharya, U.R.; Faust, O.; Sree, S.V.; Molinari, F.; Suri, J.S. ThyroScreen system: High resolution ultrasound thyroid image characterization into benign and malignant classes using novel combination of texture and discrete wavelet transform. Comput. Methods Programs Biomed. 2012, 107, 233–241. [Google Scholar] [CrossRef]
- Buda, M.; Wildman-Tobriner, B.; Hoang, J.K.; Thayer, D.; Tessler, F.N.; Middleton, W.D.; Mazurowski, M.A. Management of Thyroid Nodules Seen on US Images: Deep Learning May Match Performance of Radiologists. Radiology 2019, 292, 695–701. [Google Scholar] [CrossRef]
- Choi, Y.J.; Baek, J.H.; Park, H.S.; Shim, W.H.; Kim, T.Y.; Shong, Y.K.; Lee, J.H. A Computer-Aided Diagnosis System Using Artificial Intelligence for the Diagnosis and Characterization of Thyroid Nodules on Ultrasound: Initial Clinical Assessment. Thyroid. Off. J. Am. Thyroid Assoc. 2017, 27, 546–552. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Ha, E.J.; Cho, Y.J.; Kim, H.L.; Han, M.; Kang, S.Y. Computer-Aided Diagnosis of Thyroid Nodules via Ultrasonography: Initial Clinical Experience. Korean J. Radiol. 2018, 19, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Ha, E.J.; Han, M. Real-World Performance of Computer-Aided Diagnosis System for Thyroid Nodules Using Ultrasonography. Ultrasound Med. Biol. 2019, 45, 2672–2678. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.; Grassi, G.; De Angelis, C.; Monaco, C.G.; Sdao, S.; Sardanelli, F.; Sconfienza, L.M.; Mauri, G. A computer-aided diagnosis system for the assessment and characterization of low-to-high suspicion thyroid nodules on ultrasound. Radiol. Med. 2019, 124, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.; Li, Y.; Shen, J.; Zhang, Y.; Wang, Y. Clinical Value of a Computer-Aided Diagnosis System in Thyroid Nodules: Analysis of a Reading Map Competition. Ultrasound Med. Biol. 2019, 45, 2666–2671. [Google Scholar] [CrossRef] [PubMed]
- Galimzianova, A.; Siebert, S.M.; Kamaya, A.; Desser, T.S.; Rubin, D.L. Toward Automated Pre-Biopsy Thyroid Cancer Risk Estimation in Ultrasound. In Proceedings of the 2017 AMIA Annual Symposium, Washington, DC, USA, 4–7 November 2017; pp. 734–741. [Google Scholar]
- Jeong, E.Y.; Kim, H.L.; Ha, E.J.; Park, S.Y.; Cho, Y.J.; Han, M. Computer-aided diagnosis system for thyroid nodules on ultrasonography: Diagnostic performance and reproducibility based on the experience level of operators. Eur. Radiol. 2019, 29, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, E.K.; Kim, S.J.; Kwak, J.Y. Thyroid ultrasonography: Pitfalls and techniques. Korean J. Radiol. 2014, 15, 267–276. [Google Scholar] [CrossRef]
- Caleo, A.; Vigliar, E.; Vitale, M.; Di Crescenzo, V.; Cinelli, M.; Carlomagno, C.; Garzi, A.; Zeppa, P. Cytological diagnosis of thyroid nodules in Hashimoto thyroiditis in elderly patients. BMC Surg. 2013, 13 (Suppl. 2). [Google Scholar] [CrossRef]
- Anderson, L.; Middleton, W.D.; Teefey, S.A.; Reading, C.C.; Langer, J.E.; Desser, T.; Szabunio, M.M.; Mandel, S.J.; Hildebolt, C.F.; Cronan, J.J. Hashimoto thyroiditis: Part 2, sonographic analysis of benign and malignant nodules in patients with diffuse Hashimoto thyroiditis. Am. J. Roentgenol. 2010, 195, 216–222. [Google Scholar] [CrossRef]
- Langer, J.E.; Khan, A.; Nisenbaum, H.L.; Baloch, Z.W.; Horii, S.C.; Coleman, B.G.; Mandel, S.J. Sonographic appearance of focal thyroiditis. Am. J. Roentgenol. 2001, 176, 751–754. [Google Scholar] [CrossRef]
- Yildirim, D.; Gurses, B.; Gurpinar, B.; Ekci, B.; Colakoglu, B.; Kaur, A. Nodule or pseudonodule? Differentiation in Hashimoto’s thyroiditis with sonoelastography. J. Int. Med Res. 2011, 39, 2360–2369. [Google Scholar] [CrossRef]
- Silva de Morais, N.; Stuart, J.; Guan, H.; Wang, Z.; Cibas, E.S.; Frates, M.C.; Benson, C.B.; Cho, N.L.; Nehs, M.A.; Alexander, C.A.; et al. The Impact of Hashimoto Thyroiditis on Thyroid Nodule Cytology and Risk of Thyroid Cancer. J. Endocr. Soc. 2019, 3, 791–800. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.; Cooper, D.S. The incidence and prevalence of thyroid autoimmunity. Endocrine 2012, 42, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Cibas, E.S.; Ali, S.Z. The Bethesda System for Reporting Thyroid Cytopathology. Am. J. Clin. Pathol. 2009, 132, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Bartalena, L. Diagnosis and management of Graves disease: A global overview. Nat. Rev. Endocrinol. 2013, 9, 724–734. [Google Scholar] [CrossRef]
- Slatosky, J.; Shipton, B.; Wahba, H. Thyroiditis: Differential diagnosis and management. Am. Fam. Physician 2000, 61, 1047–1052. [Google Scholar]
- Schoonjans, F.; Zalata, A.; Depuydt, C.E.; Comhaire, F.H. MedCalc: A new computer program for medical statistics. Comput. Methods Programs Biomed. 1995, 48, 257–262. [Google Scholar] [CrossRef]
- Sahai, H.; Khurshid, A. Statistics in Epidemiology: Methods, Techniques, and Applications; CRC Press: Boca Raton, FL, USA, 1996; 321p. [Google Scholar]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Hanley, J.A.; Hajian-Tilaki, K.O. Sampling variability of nonparametric estimates of the areas under receiver operating characteristic curves: An update. Acad. Radiol. 1997, 4, 49–58. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Zweig, M.H.; Campbell, G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin. Chem. 1993, 39, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, S.; Zhang, Q.; Wei, X.; Pan, Y.; Zhao, J.; Xin, X.; Qin, C.; Wang, X.; Li, J.; et al. Diagnosis of thyroid cancer using deep convolutional neural network models applied to sonographic images: A retrospective, multicohort, diagnostic study. Lancet Oncol. 2019, 20, 193–201. [Google Scholar] [CrossRef]
- Acharya, U.R.; Faust, O.; Sree, S.V.; Molinari, F.; Garberoglio, R.; Suri, J.S. Cost-effective non-invasive automated benign malignant thyroid lesion classification in 3D contrast-enhanced ultrasound using combination of wavelets textures: A class of ThyroScan algorithms. Technol. Cancer Res. Treat. 2011, 10, 371–380. [Google Scholar] [CrossRef]
- Acharya, U.R.; Vinitha Sree, S.; Krishnan, M.M.; Molinari, F.; Garberoglio, R.; Suri, J.S. Non-invasive automated 3D thyroid lesion classification in ultrasound: A class of ThyroScan systems. Ultrasonics 2012, 52, 508–520. [Google Scholar] [CrossRef]
- Floridi, C.; Cellina, M.; Buccimazza, G.; Arrichiello, A.; Sacrini, A.; Arrigoni, F.; Pompili, G.; Barile, A.; Carrafiello, G. Ultrasound imaging classifications of thyroid nodules for malignancy risk stratification and clinical management: State of the art. Gland Surg. 2019, 8 (Suppl. 3), S233–S244. [Google Scholar] [CrossRef]

| US Feature | Outcome |
|---|---|
| Composition | Solid |
| Partially cystic | |
| Cystic | |
| Echogenicity | Hyper/isoechogenic |
| Hypoechogenic | |
| Orientation | Parallel |
| Non-parallel | |
| Margin | Well-defined |
| Microlobulated | |
| Ill-defined | |
| Spongiform | Appearance |
| Non-appearance | |
| Shape | Oval-to-round |
| Irregular | |
| Calcification | No calcification |
| Macrocalcification | |
| Microcalcification 1 |
| Case/Diagnosis/Feature | Number | |
|---|---|---|
| Malignant-surgery diagnosis/all | 15 a/200 | |
| Radiologist possible malignancies (/correct) | 35 (/13) | |
| CAD system possible malignancies (/correct) | 122 (/12) | |
| Radiologist missed malignancies | 2 | |
| CAD system missed malignancies | 3 | |
| Thyroiditis b cases | all | 43 |
| with true nodules | 9 | |
| with pseudonodules | 8 | |
| with focal inhomogeneities | 26 | |
| Nodule features of interest | coarse macrocalcification | 12 |
| non-coarse macrocalcification | 10 | |
| inspissated colloid | 5 | |
| FNAB | all | 121 |
| in focal inhomogeneity | 14 | |
| in nodule with coarse macrocalcification | 7 | |
| in nodule with non-coarse macrocalcification | 4 | |
| in inspissated colloid cystic nodule | 4 | |
| in true nodule related to thyroiditis | 5 | |
| Radiologist K-TIRADS scores 1/2/3/4/5 | 34/31/100/17/18 | |
| CAD system K-TIRADS scores 1/2/3/4/5 | 0/9/69/53/69 | |
| Lesion size (largest diameter, mm) | min | 8 |
| max | 42 | |
| average | 14 | |
| Thyroid Entities * | Rate | p2 | CAD TIRADS 3 Rates | Malignancies/Diagnosed 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAD 1 Correct Group | CAD Incorrect Group | 1 | 2 | 3 | 4 | 5 | ||||
| Mimicking lesions | Focal inhomogeneity (thyroiditis) | 0 | 26 | <0.0001 | 0 | 0 | 0 | 2 | 24 | 0/0 |
| Pseudonodule in thyroiditis | 5 | 3 | 0.48 | 0 | 0 | 5 | 0 | 3 | 0/0 | |
| True nodules | True nodule in thyroiditis | 1 | 8 | 0.019 | 0 | 0 | 0 | 8 | 1 | 1/1 |
| Macrocalcification non-coarse | 4 | 5 | 0.527 | 0 | 0 | 3 | 3 | 3 | 0/0 | |
| Coarse macrocalcification | 0 | 12 | 0.0001 | 0 | 0 | 0 | 1 | 11 | 0/0 | |
| Inspissated colloid cystic nodule | 0 | 5 | 0.014 | 0 | 0 | 0 | 3 | 2 | 0/0 | |
| US features * | ||||||||||
| Composition | Solid | 30 | 30 | 0.442 | 0 | 0 | 18 | 16 | 26 | 12/12 |
| Partially cystic | 34 | 29 | 0.878 | 0 | 2 | 33 | 22 | 6 | 1/0 | |
| Cystic | 14 | 5 | 0.1 | 0 | 7 | 7 | 4 | 1 | 0/0 | |
| Echogenicity | Hyper/isoechoic | 58 | 54 | 0.5 | 0 | 6 | 53 | 37 | 16 | 1/0 |
| Hypoechoic | 20 | 10 | 0.2 | 0 | 3 | 5 | 5 | 17 | 12/12 | |
| Orientation | Parallel | 68 | 63 | 0.487 | 0 | 9 | 57 | 41 | 24 | 4/3 |
| Non-parallel | 10 | 1 | 0.017 | 0 | 0 | 1 | 1 | 9 | 10/10 | |
| Margin | Well-defined | 65 | 58 | 0.642 | 0 | 9 | 56 | 37 | 21 | 2/1 |
| Microlobulated | 5 | 5 | 0.754 | 0 | 0 | 2 | 4 | 4 | 3/3 | |
| Ill-defined | 8 | 1 | 0.04 | 0 | 0 | 0 | 1 | 8 | 8/8 | |
| Spongiform | Appearance | 11 | 8 | 0.8 | 0 | 2 | 9 | 7 | 1 | 0/0 |
| Non-appearance | 67 | 56 | 0.919 | 0 | 7 | 49 | 35 | 32 | 13/12 | |
| Shape | Ovoid to round | 71 | 64 | 0.585 | 0 | 9 | 58 | 40 | 28 | 6/5 |
| Irregular | 7 | 0 | 0.017 | 0 | 0 | 0 | 2 | 5 | 7/7 | |
| Microcalcification | 5 | 1 | 0.162 | 0 | 0 | 1 | 1 | 4 | 6/5 | |
| Diagnostic Parameter | Human, All | CAD, All | CAD, Screened | Human, Screened | p Values of Comparisons 4 | |||
|---|---|---|---|---|---|---|---|---|
| Human vs. CAD All | Human vs. CAD Screened | CAD all vs. Screened | Human All vs. Screened | |||||
| Sensitivity | 88.67% | 80% | 78.57% | 85.71% | 1 | 1 | 0.92 | 0.94 |
| Specificity | 88.11% | 40.54% | 55.97% | 84.33% | <0.0001 | <0.0001 | 0.007 | 0.33 |
| Accuracy | 88% | 43.5% | 58.1% | 84.46% | <0.0001 | <0.0001 | 0.12 | 0.8 |
| PPV 5 | 37.14% | 9.84% | 15.71% | 36.36% | ||||
| NPV 6 | 98.79% | 96.15% | 96.15% | 98.26% | ||||
| ROC AUC 7 | 0.937 | 0.656 | 0.76 | 0.922 | 0.0002 | 0.049 | 0.289 | 0.782 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnár, K.; Kálmán, E.; Hári, Z.; Giyab, O.; Gáspár, T.; Rucz, K.; Bogner, P.; Tóth, A. False-Positive Malignant Diagnosis of Nodule Mimicking Lesions by Computer-Aided Thyroid Nodule Analysis in Clinical Ultrasonography Practice. Diagnostics 2020, 10, 378. https://doi.org/10.3390/diagnostics10060378
Molnár K, Kálmán E, Hári Z, Giyab O, Gáspár T, Rucz K, Bogner P, Tóth A. False-Positive Malignant Diagnosis of Nodule Mimicking Lesions by Computer-Aided Thyroid Nodule Analysis in Clinical Ultrasonography Practice. Diagnostics. 2020; 10(6):378. https://doi.org/10.3390/diagnostics10060378
Chicago/Turabian StyleMolnár, Krisztián, Endre Kálmán, Zsófia Hári, Omar Giyab, Tamás Gáspár, Károly Rucz, Péter Bogner, and Arnold Tóth. 2020. "False-Positive Malignant Diagnosis of Nodule Mimicking Lesions by Computer-Aided Thyroid Nodule Analysis in Clinical Ultrasonography Practice" Diagnostics 10, no. 6: 378. https://doi.org/10.3390/diagnostics10060378
APA StyleMolnár, K., Kálmán, E., Hári, Z., Giyab, O., Gáspár, T., Rucz, K., Bogner, P., & Tóth, A. (2020). False-Positive Malignant Diagnosis of Nodule Mimicking Lesions by Computer-Aided Thyroid Nodule Analysis in Clinical Ultrasonography Practice. Diagnostics, 10(6), 378. https://doi.org/10.3390/diagnostics10060378





