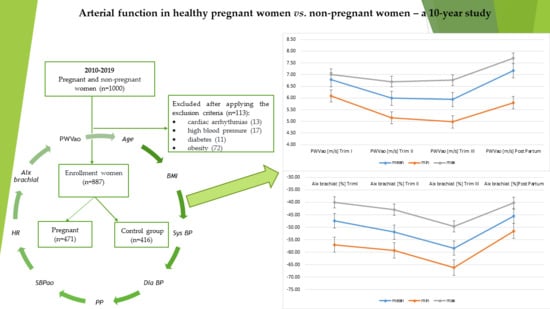
Arterial Function in Healthy Pregnant Women vs. Non-Pregnant Women—A 10-Year Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Paraclinical Evaluation
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khalil, A.; Akolekar, R.; Syngelaki, A.; Elkhouli, M.; Nicolaides, K.H. Maternal Hemodynamics at 11-13 Weeks’ Gestation and Risk of Pre-Eclampsia. Ultrasound Obs. Gynecol. 2012, 40, 28–34. [Google Scholar] [CrossRef]
- Kim, H.-L.; Kim, S.-H. Pulse Wave Velocity in Atherosclerosis. Front. Cardiovasc. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Wong, N.D. Metabolic Syndrome, Cardiovascular Risk and Screening for Subclinical Atherosclerosis. Expert Rev. Cardiovasc. Ther. 2009, 7, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-N.; Gao, H.-Q.; Li, B.-Y.; Cheng, M.; Ma, Y.-B.; Zhang, Z.-M.; Gao, X.-M.; Liu, Y.-P.; Wang, M. Pulse Wave Velocity as a marker of Artheriosclerosis and Its Comorbidities in Chinese Patients. Hypertens. Res. 2007, 30, 237–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allali, J.; Chauve, C.; Denise, A.; Drevet, C.; Ferraro, P.; Gautheret, D.; Herrbach, C.; Leclerc, F.; Ouangraoua, A.; Sagot, M.-F.; et al. BRASERO: A Resource for Benchmarking RNA Secondary Structure Comparison Algorithms. Adv. Bioinform. 2012, 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, J.; Gharib, A.M.; Garcia, A.; Heroux, J.; Yazdani, S.K.; Malvè, M.; Tracqui, P.; Martinez, M.A.; Doblare, M.; Finet, G.; et al. Is Arterial Wall-Strain Stiffening an Additional Process Responsible for Atherosclerosis in Coronary Bifurcations?: An in Vivo Study Based on Dynamic CT and MRI. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1097–H1106. [Google Scholar] [CrossRef] [PubMed]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.S.; Protogerou, A.D.; et al. Expert Consensus Document on the Measurement of Aortic Stiffness in Daily Practice Using Carotid-Femoral Pulse Wave Velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef]
- Inoue, N.; Maeda, R.; Kawakami, H.; Shokawa, T.; Yamamoto, H.; Ito, C.; Sasaki, H. Aortic Pulse Wave Velocity Predicts Cardiovascular Mortality in Middle-Aged and Elderly Japanese Men. Circ. J. 2009, 73, 549–553. [Google Scholar] [CrossRef]
- Stephanie, R.A.; Curtis, L. Heart Disease and Pregnancy. Cardiol. Ther. 2017, 6, 157–173. [Google Scholar]
- Meah, V.L.; Cockcroft, J.R.; Backx, K.; Shave, R.; Stöhr, E.J. Cardiac Output and Related Haemodynamics during Pregnancy: A Series of Meta-Analyses. Heart 2016, 102, 518–526. [Google Scholar] [CrossRef]
- Lopes van Balen, V.A.; van Gansewinkel, T.A.G.; de Haas, S.; van Kuijk, S.M.J.; van Drongelen, J.; Ghossein-Doha, C.; Spaanderman, M.E.A. Physiological Adaptation of Endothelial Function to Pregnancy: Systematic Review and Meta-Analysis. Ultrasound Obs. Gynecol. 2017, 50, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.B.; Abraham, W.T.; Zamudio, S.; Coffin, C.; Merouani, A.; Young, D.; Johnson, A.; Osorio, F.; Goldberg, C.; Moore, L.G.; et al. Temporal Relationships between Hormonal and Hemodynamic Changes in Early Human Pregnancy. Kidney Int. 1998, 54, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Elvan-Taşpinar, A.; Franx, A.; Bots, M.L.; Koomans, H.A.; Bruinse, H.W. Arterial Stiffness and Fetal Growth in Normotensive Pregnancy. Am. J. Hypertens. 2005, 18, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Lof, M.; Olausson, H.; Bostrom, K.; Janerot-Sjöberg, B.; Sohlstrom, A.; Forsum, E. Changes in Basal Metabolic Rate during Pregnancy in Relation to Changes in Body Weight and Composition, Cardiac Output, Insulin-like Growth Factor I, and Thyroid Hormones and in Relation to Fetal Growth. Am. J. Clin. Nutr. 2005, 81, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Selzer, A. Risks of Pregnancy in Women With Cardiac Disease. JAMA J. Am. Med. Assoc. 1977, 238, 892–893. [Google Scholar] [CrossRef]
- McFaul, P.B.; Dornan, J.C.; Lamki, H.; Boyle, D. Pregnancy Complicated by Maternal Heart Disease. A Review of 519 Women. Bjog Int. J. Obs. Gynaecol. 1988, 95, 861–867. [Google Scholar] [CrossRef]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological Changes in Pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef]
- Ngene, N.C.; Moodley, J. Physiology of Blood Pressure Relevant to Managing Hypertension in Pregnancy. J. Matern. Fetal Neonatal Med. 2019, 32, 1368–1377. [Google Scholar] [CrossRef]
- Foo, F.L.; McEniery, C.M.; Lees, C.; Khalil, A. Assessment of Arterial Function in Pregnancy: Recommendations of the International Working Group on Maternal Hemodynamics. Ultrasound Obs. Gynecol. 2017, 50, 324–331. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Ducimetiere, P.; Benetos, A. Aortic Stiffness Is an Independent Predictor of All-Cause and Cardiovascular Mortality in Hypertensive Patients. Hypertension 2001, 37, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Katsahian, S.; Fassot, C.; Tropeano, A.I.; Gautier, I.; Laloux, B.; Boutouyrie, P. Aortic Stiffness Is an Independent Predictor of Fatal Stroke in Essential Hypertension. Stroke 2003, 34, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Boutouyrie, P.; Tropeano, A.I.; Asmar, R.; Gautier, I.; Benetos, A.; Lacolley, P.; Laurent, S. Aortic Stiffness Is an Independent Predictor of Primary Coronary Events in Hypertensive Patients: A Longitudinal Study. Hypertension 2002, 39, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F.; Hwang, S.J.; Vasan, R.S.; Larson, M.G.; Pencina, M.J.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J. Arterial Stiffness and Cardiovascular Events: The Framingham Heart Study. Circulation 2010, 121, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Palatini, P.; Casiglia, E.; Gasowski, J.; Głuszek, J.; Jankowski, P.; Narkiewicz, K.; Saladini, F.; Stolarz-Skrzypek, K.; Tikhonoff, V.; Van Bortel, L.; et al. Arterial Stiffness, Central Hemodynamics, and Cardiovascular Risk in Hypertension. Vasc. Health Risk Manag. 2011, 2011, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-G.; Joo, S.-J. Arterial Stiffness and Cardiovascular Risk. Korean J. Intern. Med. 2019, 34, 504–506. [Google Scholar] [CrossRef]
- Ogola, B.O.; Zimmerman, M.A.; Clark, G.L.; Abshire, C.M.; Gentry, K.M.; Miller, K.S.; Lindsey, S.H. Sex Differences in Cardiovascular and Cerebrovascular Physiology, Disease, and Signaling Mechanisms: New Insights into Arterial Stiffening: Does Sex Matter? Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1073–H1087. [Google Scholar] [CrossRef]
- Mattace-Raso, F.U.S.; Van Der Cammen, T.J.M.; Hofman, A.; Van Popele, N.M.; Bos, M.L.; Schalekamp, M.A.D.H.; Asmar, R.; Reneman, R.S.; Hoeks, A.P.G.; Breteler, M.M.B.; et al. Arterial Stiffness and Risk of Coronary Heart Disease and Stroke: The Rotterdam Study. Circulation 2006, 113, 657–663. [Google Scholar] [CrossRef]
- Mzayek, F.; Sherwin, R.; Hughes, J.; Hassig, S.; Srinivasan, S.; Chen, W.; Berenson, G.S. The Association of Birth Weight with Arterial Stiffness at Mid-Adulthood: The Bogalusa Heart Study. J. Epidemiol. Community Health 2009, 63, 729–733. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Recommendations Guiding Physicians in Biomedical Research Involving Human Subjects. JAMA J. Am. Med. Assoc. 1997, 277, 925–926. [Google Scholar] [CrossRef]
- Devices—TensioMed & Arterial Stiffness. Available online: https://www.tensiomed.eu/devices/ (accessed on 1 June 2020).
- EUR-Lex-32016R0679-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2016/679/oj (accessed on 1 June 2020).
- Vermeersch, S.J.; Dynamics, B.; Society, L. Determinants of Pulse Wave Velocity in Healthy People and in the Presence of Cardiovascular Risk Factors: ‘Establishing Normal and Reference Values. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar]
- La Rocca, H.-P.B. Towards applicability of measures of arterial stiffness in clinical routine. Eur. Heart J. 2010, 31, 2320–2322. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, T.J.; Song, D.; Lee, K.J.; Kim, E.H.; Lee, H.S.; Nam, C.M.; Nam, H.S.; Kim, Y.D.; Heo, J.H. Brachial-Ankle Pulse Wave Velocity Is a Strong Predictor for Mortality in Patients with Acute Stroke. Hypertension 2014, 64, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Ki, Y.J.; Choi, D.H.; Lee, Y.M.; Lim, L.; Song, H.; Koh, Y.Y. Predictive Value of Brachial-Ankle Pulse Wave Velocity for Long-Term Clinical Outcomes after Percutaneous Coronary Intervention in a Korean Cohort. Int. J. Cardiol. 2014, 175, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, K.; Riste, L.; Anderson, S.G.; Wright, J.S.; Dunn, G.; Gosling, R.G. Aortic Pulse-Wave Velocity and Its Relationship to Mortality in Diabetes and Glucose Intolerance: An Integrated Index of Vascular Function? Circulation 2002, 106, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; Safar, M.E.; London, G.M. Impact of Aortic Stiffness on Survival in End-Stage Renal Disease. Circulation 1999, 99, 2434–2439. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.M.; Amoozegar, J.B.; McClure, E.M.; Squiers, L.B.; Broussard, C.S.; Lind, J.N.; Polen, K.N.; Frey, M.T.; Gilboa, S.M.; Biermann, J. Improving Safe Use of Medications During Pregnancy: The Roles of Patients, Physicians, and Pharmacists. Qual. Health Res. 2017, 27, 2071–2080. [Google Scholar] [CrossRef]
- Babiker, A.; El Husseini, M.; Al Nemri, A.; Al Frayh, A.; Al Juryyan, N.; Faki, M.O.; Assiri, A.; Al Saadi, M.; Shaikh, F.; Al Zamil, F. Health Care Professional Development: Working as a Team to Improve Patient Care. Sudan. J. Paediatr. 2014, 14, 9–16. [Google Scholar]
- Yannoutsos, A.; Bahous, S.A.; Safar, M.E.; Blacher, J. Clinical Relevance of Aortic Stiffness in End-Stage Renal Disease and Diabetes: Implication for Hypertension Management. J. Hypertens. 2018, 36, 1237–1246. [Google Scholar] [CrossRef]
- Burton, G.J.; Yung, H.W.; Cindrova-Davies, T.; Charnock-Jones, D.S. Placental Endoplasmic Reticulum Stress and Oxidative Stress in the Pathophysiology of Unexplained Intrauterine Growth Restriction and Early Onset Preeclampsia. Placenta 2009, 30, 43–48. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Chaemsaithong, P.; Yeo, L.; Romero, R. Pre-Eclampsia Part 1: Current Understanding of Its Pathophysiology. Nat. Rev. Nephrol. 2014, 10, 466–480. [Google Scholar] [CrossRef]
- Savvidou, M.D.; Hingorani, A.D.; Tsikas, D.; Frölich, J.C.; Vallance, P.; Nicolaides, K.H. Endothelial Dysfunction and Raised Plasma Concentrations of Asymmetric Dimethylarginine in Pregnant Women Who Subsequently Develop Pre-Eclampsia. Lancet 2003, 361, 1511–1517. [Google Scholar] [CrossRef]
- Beck, D.T.; Martin, J.S.; Casey, D.P.; Braith, R.W. Exercise Training Reduces Peripheral Arterial Stiffness and Myocardial Oxygen Demand in Young Prehypertensive Subjects. Am. J. Hypertens. 2013, 26, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc, S.; Avram, C.; Turi, V.; Militaru, A.; Avram, A.; Cimpean, A.M.; Iurciuc, M. Physical Training, Hemodynamic Parameters and Arterial Stiffness: Friends or Foes of the Hypertensive Patient? In Vivo 2016, 30, 521–528. [Google Scholar]
- Crişan, S.; Petrescu, L.; Lazăr, M.A.; Văcărescu, C.; Nicola, A.R.; Cozma, D.; Mornoş, C.; Luca, C.T. Reduced Ejection Fraction Heart Failure—New Data from Multicenter Studies and National Registries Regarding General and Elderly Populations: Hopes and Disappointments. Clin. Interv. Aging 2018, 13, 651–656. [Google Scholar] [CrossRef]
- Beetham, K.S.; Giles, C.; Noetel, M.; Clifton, V.; Jones, J.C.; Naughton, G. The Effects of Vigorous Intensity Exercise in the Third Trimester of Pregnancy: A Systematic Review and Meta-Analysis. BMC Pregnancy Childbirth 2019, 19, 281. [Google Scholar] [CrossRef]
- Cavalcante, J.L.; Lima, J.A.C.; Redheuil, A.; Al-Mallah, M.H. Aortic Stiffness: Current Understanding and Future Directions. J. Am. Coll. Cardiol. 2011, 57, 1511–1522. [Google Scholar] [CrossRef]
- Dragan, S.; Buleu, F.; Christodorescu, R.; Cobzariu, F.; Iurciuc, S.; Velimirovici, D.; Xiao, J.; Luca, C.T. Benefits of Multiple Micronutrient Supplementation in Heart Failure: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 965–981. [Google Scholar] [CrossRef]
- Vamvakis, A.; Gkaliagkousi, E.; Triantafyllou, A.; Gavriilaki, E.; Douma, S. Beneficial Effects of Nonpharmacological Interventions in the Management of Essential Hypertension. Jrsm Cardiovasc. Dis. 2017, 6, 204800401668389. [Google Scholar] [CrossRef]
- Stoicescu, M.; Csepento, C.; Muţiu, G.; Bungǎu, S. The Role of Increased Plasmatic Renin Level in the Pathogenesis of Arterial Hypertension in Young Adults. Rom. J. Morphol. Embryol. 2011, 52, 419–423. [Google Scholar]
- Vesa, C.M.; Popa, L.; Popa, A.R.; Rus, M.; Zaha, A.A.; Bungau, S.; Tit, D.M.; Corb Aron, R.A.; Zaha, D.C. Current Data Regarding the Relationship between Type 2 Diabetes Mellitus and Cardiovascular Risk Factors. Diagnostics 2020, 10, 314. [Google Scholar] [CrossRef]
- Fujime, M.; Tomimatsu, T.; Okaue, Y.; Koyama, S.; Kanagawa, T.; Taniguchi, T.; Kimura, T. Central Aortic Blood Pressure and Augmentation Index during Normal Pregnancy. Hypertens. Res. 2012, 35, 633–638. [Google Scholar] [CrossRef] [PubMed]

| Age Category (Years) | Mean (+2 SD) * | Median (10–90 pc) ** |
|---|---|---|
| <30 years | 6.2 (4.7–7.6) | 6.1 (5.3–7.1) |
| 30–39 years | 6.5 (3.8–9.2) | 6.4 (5.2–8.0) |
| 40–49 | 7.2 (4.6–9.8) | 6.9 (5.9–8.6) |
| 50–59 | 8.3 (4.5–12.1) | 8.1 (6.3–10.0) |
| 60–69 | 10.3 (5.5–15.0) | 9.7 (7.9–13.1) |
| ≥70 | 10.9 (5.5–16.3) | 10.6 (8.0–14.6) |
| Statistics | Age | BMI (Kg/m2) | SBP (mmHg) | DBP (mmHg) | PP (mmHg) | SBPao (mmHg) | HR (1/min) | Brachial AIx (%) | PWVao (m/s) |
|---|---|---|---|---|---|---|---|---|---|
| Mean | 30.15 | 21.43 | 122.30 | 72.62 | 49.68 | 115.21 | 72.28 | −47.41 | 6.78 |
| 29.61 | 21.43 | 122.59 | 74.72 | 47.87 | 109.16 | 71.57 | −49.50 | 7.43 | |
| Standard error | 0.24 | 0.10 | 0.49 | 0.28 | 0.30 | 0.14 | 0.16 | 0.20 | 0.01 |
| 0.22 | 0.09 | 0.42 | 0.30 | 0.28 | 0.55 | 0.48 | 0.79 | 0.02 | |
| Median | 27 | 20.76 | 130 | 75 | 54 | 116 | 73 | −47.47 | 6.81 |
| 31 | 21.36 | 122 | 75 | 48 | 110 | 71 | −50.7 | 7.4 | |
| Mode | 27 | 19.96 | 130 | 75 | 55 | 116 | 73 | −52.6 | 6.78 |
| 32 | 22.58 | 130 | 80 | 40 | 119 | 68 | −34.5 | 7.4 | |
| Standard deviation | 5.22 | 2.27 | 10.72 | 6.06 | 6.51 | 3.11 | 3.49 | 4.36 | 0.16 |
| 4.39 | 1.86 | 8.64 | 6.09 | 5.69 | 11.22 | 9.75 | 16.20 | 0.49 | |
| Sample variance | 27.24 | 5.14 | 114.98 | 36.70 | 42.35 | 9.66 | 12.17 | 19.02 | 0.02 |
| 19.26 | 3.47 | 74.60 | 37.12 | 32.33 | 125.80 | 95.14 | 262.60 | 0.24 | |
| Kurtosis | −0.79 | −0.87 | 0.19 | −0.06 | −0.20 | 0.81 | 0.11 | −1.25 | 6.17 |
| 0.30 | −0.81 | −0.94 | −0.58 | −0.86 | 0.04 | 0.03 | 0.45 | 7.95 | |
| Skewness | 0.77 | 0.50 | −1.15 | −0.05 | −0.94 | −0.95 | −0.68 | −0.04 | −2.18 |
| 0.11 | 0.19 | 0.04 | −0.05 | 0.28 | 0.04 | 0.45 | 0.41 | 2.26 | |
| Range | 25 | 9.65 | 36 | 25 | 26 | 13 | 16 | 17 | 0.92 |
| 25 | 7.52 | 34 | 30 | 20 | 56 | 53 | 101.6 | 3 | |
| Minimum | 20 | 17.30 | 94 | 60 | 29 | 107 | 63 | −57 | 6.09 |
| 20 | 18.20 | 108 | 62 | 40 | 82 | 52 | −85.9 | 6.7 | |
| Maximum | 45 | 26.95 | 130 | 85 | 55 | 120 | 79 | −40 | 7.01 |
| 45 | 25.71 | 142 | 92 | 60 | 138 | 105 | 15.7 | 9.7 |
| Statistics → Variables ↓ | Group | Mean | Standard Deviation | Std. Error Mean | p Value t-Test | p Value Mann–Whitney | |
|---|---|---|---|---|---|---|---|
| Age | Years | 1 | 30.15 | 5.2 | 0.241 | 0.096 | 0.607 |
| 2 | 29.61 | 4.39 | 0.22 | ||||
| BMI | Kg/m2 | 1 | 21.42 | 2.2 | 0.1045 | <0.001 * | 0.034 * |
| 2 | 21.43 | 1.86 | 0.09 | ||||
| Brachial AIx | % | 1 | −47.41 | 4.36 | 0.20 | 0.011* | 0.012 * |
| 2 | −49.50 | 16.20 | 0.79 | ||||
| PWVao | m/s | 1 | 6.77 | 0.157 | 0.007 | <0.001 * | <0.001 * |
| 2 | 7.43 | 0.49 | 0.023 | ||||
| SBP | (mm Hg) | 1 | 122.30 | 10.723 | 0.494 | <0.001 * | <0.001 * |
| 2 | 122.59 | 8.64 | 0.42 | ||||
| DBP | 1 | 72.62 | 6.058 | 0.279 | <0.001 * | <0.001 * | |
| 2 | 74.72 | 6.09 | 0.3 | ||||
| PP | 1 | 49.68 | 6.507 | 0.300 | <0.001 * | <0.001 * | |
| 2 | 47.87 | 5.69 | 0.28 | ||||
| SBPao | 1 | 115.21 | 3.108 | 0.143 | <0.001 * | <0.001 * | |
| 2 | 109.16 | 11.22 | 0.55 | ||||
| HR | 1/min | 1 | 72.28 | 3.488 | 0.161 | <0.001 * | <0.001 * |
| 2 | 71.57 | 9.75 | 0.48 | ||||
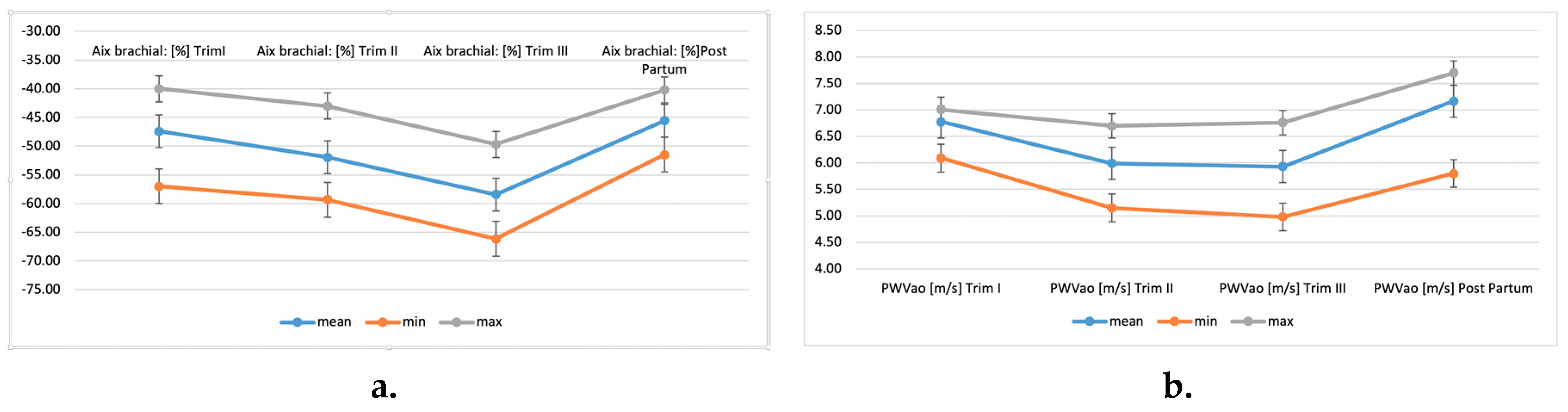
| Statistics | Brachial AIx (%) | PWVao (m/s) | Brachial AIx (%) | PWVao (m/s) | Brachial AIx (%) | PWVao (m/s) | Brachial AIx (%) | PWVao (m/s) |
|---|---|---|---|---|---|---|---|---|
| 1st trimester | 2nd trimester | 3rd trimester | Post-partum | |||||
| Mean | −47.41 | 6.78 | −51.93 | 5.99 | −58.40 | 5.93 | −45.57 | 7.17 |
| Standard error | 0.20 | 0.01 | 0.11 | 0.02 | 0.22 | 0.02 | 0.16 | 0.02 |
| Median | −47.47 | 6.81 | −52.18 | 6.1 | −58.45 | 6.07 | −45.16 | 7.35 |
| Mode | −52.6 | 6.78 | −52.6 | 6.1 | −52.6 | 6.14 | −45.16 | 7.39 |
| Standard deviation | 4.36 | 0.16 | 2.43 | 0.51 | 4.73 | 0.53 | 3.54 | 0.43 |
| Sample variance | 19.02 | 0.02 | 5.89 | 0.26 | 22.37 | 0.28 | 12.51 | 0.18 |
| Kurtosis | −1.25 | 6.17 | 1.86 | −1.65 | −1.41 | −1.18 | −1.38 | 4.64 |
| Skewness | −0.04 | −2.18 | 0.10 | −0.04 | 0.13 | −0.31 | −0.21 | −2.43 |
| Range | 17 | 0.92 | 16.3 | 1.55 | 16.46 | 1.78 | 11.28 | 1.9 |
| Minimum | −57 | 6.09 | −59.3 | 5.15 | −66.15 | 4.98 | −51.5 | 5.8 |
| Maximum | −40 | 7.01 | −43 | 6.7 | −49.69 | 6.76 | −40.22 | 7.7 |
| Statistics | Gestational Period (Weeks) | APGAR Score at 1 min | Foetus Weight (g) |
|---|---|---|---|
| Mean | 39.34 | 9.22 | 3468.97 |
| Standard error | 0.05 | 0.04 | 6.86 |
| Median | 39 | 9 | 3500 |
| Mode | 40 | 10 | 3500 |
| Standard deviation | 1.05 | 0.95 | 148.84 |
| Sample variance | 1.09 | 0.90 | 22,154.52 |
| Kurtosis | 0.97 | 11.92 | −0.09 |
| Skewness | −0.30 | −2.17 | 0.18 |
| Range | 7 | 9 | 820 |
| Minimum | 35 | 1 | 3100 |
| Maximum | 42 | 10 | 3920 |
| Variables | Yes/Yes/Girl/Natural | No/No/Boy/C-Section | ||
|---|---|---|---|---|
| Number | % | Number | % | |
| Group 1 (N1 = 471) | ||||
| Exercise | 160 | 33.97 | 311 | 66.03 |
| Smoker | 194 | 41.19 | 277 | 58.81 |
| Baby gender | 204 | 43.31 | 267 | 56.69 |
| Delivery type | 233 | 49.47 | 238 | 50.53 |
| Group 2 (Control Group, N2 = 416) | ||||
| Exercise | 191 | 45.91 | 225 | 54.09 |
| Smoker | 160 | 38.46 | 256 | 61.54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turi, V.; Dragan, S.; Iurciuc, M.; Moleriu, L.; Bungau, S.; Tit, D.M.; Toader, D.-O.; Diaconu, C.C.; Behl, T.; Petre, I. Arterial Function in Healthy Pregnant Women vs. Non-Pregnant Women—A 10-Year Study. Diagnostics 2020, 10, 374. https://doi.org/10.3390/diagnostics10060374
Turi V, Dragan S, Iurciuc M, Moleriu L, Bungau S, Tit DM, Toader D-O, Diaconu CC, Behl T, Petre I. Arterial Function in Healthy Pregnant Women vs. Non-Pregnant Women—A 10-Year Study. Diagnostics. 2020; 10(6):374. https://doi.org/10.3390/diagnostics10060374
Chicago/Turabian StyleTuri, Vladiana, Simona Dragan, Mircea Iurciuc, Lavinia Moleriu, Simona Bungau, Delia Mirela Tit, Daniela-Oana Toader, Camelia Cristina Diaconu, Tapan Behl, and Izabella Petre. 2020. "Arterial Function in Healthy Pregnant Women vs. Non-Pregnant Women—A 10-Year Study" Diagnostics 10, no. 6: 374. https://doi.org/10.3390/diagnostics10060374
APA StyleTuri, V., Dragan, S., Iurciuc, M., Moleriu, L., Bungau, S., Tit, D. M., Toader, D.-O., Diaconu, C. C., Behl, T., & Petre, I. (2020). Arterial Function in Healthy Pregnant Women vs. Non-Pregnant Women—A 10-Year Study. Diagnostics, 10(6), 374. https://doi.org/10.3390/diagnostics10060374