Evaluation of ViroTrack Sero Zika IgG/IgM, a New Rapid and Quantitative Zika Serological Diagnostic Assay
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
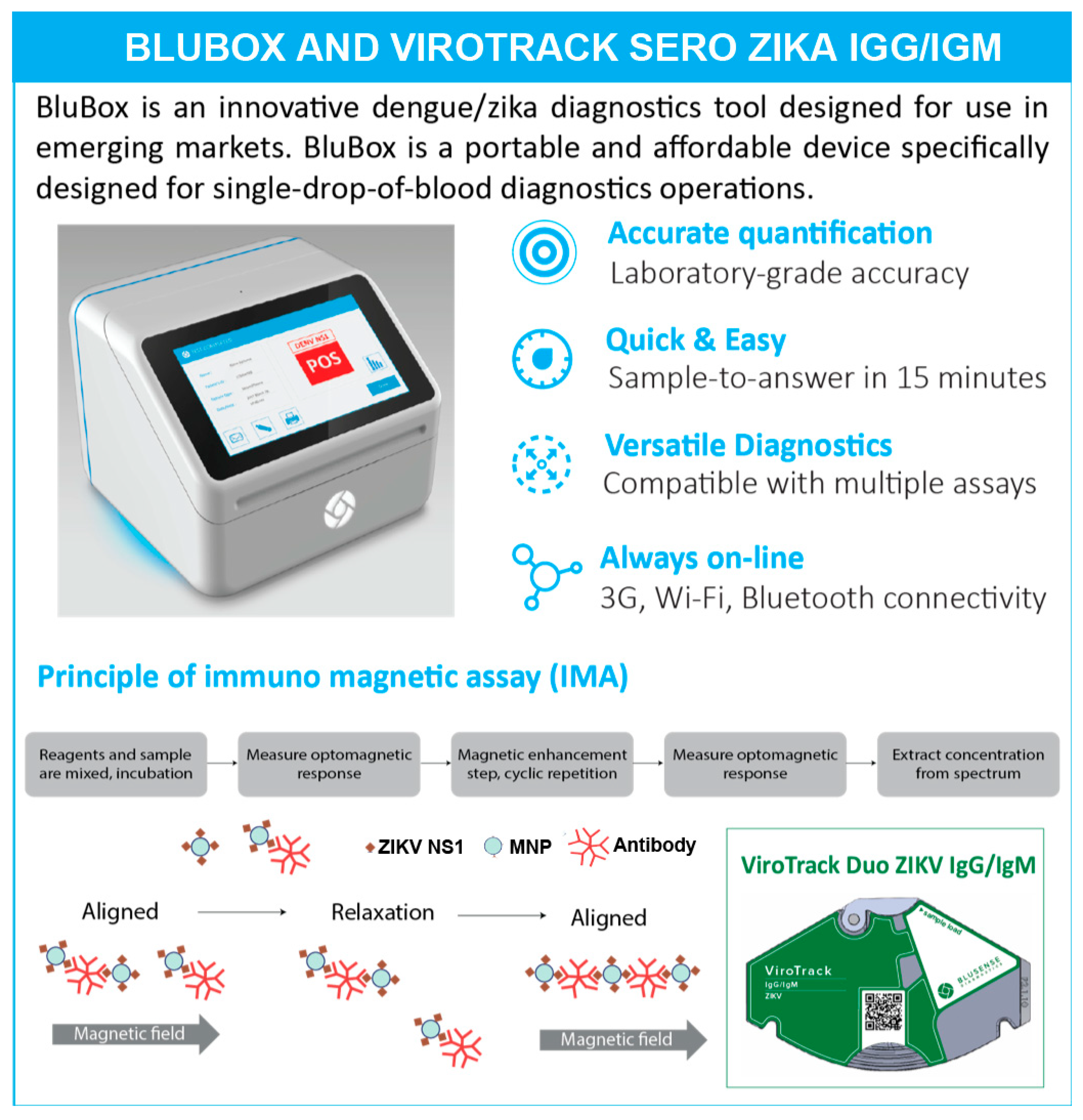
2.2. ViroTrack Sero Zika IgG/IgM Cartridge Design and BluBox
2.3. Clinical Samples and Sample Preparation
2.4. Immune Status
2.5. Serological Study 1
2.6. Serological Study 2
2.7. Serological Study 3
2.8. Statistical Analysis
3. Results
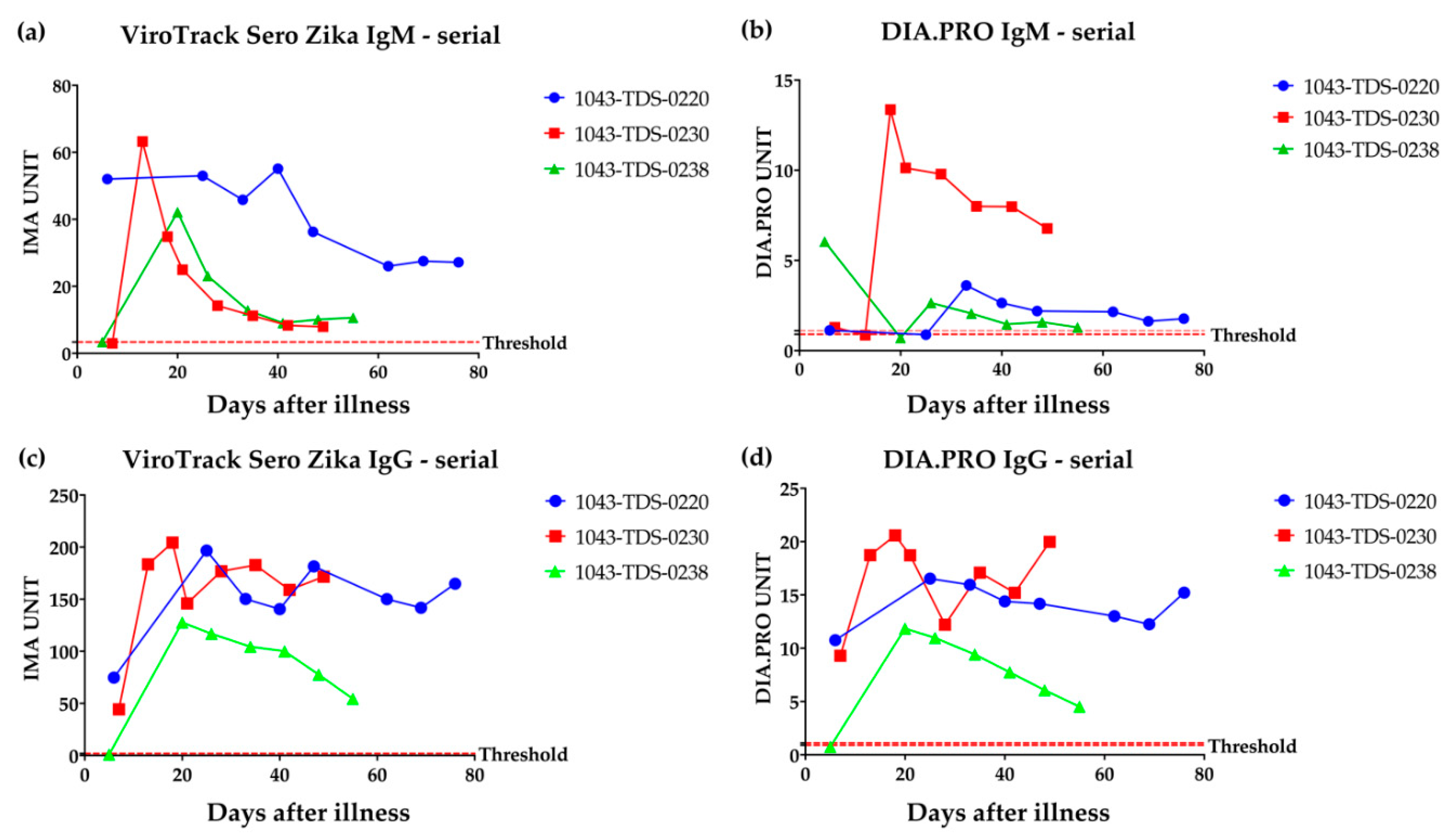
3.1. Serological Study 1: Zika IgM & IgG Measured by VirTrack Sero Zika IgG/IgM Assay Following Three Patients after Illness Onset
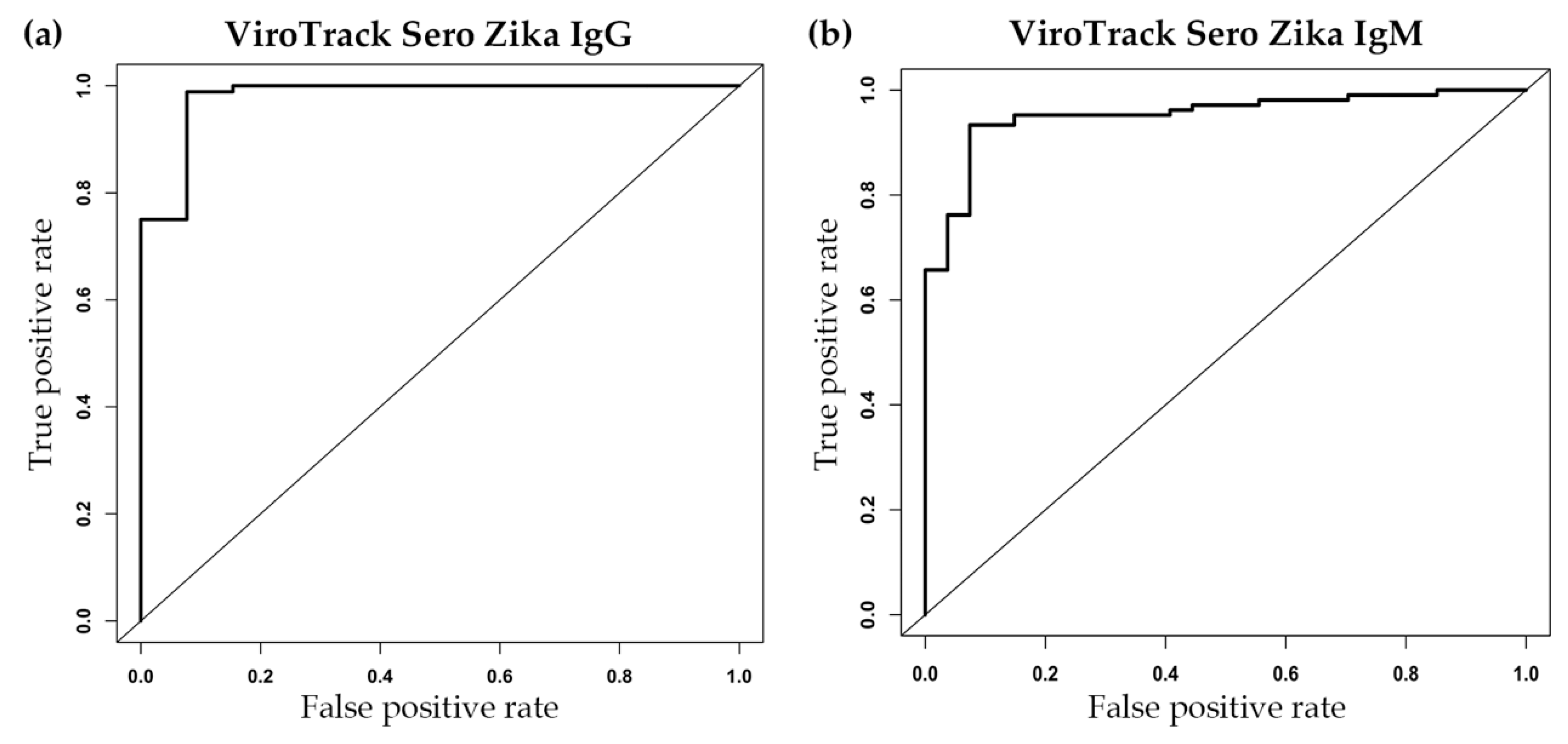
3.2. Serological Study 2: ViroTrack Sero Zika IgG/IgM Sensitivity and Specificity
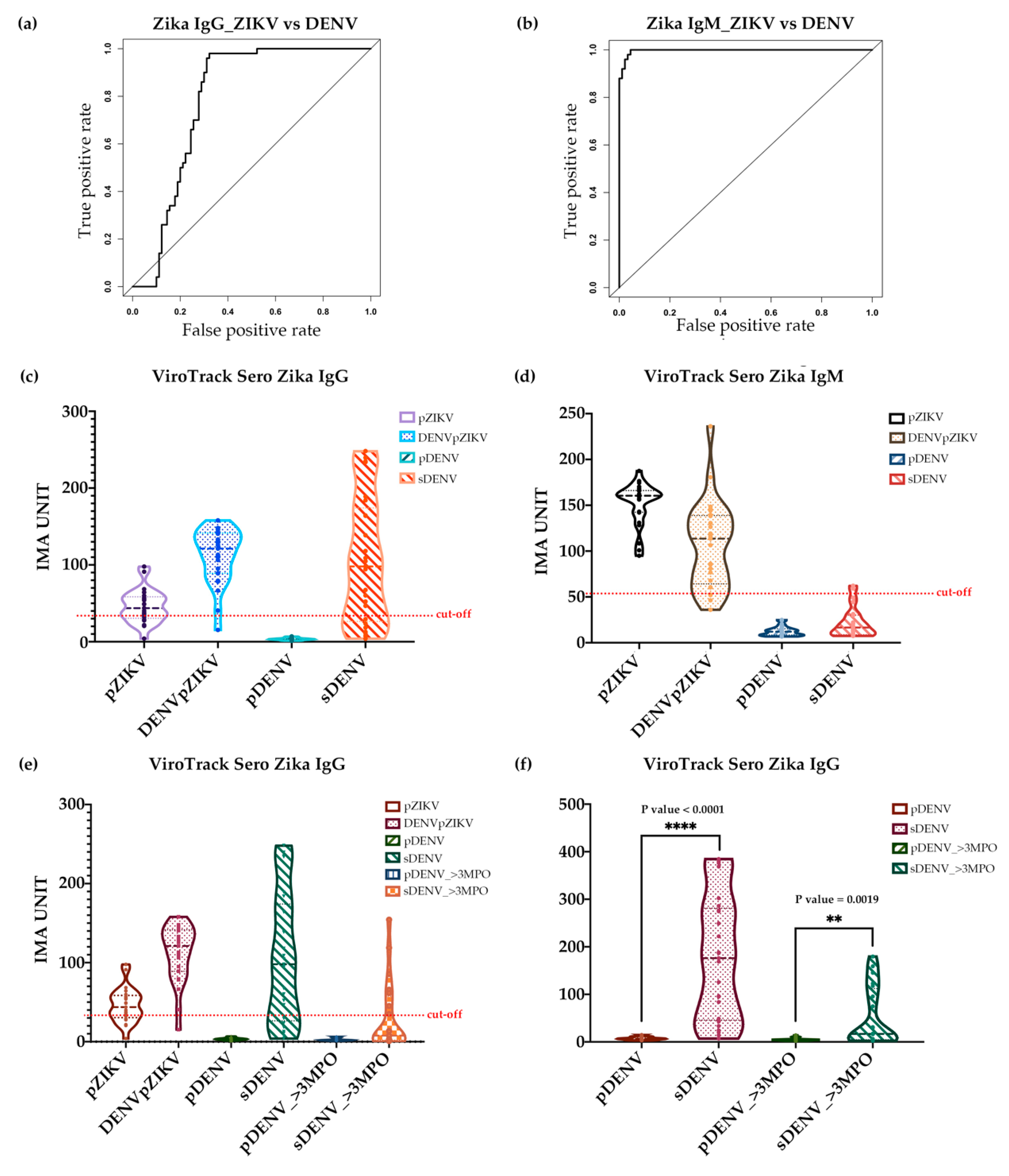
3.3. Serological Study 3: ViroTrack Sero Zika IgG/IgM to Distinguish ZIKV- and DENV-Infected Samples
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DENV | Dengue virus |
| ZIKV | Zika virus |
| CHIKV | Chikungunya virus |
| JEV | Japanese encephalitis virus |
| WNV | West Nile virus |
| YFV | Yellow fever virus |
| TBEV | Tick-borne Encephalitis virus |
| GBS | Guillain-Barré Syndrome |
| RDT | Rapid diagnostic test |
| PRNT | Plaque reduction neutralization test |
| ELISA | Enzyme-linked immunosorbent assay |
| BSD | BluSense Diagnostics |
| IMA | Immuno-Magnetic Assay |
| MNPs | Magnetic nanoparticles |
| BARDA | Biomedical Advanced Research and Development Authority |
| pZIKV | Primary Zika infection |
| pDENV | Primary Dengue infection |
| DENVpZIKV | Primary Zika with documented DENV infection |
| sDENV | Secondary Dengue infection |
| MPO | Months post-onset |
| iELISA | Inhibition ELISA |
| BOB-ELISA | Blockade-of-Binding ELISA |
| MAC-ELISA | IgM antibody capture enzyme-linked immunosorbent assay |
| ROC | Receiver operating characteristic |
| AUC | Area under curve |
References
- Faria, N.R.; Quick, J.; Claro, I.M.; Theze, J.; de Jesus, J.G.; Giovanetti, M.; Kraemer, M.U.G.; Hill, S.C.; Black, A.; da Costa, A.C.; et al. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature 2017, 546, 406–410. [Google Scholar] [CrossRef]
- Boorman, J.P.; Porterfield, J.S. A simple technique for infection of mosquitoes with viruses; transmission of Zika virus. Trans. R. Soc. Trop. Med. Hyg. 1956, 50, 238–242. [Google Scholar] [CrossRef]
- Hayes, E.B. Zika virus outside Africa. Emerg. Infect. Dis. 2009, 15, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Filipe, A.R.; Martins, C.M.; Rocha, H. Laboratory infection with Zika virus after vaccination against yellow fever. Arch. Gesamte Virusforsch. 1973, 43, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, B.; Hearn, P.; Afrough, B.; Lumley, S.; Carter, D.; Aarons, E.J.; Simpson, A.J.; Brooks, T.J.; Hewson, R. Detection of Zika virus in Semen. Emerg. Infect. Dis. 2016, 22, 940. [Google Scholar] [CrossRef] [PubMed]
- Foy, B.D.; Kobylinski, K.C.; Chilson Foy, J.L.; Blitvich, B.J.; Travassos da Rosa, A.; Haddow, A.D.; Lanciotti, R.S.; Tesh, R.B. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis. 2011, 17, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Besnard, M.; Lastere, S.; Teissier, A.; Cao-Lormeau, V.; Musso, D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Eurosurveillance 2014, 19. [Google Scholar] [CrossRef]
- Schuler-Faccini, L.; Ribeiro, E.M.; Feitosa, I.M.; Horovitz, D.D.; Cavalcanti, D.P.; Pessoa, A.; Doriqui, M.J.; Neri, J.I.; Neto, J.M.; Wanderley, H.Y.; et al. Possible association between Zika virus infection and microcephaly — Brazil, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 59–62. [Google Scholar] [CrossRef]
- Mlakar, J.; Korva, M.; Tul, N.; Popovic, M.; Poljsak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodusek, V.; et al. Zika virus associated with microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef]
- Musso, D.; Nhan, T.; Robin, E.; Roche, C.; Bierlaire, D.; Zisou, K.; Shan Yan, A.; Cao-Lormeau, V.M.; Broult, J. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Eurosurveillance 2014, 19. [Google Scholar] [CrossRef]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Saúde, M.d.S. Protocolo de Vigilância e Resposta à Ocorrência de Microcefalia Relacionada à Infecção Pelo vírus Zika; Ministério da Saúde Brasília: Brasília, Brazil, 2015. [Google Scholar]
- Krow-Lucal, E.R.; Biggerstaff, B.J.; Staples, J.E. Estimated incubation period for Zika virus disease. Emerg. Infect. Dis. 2017, 23, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.I. Zika virus infection in man. Trans. R. Soc. Trop. Med. Hyg. 1964, 58, 335–338. [Google Scholar] [CrossRef]
- Victora, C.G.; Schuler-Faccini, L.; Matijasevich, A.; Ribeiro, E.; Pessoa, A.; Barros, F.C. Microcephaly in Brazil: How to interpret reported numbers? Lancet 2016, 387, 621–624. [Google Scholar] [CrossRef]
- Liuzzi, G.; Puro, V.; Vairo, F.; Nicastri, E.; Capobianchi, M.R.; Di Caro, A.; Piacentini, M.; Zumla, A.; Ippolito, G. Zika virus and microcephaly: Is the correlation, causal or coincidental? New Microbiol. 2016, 39, 83–85. [Google Scholar]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastere, S.; Valour, F.; Baudouin, L.; Mallet, H.; Musso, D.; Ghawche, F. Zika virus infection complicated by Guillain-Barre syndrome — Case report, French Polynesia, December 2013. Eurosurveillance 2014. [Google Scholar] [CrossRef]
- Kuno, G.; Chang, G.J.; Tsuchiya, K.R.; Karabatsos, N.; Cropp, C.B. Phylogeny of the genus Flavivirus. J. Virol. 1998, 72, 73–83. [Google Scholar] [CrossRef]
- Kuno, G.; Chang, G.J. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch. Virol. 2007, 152, 687–696. [Google Scholar] [CrossRef]
- Goo, L.; DeMaso, C.R.; Pelc, R.S.; Ledgerwood, J.E.; Graham, B.S.; Kuhn, R.J.; Pierson, T.C. The Zika virus envelope protein glycan loop regulates virion antigenicity. Virology 2018, 515, 191–202. [Google Scholar] [CrossRef]
- Westaway, E.G.; Goodman, M.R. Variation in distribution of the three flavivirus-specified glycoproteins detected by immunofluorescence in infected Vero cells. Arch. Virol. 1987, 94, 215–228. [Google Scholar] [CrossRef]
- Westaway, E.G.; Mackenzie, J.M.; Kenney, M.T.; Jones, M.K.; Khromykh, A.A. Ultrastructure of Kunjin virus-infected cells: Colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J. Virol. 1997, 71, 6650–6661. [Google Scholar] [CrossRef] [PubMed]
- Rezza, G.; Ippolito, G. Emerging and Re-emerging Viral Infections; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Muller, D.A.; Young, P.R. The flavivirus NS1 protein: Molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antivir. Res. 2013, 98, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.W.; Paploski, I.A.; Kikuti, M.; Rodrigues, M.S.; Silva, M.M.; Campos, G.S.; Sardi, S.I.; Kitron, U.; Reis, M.G.; Ribeiro, G.S. Outbreak of exanthematous illness associated with Zika, Chikungunya, and Dengue viruses, Salvador, Brazil. Emerg. Infect. Dis. 2015, 21, 2274–2276. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef]
- Freire, M.; Pol-Fachin, L.; Coelho, D.F.; Viana, I.F.T.; Magalhaes, T.; Cordeiro, M.T.; Fischer, N.; Loeffler, F.F.; Jaenisch, T.; Franca, R.F.; et al. Mapping putative B-cell Zika virus NS1 epitopes provides molecular basis for anti-NS1 antibody discrimination between Zika and Dengue viruses. ACS Omega 2017, 2, 3913–3920. [Google Scholar] [CrossRef]
- George, J.; Valiant, W.G.; Mattapallil, M.J.; Walker, M.; Huang, Y.S.; Vanlandingham, D.L.; Misamore, J.; Greenhouse, J.; Weiss, D.E.; Verthelyi, D.; et al. Prior exposure to Zika virus significantly enhances peak Dengue-2 viremia in Rhesus Macaques. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Fowler, A.M.; Tang, W.W.; Young, M.P.; Mamidi, A.; Viramontes, K.M.; McCauley, M.D.; Carlin, A.F.; Schooley, R.T.; Swanstrom, J.; Baric, R.S.; et al. Maternally acquired Zika antibodies enhance Dengue disease severity in mice. Cell Host Microbe 2018, 24, 743–750 e5. [Google Scholar] [CrossRef]
- Valiant, W.G.; Huang, Y.S.; Vanlandingham, D.L.; Higgs, S.; Lewis, M.G.; Mattapallil, J.J. Zika convalescent macaques display delayed induction of anamnestic cross-neutralizing antibody responses after dengue infection. Emerg. Microbes. Infect. 2018, 7, 130. [Google Scholar] [CrossRef]
- Valiant, W.G.; Lalani, T.; Yun, H.C.; Kunz, A.; Burgess, T.H.; Mattapallil, J.J. Human serum with high neutralizing antibody titers against both Zika and Dengue virus shows delayed in vitro antibody-dependent enhancement of Dengue virus infection. Open Forum Infect. Dis. 2018, 5. [Google Scholar] [CrossRef]
- Valiant, W.G.; Mattapallil, M.J.; Higgs, S.; Huang, Y.S.; Vanlandingham, D.L.; Lewis, M.G.; Mattapallil, J.J. Simultaneous coinfection of macaques with Zika and Dengue viruses does not enhance acute plasma viremia but leads to activation of monocyte subsets and biphasic release of pro-inflammatory cytokines. Sci. Rep. 2019, 9, 7877. [Google Scholar] [CrossRef]
- Antunes, P.; Watterson, D.; Parmvi, M.; Burger, R.; Boisen, A.; Young, P.; Cooper, M.A.; Hansen, M.F.; Ranzoni, A.; Donolato, M. Quantification of NS1 dengue biomarker in serum via optomagnetic nanocluster detection. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef]
- Balmaseda, A.; Stettler, K.; Medialdea-Carrera, R.; Collado, D.; Jin, X.; Zambrana, J.V.; Jaconi, S.; Cameroni, E.; Saborio, S.; Rovida, F.; et al. Antibody-based assay discriminates Zika virus infection from other flaviviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 8384–8389. [Google Scholar] [CrossRef] [PubMed]
- CDC Zika Mac-ELISA Instructions for Use. Available online: https://www.cdc.gov/zika/pdfs/zika-mac-elisa-instructions-for-use.pdf (accessed on 2 February 2018).
- Tsai, W.Y.; Youn, H.H.; Brites, C.; Tsai, J.J.; Tyson, J.; Pedroso, C.; Drexler, J.F.; Stone, M.; Simmons, G.; Busch, M.P.; et al. Distinguishing secondary Dengue virus infection from Zika virus infection with previous dengue by a combination of 3 simple serological tests. Clin. Infect. Dis. 2017, 65, 1829–1836. [Google Scholar] [CrossRef]
- Priyamvada, L.; Quicke, K.M.; Hudson, W.H.; Onlamoon, N.; Sewatanon, J.; Edupuganti, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Mulligan, M.J.; Wilson, P.C.; et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. USA 2016, 113, 7852–7857. [Google Scholar] [CrossRef] [PubMed]
- Swanstrom, J.A.; Plante, J.A.; Plante, K.S.; Young, E.F.; McGowan, E.; Gallichotte, E.N.; Widman, D.G.; Heise, M.T.; de Silva, A.M.; Baric, R.S. Dengue virus envelope dimer epitope monoclonal antibodies isolated from dengue patients are protective against Zika virus. mBio 2016, 7. [Google Scholar] [CrossRef]
- Andrade, P.; Narvekar, P.; Montoya, M.; Michlmayr, D.; Balmaseda, A.; Coloma, J.; Harris, E. Primary and secondary dengue virus infections elicit similar memory B cell responses but breadth to other serotypes and cross-reactivity to Zika virus is higher in secondary dengue. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
| Samples n = 137 | IgG a | IgM b |
|---|---|---|
| Sensitivity | 92% | 93% |
| Specificity | 92% | 93% |
| Cutoff | 11.5 | 12.14 |
| AUC | 0.98 | 0.95 |
| Agreement to ELISA (%) | 98 | 93 |
| ViroTrack Sero Zika IgG/IgM | Sensitivity | Specificity | AUC |
|---|---|---|---|
| Zika IgG | 86% | 62% | 0.69 |
| Zika IgM | 96% | 96% | 0.96 |
| ViroTrack Sero Zika IgG/IgM | pZIKV | DENVpZIKV | pDENV | sDENV |
|---|---|---|---|---|
| Zika IgG | 19/25 (76%) | 24/25 (96%) | 1/25 (4%) | 18/25 (72%) |
| Zika IgM | 25/25 (100%) | 23/25 (92%) | 0/25 (0%) | 2/25 (8%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, T.; Wang, X.; Donolato, M.; Harris, E.; Cruz, M.M.; Balmaseda, A.; Wang, R.Y.L. Evaluation of ViroTrack Sero Zika IgG/IgM, a New Rapid and Quantitative Zika Serological Diagnostic Assay. Diagnostics 2020, 10, 372. https://doi.org/10.3390/diagnostics10060372
Liao T, Wang X, Donolato M, Harris E, Cruz MM, Balmaseda A, Wang RYL. Evaluation of ViroTrack Sero Zika IgG/IgM, a New Rapid and Quantitative Zika Serological Diagnostic Assay. Diagnostics. 2020; 10(6):372. https://doi.org/10.3390/diagnostics10060372
Chicago/Turabian StyleLiao, Tony, Xiaole Wang, Marco Donolato, Eva Harris, Magelda Montoya Cruz, Angel Balmaseda, and Robert Y.L. Wang. 2020. "Evaluation of ViroTrack Sero Zika IgG/IgM, a New Rapid and Quantitative Zika Serological Diagnostic Assay" Diagnostics 10, no. 6: 372. https://doi.org/10.3390/diagnostics10060372
APA StyleLiao, T., Wang, X., Donolato, M., Harris, E., Cruz, M. M., Balmaseda, A., & Wang, R. Y. L. (2020). Evaluation of ViroTrack Sero Zika IgG/IgM, a New Rapid and Quantitative Zika Serological Diagnostic Assay. Diagnostics, 10(6), 372. https://doi.org/10.3390/diagnostics10060372





