Comparison of Clinical Outcomes between Idiopathic Frozen Shoulder and Diabetic Frozen Shoulder After a Single Ultrasound-Guided Intra-Articular Corticosteroid Injection
Abstract
1. Introduction
2. Materials and Methods
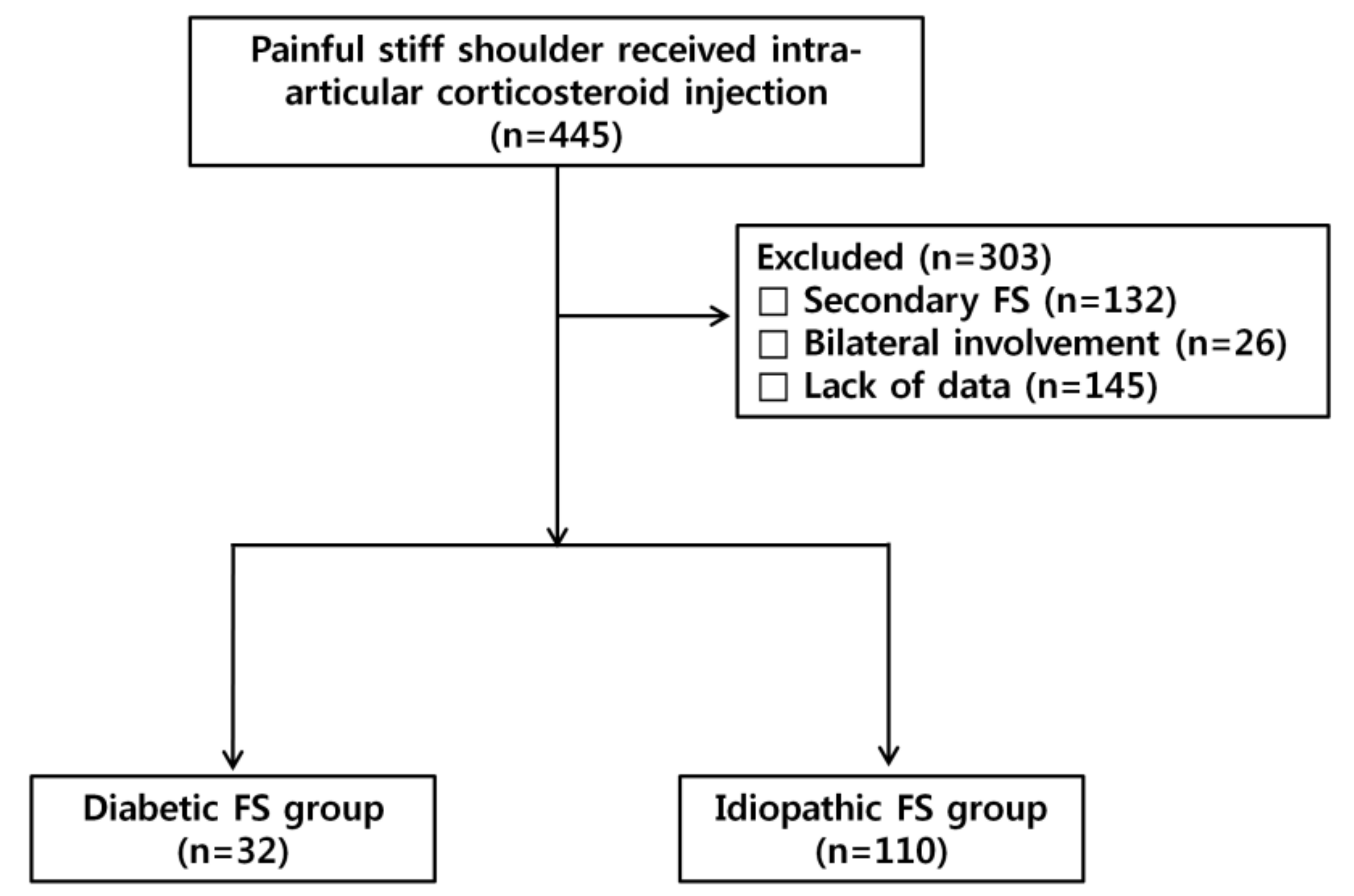
2.1. Patients
2.2. Treatment Protocol
2.3. Outcome Assessment
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
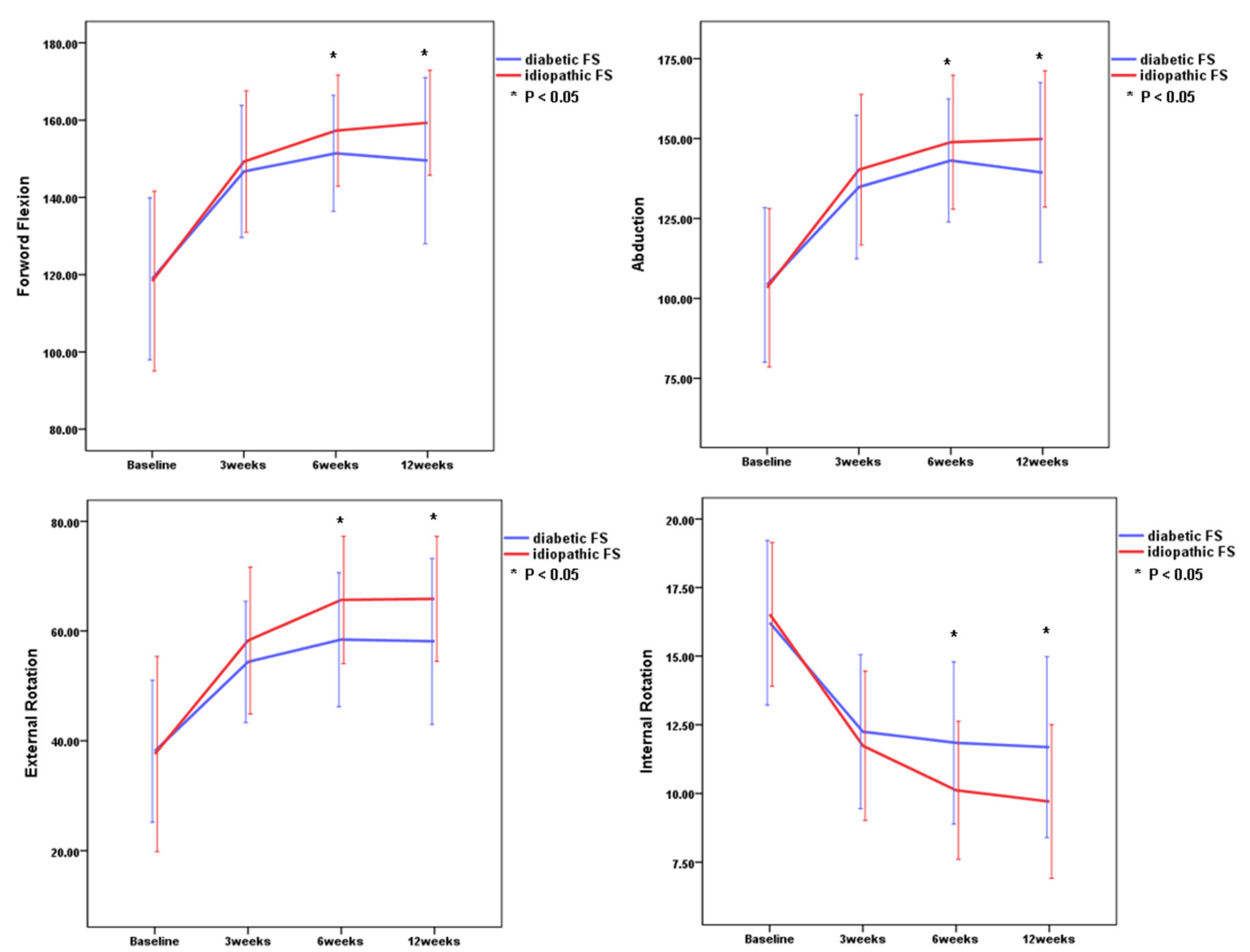
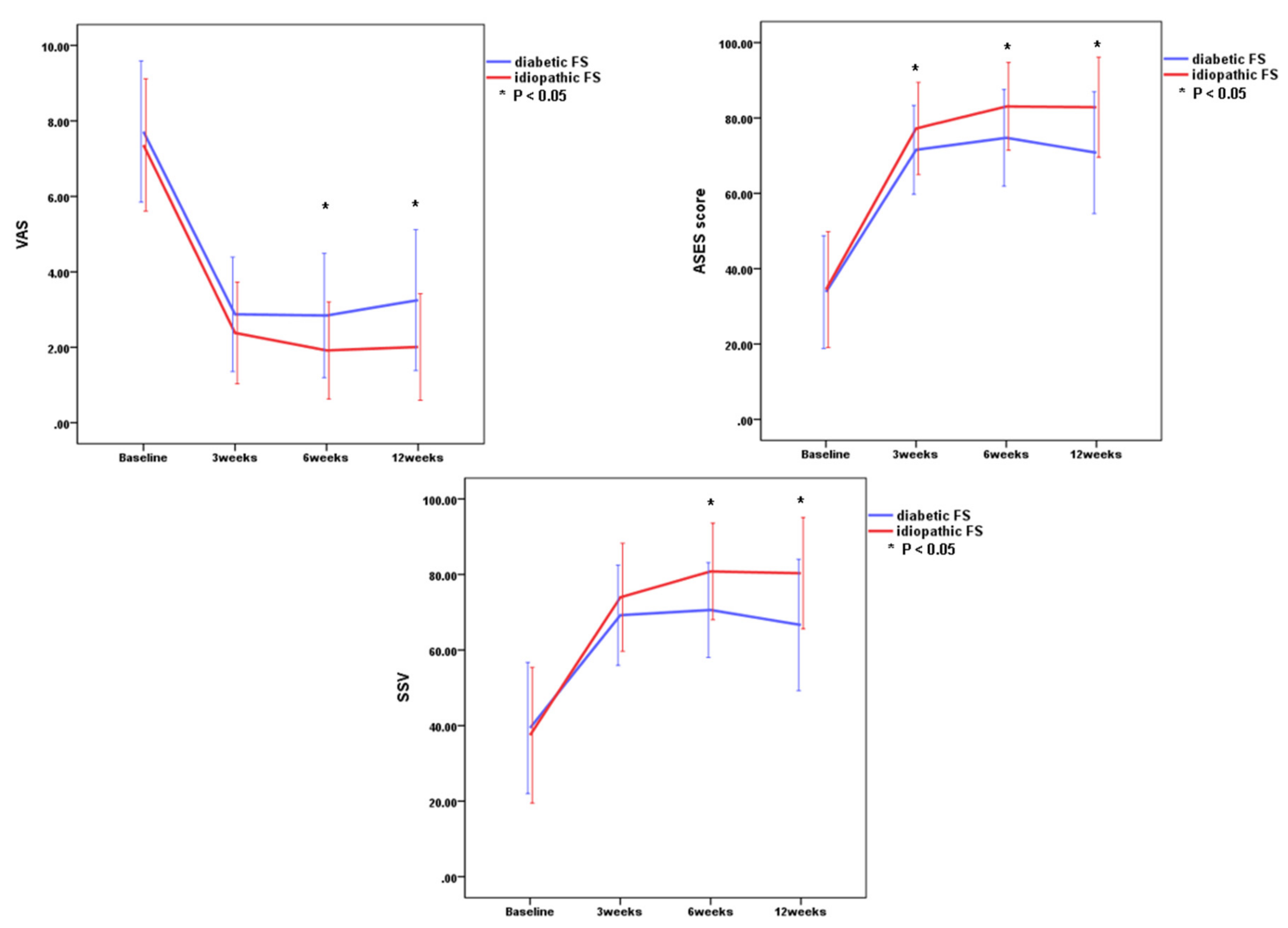
3.2. Changes in Outcome Measurements for Patients with Diabetic or Idiopathic FS
3.3. Comparison of Clinical Outcomes Between Idiopathic and Diabetic FS at Each Point
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hannafin, J.A.; Chiaia, T.A. Adhesive capsulitis. A treatment approach. Clin. Orthop. Relat. Res. 2000, 95–109. [Google Scholar] [CrossRef]
- Neviaser, A.S.; Hannafin, J.A. Adhesive capsulitis: A review of current treatment. Am. J. Sports Med. 2010, 38, 2346–2356. [Google Scholar] [CrossRef] [PubMed]
- Itoi, E.; Arce, G.; Bain, G.I.; Diercks, R.L.; Guttmann, D.; Imhoff, A.B.; Mazzocca, A.D.; Sugaya, H.; Yoo, Y.S. Shoulder stiffness: Current concepts and concerns. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. North Am. Int. Arthrosc. Assoc. 2016, 32, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Brue, S.; Valentin, A.; Forssblad, M.; Werner, S.; Mikkelsen, C.; Cerulli, G. Idiopathic adhesive capsulitis of the shoulder: A review. Knee Surg. Sports Traumatol. Arthrosc. Off. J. Esska 2007, 15, 1048–1054. [Google Scholar] [CrossRef]
- Eljabu, W.; Klinger, H.M.; von Knoch, M. Prognostic factors and therapeutic options for treatment of frozen shoulder: A systematic review. Arch. Orthop. Trauma Surg. 2016, 136, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.E.; Anakwenze, O.A.; Warrender, W.J.; Abboud, J.A. Current review of adhesive capsulitis. J. Shoulder Elb. Surg. 2011, 20, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, M. Adhesive capsulitis: An age related symptom of metabolic syndrome and chronic low-grade inflammation? Med. Hypotheses 2016, 88, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Whelton, C.; Peach, C.A. Review of diabetic frozen shoulder. Eur. J. Orthop. Surg. Traumatol. Orthop. Traumatol. 2018, 28, 363–371. [Google Scholar] [CrossRef]
- Ando, A.; Sugaya, H.; Hagiwara, Y.; Takahashi, N.; Watanabe, T.; Kanazawa, K.; Itoi, E. Identification of prognostic factors for the nonoperative treatment of stiff shoulder. Int. Orthop. 2013, 37, 859–864. [Google Scholar] [CrossRef]
- Cho, C.H.; Kim, D.H.; Lee, Y.K. Serial comparison of clinical outcomes after arthroscopic capsular release for refractory frozen shoulder with and without diabetes. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. North Am. Int. Arthrosc. Assoc. 2016, 32, 1515–1520. [Google Scholar] [CrossRef]
- Dehghan, A.; Pishgooei, N.; Salami, M.A.; Zarch, S.M.; Nafisi-Moghadam, R.; Rahimpour, S.; Soleimani, H.; Owlia, M.B. Comparison between NSAID and intra-articular corticosteroid injection in frozen shoulder of diabetic patients; a randomized clinical trial. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2013, 121, 75–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roh, Y.H.; Yi, S.R.; Noh, J.H.; Lee, S.Y.; Oh, J.H.; Gong, H.S.; Baek, G.H. Intra-articular corticosteroid injection in diabetic patients with adhesive capsulitis: A randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. Off. J. Esska 2012, 20, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Kim, D.H.; Bae, K.C.; Lee, D.; Kim, K. Proper site of corticosteroid injection for the treatment of idiopathic frozen shoulder: Results from a randomized trial. Jt. Bone Spine Rev. Du Rhum. 2016, 83, 324–329. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, Y.S.; Kim, B.S.; Sung, D.H.; Song, K.S.; Cho, C.H. Is frozen shoulder completely resolved at 2 years after the onset of disease? J. Orthop. Sci. 2020, 25, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Alhashimi, R.A.H. Analytical observational study of frozen shoulder among patients with diabetes mellitus. Joints 2018, 6, 141–144. [Google Scholar] [CrossRef][Green Version]
- Alsubheen, S.A.; Nazari, G.; Bobos, P.; MacDermid, J.C.; Overend, T.J.; Faber, K. Effectiveness of nonsurgical interventions for managing adhesive capsulitis in patients with diabetes: A systematic review. Arch. Phys. Med. Rehabil. 2019, 100, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.; Swamy, G.; Salem, H.; Creswell, T.; Espag, M.; Tambe, A.; Clark, D. Chronic adhesive capsulitis (Frozen shoulder): Comparative outcomes of treatment in patients with diabetes and obesity. J. Clin. Orthop. Trauma 2019, 10, 265–268. [Google Scholar] [CrossRef]
- Boutefnouchet, T.; Jordan, R.; Bhabra, G.; Modi, C.; Saithna, A. Comparison of outcomes following arthroscopic capsular release for idiopathic, diabetic and secondary shoulder adhesive capsulitis: A systematic review. Orthop. Traumatol. Surg. Res. 2019, 105, 839–846. [Google Scholar] [CrossRef]
- Gundtoft, P.H.; Kristensen, A.K.; Attrup, M.; Vobbe, J.W.; Luxhøi, T.; Rix, F.G.; Hölmich, P.; Sørensen, L. Prevalence and impact of diabetes mellitus on the frozen shoulder. South. Med. J. 2018, 111, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Lyhne, J.M.; Jacobsen, J.R.; Hansen, S.J.; Jensen, C.M.; Deutch, S.R. Diabetic and non-diabetic patients report equal symptom relief after arthroscopic capsular release of frozen shoulder. J. Clin. Orthop. Trauma 2019, 10, 261–264. [Google Scholar] [CrossRef]
- Monnier, V.M.; Glomb, M.; Elgawish, A.; Sell, D.R. The mechanism of collagen cross-linking in diabetes: A puzzle nearing resolution. Diabetes 1996, 45 (Suppl. 3), S67–S72. [Google Scholar] [CrossRef]
- Mueller, M.J.; Sorensen, C.J.; McGill, J.B.; Clark, B.R.; Lang, C.E.; Chen, L.; Bohnert, K.L.; Hastings, M.K. Effect of a shoulder movement intervention on joint mobility, pain, and disability in people with diabetes: A randomized controlled trial. Phys. Ther. 2018, 98, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.K.; Kashid, M.; Chakrabarty, B.; Upreti, V.; Shaki, O. Is it necessary to screen patient with adhesive capsulitis of shoulder for diabetes mellitus? J. Fam. Med. Prim. Care 2019, 8, 2927–2932. [Google Scholar] [CrossRef]
- Shaffer, B.; Tibone, J.E.; Kerlan, R.K. Frozen shoulder. A long-term follow-up. J. Bone Jt. Surg. Am. Vol. 1992, 74, 738–746. [Google Scholar] [CrossRef]
- Cinar, M.; Akpinar, S.; Derincek, A.; Circi, E.; Uysal, M. Comparison of arthroscopic capsular release in diabetic and idiopathic frozen shoulder patients. Arch. Orthop. Trauma Surg. 2010, 130, 401–406. [Google Scholar] [CrossRef]
- Habib, G.S.; Abu-Ahmad, R. Lack of effect of corticosteroid injection at the shoulder joint on blood glucose levels in diabetic patients. Clin. Rheumatol. 2007, 26, 566–568. [Google Scholar] [CrossRef]
- Jenkins, E.F.; Thomas, W.J.; Corcoran, J.P.; Kirubanandan, R.; Beynon, C.R.; Sayers, A.E.; Woods, D.A. The outcome of manipulation under general anesthesia for the management of frozen shoulder in patients with diabetes mellitus. J. Shoulder Elb. Surg. 2012, 21, 1492–1498. [Google Scholar] [CrossRef]
- Kraeutler, M.J.; Cohen, S.B.; Ciccotti, M.G.; Dodson, C.C. Accuracy of intra-articular injections of the glenohumeral joint through an anterior approach: Arthroscopic correlation. J. Shoulder Elb. Surg. 2012, 21, 380–383. [Google Scholar] [CrossRef]
- Rill, B.K.; Fleckenstein, C.M.; Levy, M.S.; Nagesh, V.; Hasan, S.S. Predictors of outcome after nonoperative and operative treatment of adhesive capsulitis. Am. J. Sports Med. 2011, 39, 567–574. [Google Scholar] [CrossRef]
- Natali, A.; Toschi, E.; Baldeweg, S.; Ciociaro, D.; Favilla, S.; Saccà, L.; Ferrannini, E. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes 2006, 55, 1133–1140. [Google Scholar] [CrossRef]
- Yanlei, G.L.; Keong, M.W.; Tjoen, D.L.T. Do diabetic patients have different outcomes after arthroscopic capsular release for frozen shoulder? J. Orthop. 2019, 16, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Kabbabe, B.; Ramkumar, S.; Richardson, M. Cytogenetic analysis of the pathology of frozen shoulder. Int. J. Shoulder Surg. 2010, 4, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.; Gotoh, M.; Hamada, K.; Yanagisawa, K.; Yamazaki, H.; Nakamura, M.; Ueyama, Y.; Mochida, J.; Fukuda, H. Vascular endothelial growth factor 121 and 165 in the subacromial bursa are involved in shoulder joint contracture in type II diabetics with rotator cuff disease. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2003, 21, 1138–1144. [Google Scholar] [CrossRef]
- Rosenbloom, A.L.; Silverstein, J.H. Connective tissue and joint disease in diabetes mellitus. Endocrinol. Metab. Clin. North. Am. 1996, 25, 473–483. [Google Scholar] [CrossRef]
- Yan, Y.; Li, S.; Liu, Y.; Bazzano, L.; He, J.; Mi, J.; Chen, W. Temporal relationship between inflammation and insulin resistance and their joint effect on hyperglycemia: The Bogalusa Heart Study. Cardiovasc. Diabetol. 2019, 18, 109. [Google Scholar] [CrossRef]
| Variable | Diabetic FS Group | Idiopathic FS Group | p-Value |
|---|---|---|---|
| Number of patients | 32 | 110 | |
| Age | 56.8 ± 8.2 | 56.6 ± 8.6 | 0.497 |
| Male:female (no.) | 18:14 | 38:72 | 0.039 |
| Right:left (no.) | 13:19 | 52:58 | 0.550 |
| Duration of symptoms (months) | 7.2 ± 6.0 | 6.8 ± 6.8 | 0.364 |
| Initial clinical score | |||
| VAS | 7.7 ± 1.8 | 7.3 ± 1.7 | 0.276 |
| ASES | 33.8 ± 14.9 | 34.4 ± 15.3 | 0.698 |
| SSV | 39.4 ± 17.4 | 37.4 ± 17.9 | 0.546 |
| Initial ROM | |||
| Forward flexion | 118.9 o ± 20.9° | 118.3 o ± 23.2° | 0.933 |
| Abduction | 104.2 o ± 24.1° | 103.3 o ± 24.7° | 0.747 |
| External rotation | 37.7 o ± 12.0° | 37.8 o ± 16.8° | 0.706 |
| Internal rotation † | 16.2 ± 3.0 | 16.5 ± 2.6 | 0.419 |
| Baseline | 3 Weeks | 6 Weeks | 12 Weeks | Time Effect (p-Values) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diabetic FS Group | Idiopathic FS Group | Diabetic FS Group | Idiopathic FS Group | Diabetic FS Group | Idiopathic FS Group | Diabetic FS Group | Idiopathic FS Group | Diabetic FS Group | Idiopathic FS Group | |
| VAS | 7.7 ± 1.8 | 7.3 ± 1.7 | 2.8 ± 1.5 | 2.4 ± 1.3 | 2.8 ± 1.6 | 1.9 ± 1.3 | 3.3 ± 1.9 | 2.0 ± 1.4 | <0.001 | <0.001 |
| ASES | 33.8 ± 14.9 | 34.4 ± 14.3 | 71.6 ± 11.8 | 77.2 ± 12.2 | 74.7 ± 12.8 | 83.1 ± 11.6 | 70.8 ± 16.2 | 82.8 ± 13.3 | <0.001 | <0.001 |
| SSV | 39.4 ± 17.4 | 37.4 ± 17.9 | 69.2 ± 13.3 | 74.0 ± 12.2 | 70.6 ± 12.6 | 80.8 ± 12.8 | 66.6 ± 17.4 | 80.4 ± 14.8 | <0.001 | <0.001 |
| FF | 118.9° ± 20.9° | 112.3° ± 23.1° | 146.7° ± 17.1° | 149.3° ± 18.3° | 151.4° ± 15.0° | 157.3° ± 14.4° | 149.5° ± 21.5° | 159.3° ± 13.6° | <0.001 | <0.001 |
| ABD | 104.7° ± 24.1° | 103.3° ± 24.7° | 134.8° ± 22.5° | 140.3° ± 23.6° | 143.1° ± 19.3° | 148.9° ± 21.0° | 139.4° ± 28.2° | 149.9° ± 21.4° | <0.001 | <0.001 |
| ER | 37.7° ± 12.0° | 37.8° ± 16.8° | 54.4° ± 11.1° | 58.3° ± 13.4° | 58.4° ± 12.2° | 65.7° ± 11.6° | 58.1° ± 15.1° | 65.9° ± 11.4° | <0.001 | <0.001 |
| IR | 16.2 ± 3.0 | 16.5 ± 2.6 | 12.3 ± 2.8 | 11.7 ± 2.7 | 11.8 ± 3.0 | 10.1 ± 2.5 | 11.7 ± 3.3 | 9.7 ± 2.8 | <0.001 | <0.001 |
| VAS | ASES | SSV | FF | ABD | ER | IR | |
|---|---|---|---|---|---|---|---|
| Baseline | 0.276 | 0.698 | 0.546 | 0.933 | 0.747 | 0.706 | 0.419 |
| 3 weeks | 0.110 | 0.027 * | 0.065 | 0.322 | 0.172 | 0.128 | 0.392 |
| 6 weeks | 0.003 * | 0.001 * | <0.001 * | 0.034 * | 0.096 | 0.008 * | 0.002 * |
| 12 weeks | <0.001 * | 0.001 * | <0.001 * | 0.021 * | 0.066 | 0.009 * | 0.002 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, C.-H.; Jin, H.-J.; Kim, D.H. Comparison of Clinical Outcomes between Idiopathic Frozen Shoulder and Diabetic Frozen Shoulder After a Single Ultrasound-Guided Intra-Articular Corticosteroid Injection. Diagnostics 2020, 10, 370. https://doi.org/10.3390/diagnostics10060370
Cho C-H, Jin H-J, Kim DH. Comparison of Clinical Outcomes between Idiopathic Frozen Shoulder and Diabetic Frozen Shoulder After a Single Ultrasound-Guided Intra-Articular Corticosteroid Injection. Diagnostics. 2020; 10(6):370. https://doi.org/10.3390/diagnostics10060370
Chicago/Turabian StyleCho, Chul-Hyun, Hyo-Joon Jin, and Du Hwan Kim. 2020. "Comparison of Clinical Outcomes between Idiopathic Frozen Shoulder and Diabetic Frozen Shoulder After a Single Ultrasound-Guided Intra-Articular Corticosteroid Injection" Diagnostics 10, no. 6: 370. https://doi.org/10.3390/diagnostics10060370
APA StyleCho, C.-H., Jin, H.-J., & Kim, D. H. (2020). Comparison of Clinical Outcomes between Idiopathic Frozen Shoulder and Diabetic Frozen Shoulder After a Single Ultrasound-Guided Intra-Articular Corticosteroid Injection. Diagnostics, 10(6), 370. https://doi.org/10.3390/diagnostics10060370






