Advances in the Diagnosis of Venous Thromboembolism: A Literature Review
Abstract
:1. Introduction
2. Diagnosis of the Pulmonary Embolism in a Non-Pregnant Individual
2.1. Clinical Presentation
2.2. Pulmonary Embolism Rule-Out Criteria (PERC)
- (i)
- Age of 50 years and above
- (ii)
- Pulse rate of at-least 100 beats per minute
- (iii)
- Pulse oximetry with oxygen saturation below 95%
- (iv)
- Unilateral leg swelling
- (v)
- Hemoptysis
- (vi)
- Recent surgery or trauma
- (vii)
- Prior history VTE (DVT or PE)
- (viii)
- Oral hormone use
2.3. Assessment of Pre-Test Probability
2.4. D-Dimer
2.5. Biomarker for VTE
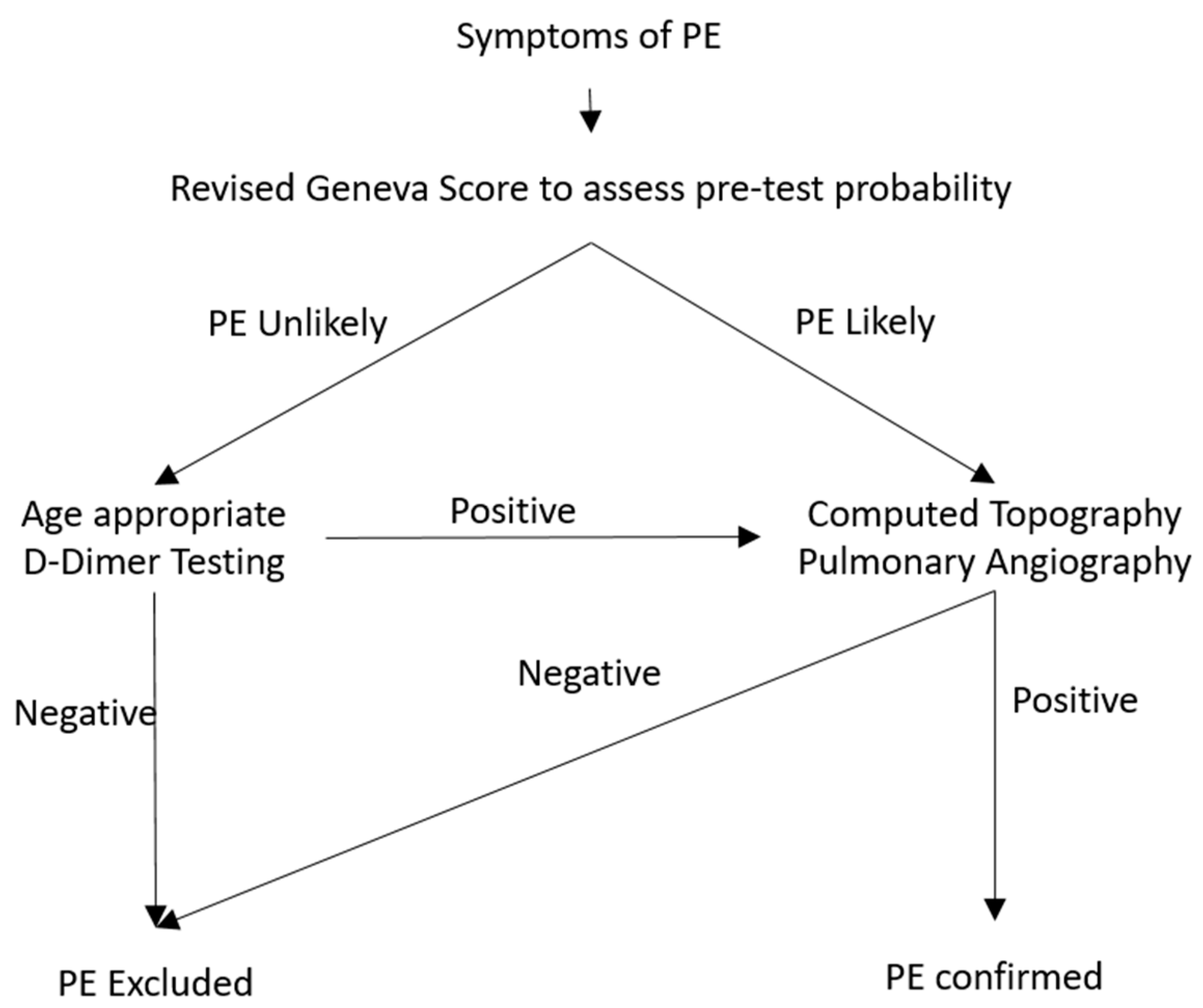
2.6. Diagnostic Algorithm for Pulmonary Embolism
2.7. Imaging Modality for Diagnosis of Pulmonary Embolism
2.8. Assessing Severity for Pulmonary Embolism and Diagnostic Approach for Critically Ill Patients with Suspected Pulmonary Embolism
3. Diagnosis of Lower Deep Vein Thrombosis in a Non-Pregnant Individual
3.1. Pre-Test Probability of First Event of Lower Extremity DVT
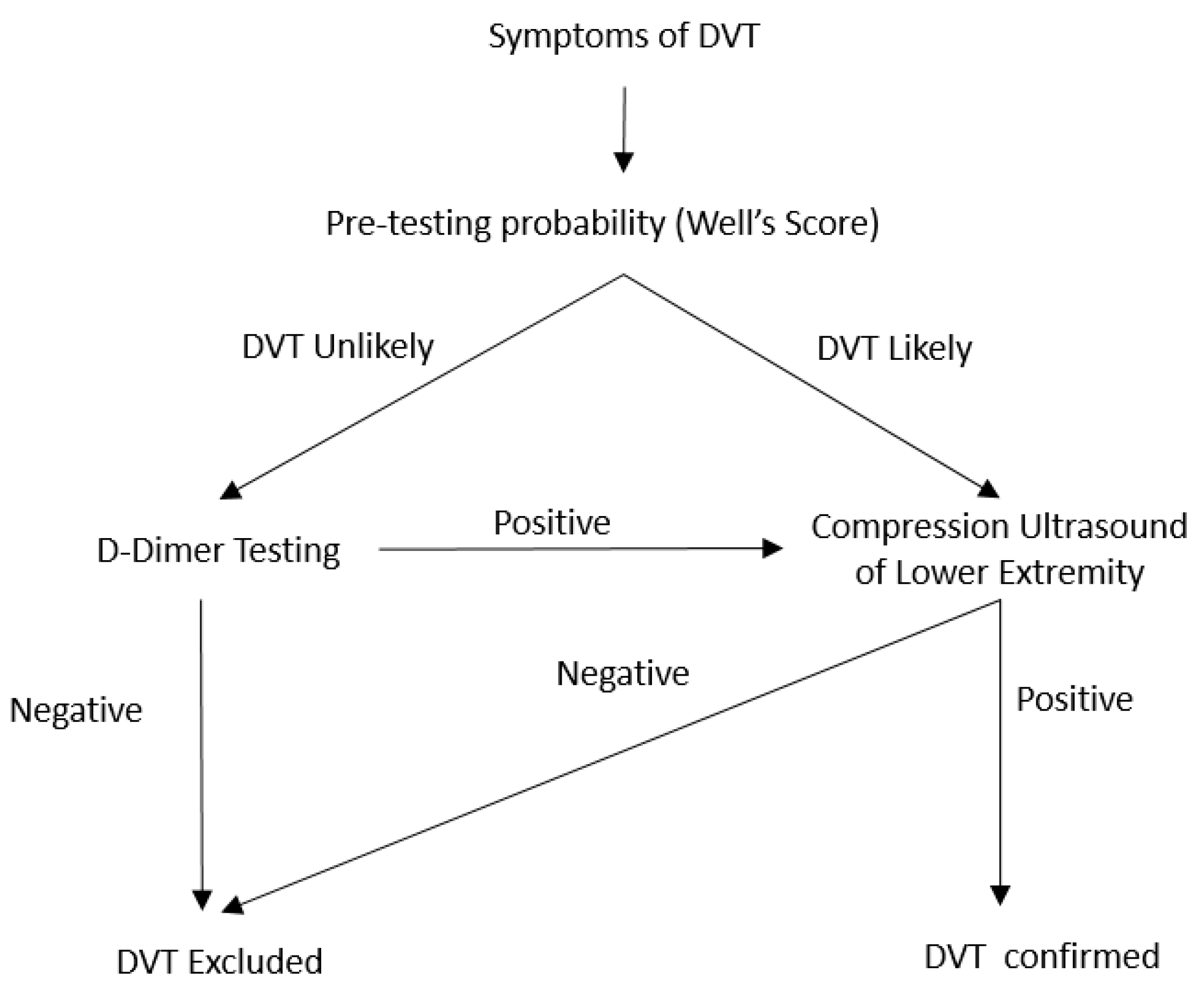
3.2. D-Dimer Level and Diagnostic Algorithm for the Diagnosis of Lower Extremity DVT
3.3. Imaging Modality for the Diagnosis of Acute DVT
4. Diagnosis of the PE in Pregnant Individuals
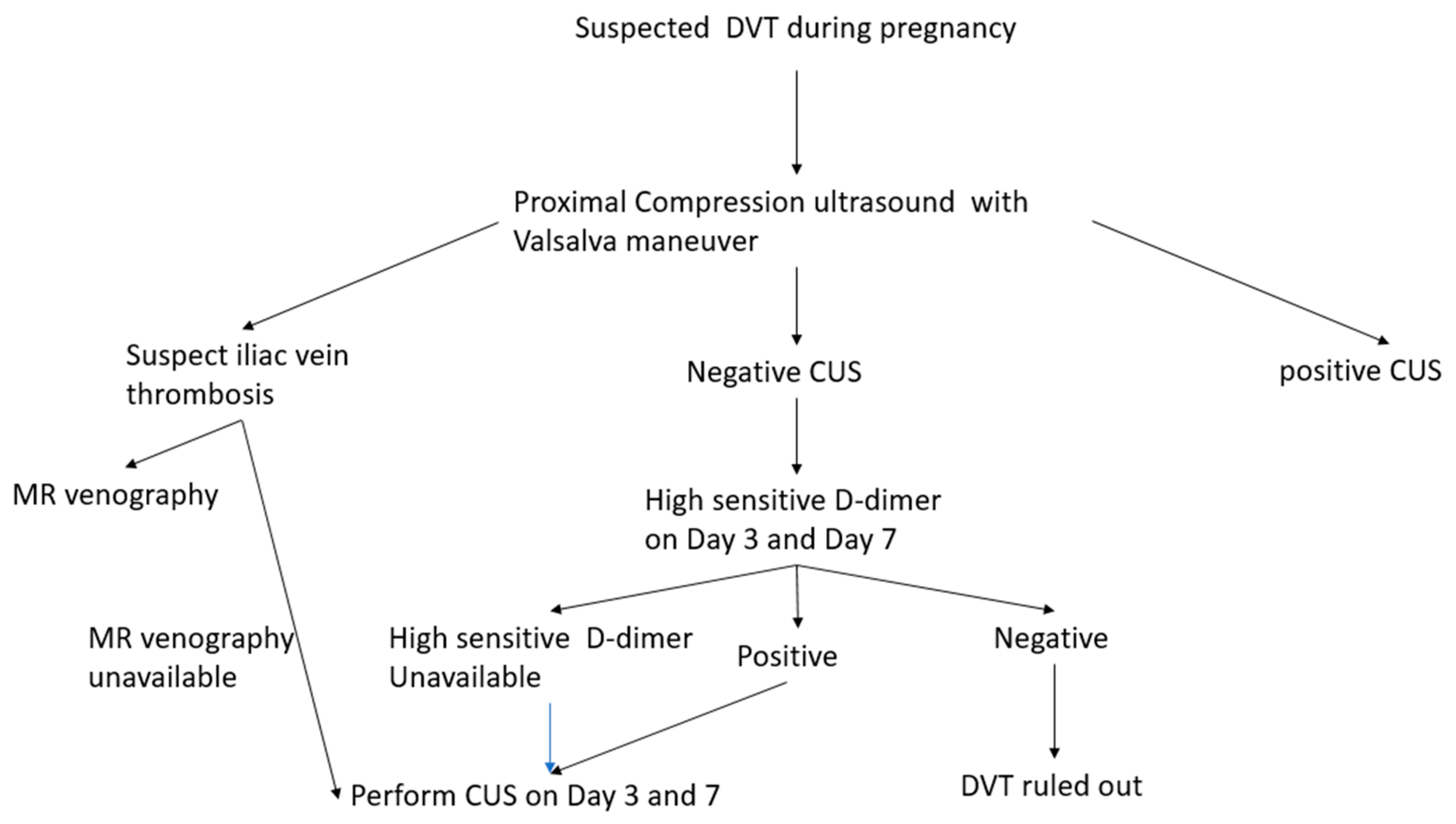
5. Diagnosis of the DVT during Pregnancy
6. Diagnosis of VTE in-Patient with Malignancy
6.1. Limitation of the Pre-Test Probability and D-Dimer in Assessing VTE in Patients with Malignancy
6.2. Evaluating the Risk of the VTE in Patient with Malignancy
7. Conclusions
Funding
Conflicts of Interest
References
- Anderson, F.A., Jr.; Wheeler, H.B.; Goldberg, R.J.; Hosmer, D.W.; Patwardhan, N.A.; Jovanovic, B.; Forcier, A.; Dalen, J.E. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch. Intern. Med. 1991, 151, 933–938. [Google Scholar] [CrossRef]
- Silverstein, M.D.; Heit, J.A.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch. Intern. Med. 1998, 158, 585–593. [Google Scholar] [CrossRef] [Green Version]
- Naess, I.A.; Christiansen, S.C.; Romundstad, P.; Cannegieter, S.C.; Rosendaal, F.R.; Hammerstrom, J. Incidence and mortality of venous thrombosis: A population-based study. J. Thromb. Haemost. 2007, 5, 692–699. [Google Scholar] [CrossRef]
- Beckman, M.G. Venous Thromboembolism. Am. J. Prev. Med. 2010, 38, S495–S501. [Google Scholar] [CrossRef]
- Heit, J.A. Epidemiology of venous thromboembolism. Nat. Rev. Cardiol. 2015, 12, 464–474. [Google Scholar] [CrossRef]
- Huang, W.; Goldberg, R.J.; Anderson, F.A.; Kiefe, C.I.; Spencer, F.A. Secular trends in occurrence of acute venous thromboembolism: The Worcester VTE study (1985–2009). Am. J. Med. 2014, 127, 829–839.e825. [Google Scholar] [CrossRef]
- Smith, S.B.; Geske, J.B.; Kathuria, P.; Cuttica, M.; Schimmel, D.R.; Courtney, D.M.; Waterer, G.W.; Wunderink, R.G. Analysis of National Trends in Admissions for Pulmonary Embolism. Chest 2016, 150, 35–45. [Google Scholar] [CrossRef]
- Stein, P.D.; Matta, F.; Alrifai, A.; Rahman, A. Trends in case fatality rate in pulmonary embolism according to stability and treatment. Thromb. Res. 2012, 130, 841–846. [Google Scholar] [CrossRef]
- Cranley, J.J.; Canos, A.J.; Sull, W.J. The diagnosis of deep venous thrombosis. Fallibility of clinical symptoms and signs. Arch. Surg. 1976, 111, 34–36. [Google Scholar] [CrossRef]
- Dronkers, C.E.A.; Ende-Verhaar, Y.M.; Kyrle, P.A.; Righini, M.; Cannegieter, S.C.; Huisman, M.V.; Klok, F.A. Disease prevalence dependent failure rate in diagnostic management studies on suspected deep vein thrombosis: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2017, 15, 2270–2273. [Google Scholar] [CrossRef] [Green Version]
- Geersing, G.J.; Zuithoff, N.P.A.; Kearon, C.; Anderson, D.R.; ten Cate-Hoek, A.J.; Elf, J.L.; Bates, S.M.; Hoes, A.W.; Kraaijenhagen, R.A.; Oudega, R.; et al. Exclusion of deep vein thrombosis using the Wells rule in clinically important subgroups: Individual patient data meta-analysis. Br. Med. J. 2014, 348, g1340. [Google Scholar] [CrossRef] [Green Version]
- Van Es, N.; van der Hulle, T.; van Es, J.; den Exter, P.L.; Douma, R.A.; Goekoop, R.J.; Mos, I.C.; Galipienzo, J.; Kamphuisen, P.W.; Huisman, M.V.; et al. Wells Rule and D-Dimer Testing to Rule Out Pulmonary Embolism: A Systematic Review and Individual-Patient Data Meta-analysis. Ann. Intern. Med. 2016, 165, 253–261. [Google Scholar] [CrossRef]
- Battinelli, E.M.; Marshall, A.; Connors, J.M. The role of thrombophilia in pregnancy. Thrombosis 2013, 2013, 516420. [Google Scholar] [CrossRef] [Green Version]
- Heit, J.A.; Kobbervig, C.E.; James, A.H.; Petterson, T.M.; Bailey, K.R.; Melton, L.J., 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: A 30-year population-based study. Ann. Intern. Med. 2005, 143, 697–706. [Google Scholar] [CrossRef]
- Heit, J.A.; O’Fallon, W.M.; Petterson, T.M.; Lohse, C.M.; Silverstein, M.D.; Mohr, D.N.; Melton, L.J., 3rd. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: A population-based study. Arch. Intern. Med. 2002, 162, 1245–1248. [Google Scholar] [CrossRef] [Green Version]
- Haddad, T.C.; Greeno, E.W. Chemotherapy-induced thrombosis. Thromb. Res. 2006, 118, 555–568. [Google Scholar] [CrossRef]
- Peterson, E.A.; Lee, A.Y.Y. Update from the clinic: What’s new in the diagnosis of cancer-associated thrombosis? Hematology Am. Soc. Hematol. Educ. Program 2019, 2019, 167–174. [Google Scholar] [CrossRef]
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis: Epidemiologic Aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Cohen, A.T.; Agnelli, G.; Anderson, F.A.; Arcelus, J.I.; Bergqvist, D.; Brecht, J.G.; Greer, I.A.; Heit, J.A.; Hutchinson, J.L.; Kakkar, A.K.; et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb. Haemost. 2007, 98, 756–764. [Google Scholar]
- Righini, M.; Robert-Ebadi, H.; Le Gal, G. Diagnosis of acute pulmonary embolism. J. Thromb. Haemost. 2017, 15, 1251–1261. [Google Scholar] [CrossRef] [Green Version]
- White, R.H. The epidemiology of venous thromboembolism. Circulation 2003, 107, I4–I8. [Google Scholar] [CrossRef] [Green Version]
- Miniati, M.; Prediletto, R.; Formichi, B.; Marini, C.; Di Ricco, G.; Tonelli, L.; Allescia, G.; Pistolesi, M. Accuracy of clinical assessment in the diagnosis of pulmonary embolism. Am. J. Respir. Crit. Care Med. 1999, 159, 864–871. [Google Scholar] [CrossRef]
- Barco, S.; Ende-Verhaar, Y.M.; Becattini, C.; Jimenez, D.; Lankeit, M.; Huisman, M.V.; Konstantinides, S.V.; Klok, F.A. Differential impact of syncope on the prognosis of patients with acute pulmonary embolism: A systematic review and meta-analysis. Eur. Heart J. 2018, 39, 4186–4195. [Google Scholar] [CrossRef] [Green Version]
- Kline, J.A.; Mitchell, A.M.; Kabrhel, C.; Richman, P.B.; Courtney, D.M. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J. Thromb. Haemost. 2004, 2, 1247–1255. [Google Scholar] [CrossRef]
- Penaloza, A.; Soulie, C.; Moumneh, T.; Delmez, Q.; Ghuysen, A.; El Kouri, D.; Brice, C.; Marjanovic, N.S.; Bouget, J.; Moustafa, F.; et al. Pulmonary embolism rule-out criteria (PERC) rule in European patients with low implicit clinical probability (PERCEPIC): A multicentre, prospective, observational study. Lancet Haematol. 2017, 4, e615–e621. [Google Scholar] [CrossRef]
- Freund, Y.; Cachanado, M.; Aubry, A.; Orsini, C.; Raynal, P.A.; Feral-Pierssens, A.L.; Charpentier, S.; Dumas, F.; Baarir, N.; Truchot, J.; et al. Effect of the Pulmonary Embolism Rule-Out Criteria on Subsequent Thromboembolic Events Among Low-Risk Emergency Department Patients: The PROPER Randomized Clinical Trial. JAMA 2018, 319, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Sanders, S.; Doust, J.; Glasziou, P. A systematic review of studies comparing diagnostic clinical prediction rules with clinical judgment. PLoS ONE 2015, 10, e0128233. [Google Scholar] [CrossRef]
- Van Es, N. Wells Rule and d-Dimer Testing to Rule Out Pulmonary Embolism: A Systematic Review and Individual-Patient Data Meta-analysis. Ann. Intern. Med. 2016, 165, 253–261. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruisman, E.; Spaan, J.; Nijkeuter, M.; Righini, M.; Aujesky, D.; Roy, P.M.; Perrier, A.; le Gal, G.; Huisman, M.V. Comparison of the revised Geneva score with the Wells rule for assessing clinical probability of pulmonary embolism. J. Thromb. Haemost. 2008, 6, 40–44. [Google Scholar] [CrossRef]
- Di Marca, S.; Cilia, C.; Campagna, A.; D’Arrigo, G.; Abd ElHafeez, S.; Tripepi, G.; Puccia, G.; Pisano, M.; Mastrosimone, G.; Terranova, V.; et al. Comparison of Wells and Revised Geneva Rule to Assess Pretest Probability of Pulmonary Embolism in High-Risk Hospitalized Elderly Adults. J. Am. Geriatr. Soc. 2015, 63, 1091–1097. [Google Scholar] [CrossRef]
- Klok, F.A.; Mos, I.C.; Nijkeuter, M.; Righini, M.; Perrier, A.; Le Gal, G.; Huisman, M.V. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch. Intern. Med. 2008, 168, 2131–2136. [Google Scholar] [CrossRef]
- Ceriani, E.; Combescure, C.; le Gal, G.; Nendaz, M.; Perneger, T.; Bounameaux, H.; Perrier, A.; Righini, M. Clinical prediction rules for pulmonary embolism: A systematic review and meta-analysis. J. Thromb. Haemost. 2010, 8, 957–970. [Google Scholar] [CrossRef]
- Pulivarthi, S.; Gurram, M.K. Effectiveness of D-dimer as a screening test for venous thromboembolism: An update. N. Am. J. Med. Sci. 2014, 6, 491–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Righini, M.; Van Es, J.; Den Exter, P.L.; Roy, P.M.; Verschuren, F.; Ghuysen, A.; Rutschmann, O.T.; Sanchez, O.; Jaffrelot, M.; Trinh-Duc, A.; et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: The ADJUST-PE study. JAMA 2014, 311, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Van der Hulle, T.; Cheung, W.Y.; Kooij, S.; Beenen, L.F.M.; van Bemmel, T.; van Es, J.; Faber, L.M.; Hazelaar, G.M.; Heringhaus, C.; Hofstee, H.; et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): A prospective, multicentre, cohort study. Lancet 2017, 390, 289–297. [Google Scholar] [CrossRef]
- Prisco, D.; Grifoni, E. The role of D-dimer testing in patients with suspected venous thromboembolism. Semin. Thromb. Hemost. 2009, 35, 50–59. [Google Scholar] [CrossRef]
- Howick, J.; Cals, J.W.; Jones, C.; Price, C.P.; Pluddemann, A.; Heneghan, C.; Berger, M.Y.; Buntinx, F.; Hickner, J.; Pace, W.; et al. Current and future use of point-of-care tests in primary care: An international survey in Australia, Belgium, The Netherlands, the UK and the USA. BMJ Open 2014, 4, e005611. [Google Scholar] [CrossRef]
- Geersing, G.J.; Janssen, K.J.; Oudega, R.; Bax, L.; Hoes, A.W.; Reitsma, J.B.; Moons, K.G. Excluding venous thromboembolism using point of care D-dimer tests in outpatients: A diagnostic meta-analysis. BMJ 2009, 339, b2990. [Google Scholar] [CrossRef] [Green Version]
- Bounameaux, H.; Perrier, A.; Righini, M. Diagnosis of venous thromboembolism: An update. Vasc. Med. 2010, 15, 399–406. [Google Scholar] [CrossRef]
- Antonopoulos, C.N.; Sfyroeras, G.S.; Kakisis, J.D.; Moulakakis, K.G.; Liapis, C.D. The role of soluble P selectin in the diagnosis of venous thromboembolism. Thromb. Res. 2014, 133, 17–24. [Google Scholar] [CrossRef]
- Andre, P. Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proc. Natl. Acad. Sci. USA 2000, 97, 13835–13840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlop, L.C.; Skinner, M.P.; Bendall, L.J.; Favaloro, E.J.; Castaldi, P.A.; Gorman, J.J.; Gamble, J.R.; Vadas, M.A.; Berndt, M.C. Characterization of GMP-140 (P-selectin) as a circulating plasma protein. J. Exp. Med. 1992, 175, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Rectenwald, J.E.; Myers, D.D., Jr.; Hawley, A.E.; Longo, C.; Henke, P.K.; Guire, K.E.; Schmaier, A.H.; Wakefield, T.W. D-dimer, P-selectin, and microparticles: Novel markers to predict deep venous thrombosis. A pilot study. Thromb. Haemost. 2005, 94, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Simanek, R.; Vormittag, R.; Dunkler, D.; Alguel, G.; Koder, S.; Kornek, G.; Marosi, C.; Wagner, O.; Zielinski, C.; et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: Results from the Vienna Cancer and Thrombosis Study (CATS). Blood 2008, 112, 2703–2708. [Google Scholar] [CrossRef] [Green Version]
- Vormittag, R.; Simanek, R.; Ay, C.; Dunkler, D.; Quehenberger, P.; Marosi, C.; Zielinski, C.; Pabinger, I. High factor VIII levels independently predict venous thromboembolism in cancer patients: The cancer and thrombosis study. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2176–2181. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, G.E.; Sims, P.J.; Wiedmer, T.; Furie, B.; Furie, B.C.; Shattil, S.J. Platelet-derived microparticles express high affinity receptors for factor VIII. J. Biol. Chem. 1991, 266, 17261–17268. [Google Scholar]
- Mesri, M.; Altieri, D.C. Endothelial cell activation by leukocyte microparticles. J. Immunol. 1998, 161, 4382–4387. [Google Scholar]
- Myers, D., Jr.; Farris, D.; Hawley, A.; Wrobleski, S.; Chapman, A.; Stoolman, L.; Knibbs, R.; Strieter, R.; Wakefield, T. Selectins influence thrombosis in a mouse model of experimental deep venous thrombosis. J. Surg. Res. 2002, 108, 212–221. [Google Scholar] [CrossRef]
- Thaler, J.; Ay, C.; Weinstabl, H.; Dunkler, D.; Simanek, R.; Vormittag, R.; Freyssinet, J.M.; Zielinski, C.; Pabinger, I. Circulating procoagulant microparticles in cancer patients. Ann. Hematol. 2011, 90, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Zwicker, J.I.; Liebman, H.A.; Neuberg, D.; Lacroix, R.; Bauer, K.A.; Furie, B.C.; Furie, B. Tumor-Derived Tissue Factor–Bearing Microparticles Are Associated With Venous Thromboembolic Events in Malignancy. Clin. Cancer Res. 2009, 15, 6830–6840. [Google Scholar] [CrossRef] [Green Version]
- Prandoni, P.; Lensing, A.W.; Cogo, A.; Cuppini, S.; Villalta, S.; Carta, M.; Cattelan, A.M.; Polistena, P.; Bernardi, E.; Prins, M.H. The long-term clinical course of acute deep venous thrombosis. Ann. Intern. Med. 1996, 125, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Souto, J.C.; Almasy, L.; Borrell, M.; Blanco-Vaca, F.; Mateo, J.; Soria, J.M.; Coll, I.; Felices, R.; Stone, W.; Fontcuberta, J.; et al. Genetic susceptibility to thrombosis and its relationship to physiological risk factors: The GAIT study. Genetic Analysis of Idiopathic Thrombophilia. Am. J. Hum. Genet. 2000, 67, 1452–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, A.; Sundquist, K.; Palmér, K.; Svensson, P.J.; Sundquist, J.; Memon, A.A. Risk prediction of recurrent venous thromboembolism: A multiple genetic risk model. J. Thromb. Thrombolysis 2019, 47, 216–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Belle, A.; Buller, H.R.; Huisman, M.V.; Huisman, P.M.; Kaasjager, K.; Kamphuisen, P.W.; Kramer, M.H.; Kruip, M.J.; Kwakkel-van Erp, J.M.; Leebeek, F.W.; et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006, 295, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Michael, J.; Zappa, M.D. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990, 263, 2753–2759. [Google Scholar] [CrossRef]
- Qanadli, S.D.; Hajjam, M.E.; Mesurolle, B.; Barre, O.; Bruckert, F.; Joseph, T.; Mignon, F.; Vieillard-Baron, A.; Dubourg, O.; Lacombe, P. Pulmonary embolism detection: Prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 2000, 217, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Leung, A.N.; Bull, T.M.; Jaeschke, R.; Lockwood, C.J.; Boiselle, P.M.; Hurwitz, L.M.; James, A.H.; McCullough, L.B.; Menda, Y.; Paidas, M.J.; et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: Evaluation of suspected pulmonary embolism in pregnancy. Am. J. Respir. Crit. Care Med. 2011, 184, 1200–1208. [Google Scholar] [CrossRef]
- Carrier, M.; Righini, M.; Wells, P.S.; Perrier, A.; Anderson, D.R.; Rodger, M.A.; Pleasance, S.; Le Gal, G. Subsegmental pulmonary embolism diagnosed by computed tomography: Incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J. Thromb. Haemost. 2010, 8, 1716–1722. [Google Scholar] [CrossRef]
- Stein, P.D.; Fowler, S.E.; Goodman, L.R.; Gottschalk, A.; Hales, C.A.; Hull, R.D.; Leeper, K.V., Jr.; Popovich, J., Jr.; Quinn, D.A.; Sos, T.A.; et al. Multidetector computed tomography for acute pulmonary embolism. N. Engl. J. Med. 2006, 354, 2317–2327. [Google Scholar] [CrossRef] [Green Version]
- Gottschalk, A.; Sostman, H.D.; Coleman, R.E.; Juni, J.E.; Thrall, J.; McKusick, K.A.; Froelich, J.W.; Alavi, A. Ventilation-perfusion scintigraphy in the PIOPED study. Part II. Evaluation of the scintigraphic criteria and interpretations. J. Nucl. Med. 1993, 34, 1119–1126. [Google Scholar]
- Waxman, A.D.; Bajc, M.; Brown, M.; Fahey, F.H.; Freeman, L.M.; Haramati, L.B.; Julien, P.; Le Gal, G.; Neilly, B.; Rabin, J.; et al. Appropriate Use Criteria for Ventilation-Perfusion Imaging in Pulmonary Embolism: Summary and Excerpts. J. Nucl. Med. 2017, 58, 13n–15n. [Google Scholar] [PubMed]
- Sostman, H.D.; Stein, P.D.; Gottschalk, A.; Matta, F.; Hull, R.; Goodman, L. Acute pulmonary embolism: Sensitivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. Radiology 2008, 246, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Harjola, V.P.; Mebazaa, A.; Čelutkienė, J.; Bettex, D.; Bueno, H.; Chioncel, O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S.; et al. Contemporary management of acute right ventricular failure: A statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Kurnicka, K.; Lichodziejewska, B.; Goliszek, S.; Dzikowska-Diduch, O.; Zdończyk, O.; Kozłowska, M.; Kostrubiec, M.; Ciurzyński, M.; Palczewski, P.; Grudzka, K.; et al. Echocardiographic Pattern of Acute Pulmonary Embolism: Analysis of 511 Consecutive Patients. J. Am. Soc. Echocardiogr. 2016, 29, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Kurzyna, M.; Torbicki, A.; Pruszczyk, P.; Burakowska, B.; Fijałkowska, A.; Kober, J.; Oniszh, K.; Kuca, P.; Tomkowski, W.; Burakowski, J.; et al. Disturbed right ventricular ejection pattern as a new Doppler echocardiographic sign of acute pulmonary embolism. Am. J. Cardiol. 2002, 90, 507–511. [Google Scholar] [CrossRef]
- Pruszczyk, P.; Goliszek, S.; Lichodziejewska, B.; Kostrubiec, M.; Ciurzyński, M.; Kurnicka, K.; Dzikowska-Diduch, O.; Palczewski, P.; Wyzgal, A. Prognostic value of echocardiography in normotensive patients with acute pulmonary embolism. JACC Cardiovasc. Imaging 2014, 7, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Becattini, C.; Agnelli, G.; Vedovati, M.C.; Pruszczyk, P.; Casazza, F.; Grifoni, S.; Salvi, A.; Bianchi, M.; Douma, R.; Konstantinides, S.; et al. Multidetector computed tomography for acute pulmonary embolism: Diagnosis and risk stratification in a single test. Eur. Heart J. 2011, 32, 1657–1663. [Google Scholar] [CrossRef]
- Becattini, C.; Vedovati, M.C.; Agnelli, G. Prognostic value of troponins in acute pulmonary embolism: A meta-analysis. Circulation 2007, 116, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Galanaud, J.P.; Kahn, S.R.; Khau Van Kien, A.; Laroche, J.P.; Quere, I. Epidemiology and management of isolated distal deep venous thrombosis. Rev. Med. Interne. 2012, 33, 678–685. [Google Scholar] [CrossRef]
- Righini, M.; Perrier, A.; De Moerloose, P.; Bounameaux, H. D-Dimer for venous thromboembolism diagnosis: 20 years later. J. Thromb. Haemost. 2008, 6, 1059–1071. [Google Scholar] [CrossRef]
- Perrier, A.; Desmarais, S.; Miron, M.J.; de Moerloose, P.; Lepage, R.; Slosman, D.; Didier, D.; Unger, P.F.; Patenaude, J.V.; Bounameaux, H. Non-invasive diagnosis of venous thromboembolism in outpatients. Lancet 1999, 353, 190–195. [Google Scholar] [CrossRef]
- Nybo, M.; Hvas, A.M. Age-adjusted D-dimer cut-off in the diagnostic strategy for deep vein thrombosis: A systematic review. Scand. J. Clin. Lab. Investig. 2017, 77, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.; Hirsh, J.; Sackett, D.L.; Taylor, D.W.; Carter, C.; Turpie, A.G.; Powers, P.; Gent, M. Clinical validity of a negative venogram in patients with clinically suspected venous thrombosis. Circulation 1981, 64, 622–625. [Google Scholar] [CrossRef] [Green Version]
- Hull, R.D.; Hirsh, J.; Carter, C.J.; Jay, R.M.; Ockelford, P.A.; Buller, H.R.; Turpie, A.G.; Powers, P.; Kinch, D.; Dodd, P.E.; et al. Diagnostic efficacy of impedance plethysmography for clinically suspected deep-vein thrombosis. A randomized trial. Ann. Intern. Med. 1985, 102, 21–28. [Google Scholar] [CrossRef]
- Lensing, A.W.; Buller, H.R.; Prandoni, P.; Batchelor, D.; Molenaar, A.H.; Cogo, A.; Vigo, M.; Huisman, P.M.; ten Cate, J.W. Contrast venography, the gold standard for the diagnosis of deep-vein thrombosis: Improvement in observer agreement. Thromb. Haemost. 1992, 67, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Berge, T.; Bergqvist, D.; Efsing, H.O.; Hallbook, T.; Lindblad, B.; Lindhagen, A. Complications of phlebography: A randomised comparison between an ionic and a non-ionic contrast medium. Clin. Radiol. 1981, 32, 595–598. [Google Scholar] [CrossRef]
- Kearon, C.; Julian, J.A.; Newman, T.E.; Ginsberg, J.S. Noninvasive diagnosis of deep venous thrombosis. McMaster Diagnostic Imaging Practice Guidelines Initiative. Ann. Intern. Med. 1998, 128, 663–677. [Google Scholar] [CrossRef]
- Goodacre, S.; Sampson, F.; Thomas, S.; van Beek, E.; Sutton, A. Systematic review and meta-analysis of the diagnostic accuracy of ultrasonography for deep vein thrombosis. BMC Med. Imaging 2005, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Pomero, F.; Dentali, F.; Borretta, V.; Bonzini, M.; Melchio, R.; Douketis, J.D.; Fenoglio, L.M. Accuracy of emergency physician-performed ultrasonography in the diagnosis of deep-vein thrombosis: A systematic review and meta-analysis. Thromb. Haemost. 2013, 109, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Pomp, E.R.; Lenselink, A.M.; Rosendaal, F.R.; Doggen, C.J. Pregnancy, the postpartum period and prothrombotic defects: Risk of venous thrombosis in the MEGA study. J. Thromb. Haemost. 2008, 6, 632–637. [Google Scholar] [CrossRef]
- Creanga, A.A.; Syverson, C.; Seed, K.; Callaghan, W.M. Pregnancy-Related Mortality in the United States, 2011-2013. Obstet. Gynecol. 2017, 130, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.A.; West, J.; Grainge, M.J.; Riley, R.D.; Tata, L.J.; Stephansson, O.; Fleming, K.M.; Nelson-Piercy, C.; Ludvigsson, J.F. Development and validation of risk prediction model for venous thromboembolism in postpartum women: Multinational cohort study. BMJ 2016, 355, i6253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, E.L.; Lawrenson, R.A.; Nightingale, A.L.; Farmer, R.D. Venous thromboembolism in pregnancy and the puerperium: Incidence and additional risk factors from a London perinatal database. BJOG 2001, 108, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Gherman, R.B.; Goodwin, T.M.; Leung, B.; Byrne, J.D.; Hethumumi, R.; Montoro, M. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet. Gynecol. 1999, 94, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Plante, L.A. Venous Thromboembolic Disease and Pregnancy. N. Engl. J. Med. 2008, 359, 2025–2033. [Google Scholar] [CrossRef]
- Van Mens, T.E.; Scheres, L.J.J.; de Jong, P.G.; Leeflang, M.M.G.; Nijkeuter, M.; Middeldorp, S. Imaging for the exclusion of pulmonary embolism in pregnancy. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- Murphy, N.; Broadhurst, D.I.; Khashan, A.S.; Gilligan, O.; Kenny, L.C.; O’Donoghue, K. Gestation-specific D-dimer reference ranges: A cross-sectional study. BJOG 2015, 122, 395–400. [Google Scholar] [CrossRef]
- Righini, M.; Robert-Ebadi, H.; Elias, A.; Sanchez, O.; le Moigne, E.; Schmidt, J.; le Gall, C.; Cornuz, J.; Aujesky, D.; Roy, P.-M.; et al. Diagnosis of Pulmonary Embolism During Pregnancy: A Multicenter Prospective Management Outcome Study. Ann. Intern. Med. 2018, 169, 766–773. [Google Scholar] [CrossRef]
- Schembri, G.P.; Miller, A.E.; Smart, R. Radiation dosimetry and safety issues in the investigation of pulmonary embolism. Semin. Nucl. Med. 2010, 40, 442–454. [Google Scholar] [CrossRef]
- Mitchell, D.P.; Rowan, M.; Loughman, E.; Ridge, C.A.; MacMahon, P.J. Contrast monitoring techniques in CT pulmonary angiography: An important and underappreciated contributor to breast dose. Eur. J. Radiol. 2017, 86, 184–189. [Google Scholar] [CrossRef]
- Leung, A.N.; Bull, T.M.; Jaeschke, R.; Lockwood, C.J.; Boiselle, P.M.; Hurwitz, L.M.; James, A.H.; McCullough, L.B.; Menda, Y.; Paidas, M.J.; et al. American Thoracic Society documents: An official American Thoracic Society/Society of Thoracic Radiology Clinical Practice Guideline—Evaluation of Suspected Pulmonary Embolism in Pregnancy. Radiology 2012, 262, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Chenevert, T.L.; Fowler, S.E.; Goodman, L.R.; Gottschalk, A.; Hales, C.A.; Hull, R.D.; Jablonski, K.A.; Leeper, K.V., Jr.; Naidich, D.P.; et al. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: A multicenter prospective study (PIOPED III). Ann. Intern. Med. 2010, 152, 434–443, W142–W143. [Google Scholar] [CrossRef]
- Turrentine, M.A.; Braems, G.; Ramirez, M.M. Use of thrombolytics for the treatment of thromboembolic disease during pregnancy. Obstet. Gynecol. Surv. 1995, 50, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Ahearn, G.S.; Hadjiliadis, D.; Govert, J.A.; Tapson, V.F. Massive pulmonary embolism during pregnancy successfully treated with recombinant tissue plasminogen activator: A case report and review of treatment options. Arch. Intern. Med. 2002, 162, 1221–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gartman, E.J. The use of thrombolytic therapy in pregnancy. Obstet Med 2013, 6, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldbach, D. Catheter Directed Thrombolysis for Massive Pulmonary Embolism in a Pregnant Patient. Chest 2018, 154, 330A. [Google Scholar] [CrossRef]
- Ray, J.G.; Chan, W.S. Deep vein thrombosis during pregnancy and the puerperium: A meta-analysis of the period of risk and the leg of presentation. Obstet. Gynecol. Surv. 1999, 54, 265–271. [Google Scholar] [CrossRef]
- Gardenghi, L.A.; Dezotti, N.R.; Dalio, M.B.; Joviliano, E.E.; Piccinato, C.E. Gestational lower limb edema and venous reflux in healthy primigravidae. Int. Angiol. 2017, 36, 569–573. [Google Scholar] [CrossRef]
- Chan, W.S.; Spencer, F.A.; Ginsberg, J.S. Anatomic distribution of deep vein thrombosis in pregnancy. CMAJ 2010, 182, 657–660. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.S.; Lee, A.; Spencer, F.A.; Crowther, M.; Rodger, M.; Ramsay, T.; Ginsberg, J.S. Predicting deep venous thrombosis in pregnancy: Out in “LEFt” field? Ann. Intern. Med. 2009, 151, 85–92. [Google Scholar] [CrossRef]
- Chan, W.S.; Ginsberg, J.S. Diagnosis of venous thromboembolism in pregnancy: A study in extrapolation or a science in evolution? Expert. Rev. Cardiovasc. Ther. 2009, 7, 1479–1482. [Google Scholar] [CrossRef] [Green Version]
- To, M.S.; Hunt, B.J.; Nelson-Piercy, C. A negative D-dimer does not exclude venous thromboembolism (VTE) in pregnancy. J. Obstet. Gynaecol. 2008, 28, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.S.; Chunilal, S.; Lee, A.; Crowther, M.; Rodger, M.; Ginsberg, J.S. A red blood cell agglutination D-dimer test to exclude deep venous thrombosis in pregnancy. Ann. Intern. Med. 2007, 147, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Scarsbrook, A.F.; Evans, A.L.; Owen, A.R.; Gleeson, F.V. Diagnosis of suspected venous thromboembolic disease in pregnancy. Clin. Radiol. 2006, 61, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, J.; Kirkinen, P. Venous circulation in the maternal lower limb: A Doppler study with the Valsalva maneuver. Ultrasound Obstet. Gynecol. 1996, 8, 93–97. [Google Scholar] [CrossRef]
- Rademaker, J.; Griesshaber, V.; Hidajat, N.; Oestmann, J.W.; Felix, R. Combined CT pulmonary angiography and venography for diagnosis of pulmonary embolism and deep vein thrombosis: Radiation dose. J. Thorac. Imaging 2001, 16, 297–299. [Google Scholar] [CrossRef]
- Torkzad, M.R.; Bremme, K.; Hellgren, M.; Eriksson, M.J.; Hagman, A.; Jörgensen, T.; Lund, K.; Sandgren, G.; Blomqvist, L.; Kälebo, P. Magnetic resonance imaging and ultrasonography in diagnosis of pelvic vein thrombosis during pregnancy. Thromb. Res. 2010, 126, 107–112. [Google Scholar] [CrossRef]
- Blann, A.D.; Lip, G.Y. Venous thromboembolism. BMJ 2006, 332, 215–219. [Google Scholar] [CrossRef]
- Blann, A.D.; Dunmore, S. Arterial and venous thrombosis in cancer patients. Cardiol. Res. Pract. 2011, 2011, 394740. [Google Scholar] [CrossRef] [Green Version]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef]
- Lucassen, W.; Geersing, G.J.; Erkens, P.M.; Reitsma, J.B.; Moons, K.G.; Buller, H.; van Weert, H.C. Clinical decision rules for excluding pulmonary embolism: A meta-analysis. Ann. Intern. Med. 2011, 155, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Douma, R.A.; van Sluis, G.L.; Kamphuisen, P.W.; Sohne, M.; Leebeek, F.W.; Bossuyt, P.M.; Buller, H.R. Clinical decision rule and D-dimer have lower clinical utility to exclude pulmonary embolism in cancer patients. Explanations and potential ameliorations. Thromb. Haemost. 2010, 104, 831–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streiff, M.B. NCCN Guidelines Insights: Cancer-Associated Venous Thromboembolic Disease, Version 2.2018. J. Natl. Compr. Canc. Netw. 2018, 16, 1289–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Key, N.S.; Bohlke, K.; Falanga, A. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update Summary. J. Oncol. Pract. 2019, 15, 661–664. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulder, F.I.; Candeloro, M.; Kamphuisen, P.W.; Di Nisio, M.; Bossuyt, P.M.; Guman, N.; Smit, K.; Büller, H.R.; van Es, N.; CAT-prediction collaborators. The Khorana score for prediction of venous thromboembolism in cancer patients: A systematic review and meta-analysis. Haematologica 2019, 104, 1277–1287. [Google Scholar] [CrossRef] [Green Version]
- Van Es, N.; Di Nisio, M.; Cesarman, G.; Kleinjan, A.; Otten, H.-M.; Mahé, I.; Wilts, I.T.; Twint, D.C.; Porreca, E.; Arrieta, O.; et al. Comparison of risk prediction scores for venous thromboembolism in cancer patients: A prospective cohort study. Haematologica 2017, 102, 1494–1501. [Google Scholar] [CrossRef] [Green Version]
- Gerotziafas, G.T.; Taher, A.; Abdel-Razeq, H.; AboElnazar, E.; Spyropoulos, A.C.; El Shemmari, S.; Larsen, A.K.; Elalamy, I.; Group, C.C.W. A Predictive Score for Thrombosis Associated with Breast, Colorectal, Lung, or Ovarian Cancer: The Prospective COMPASS-Cancer-Associated Thrombosis Study. Oncologist 2017, 22, 1222–1231. [Google Scholar] [CrossRef] [Green Version]

| Items | Rule Points |
|---|---|
| Previous PE or DVT | 1 |
| Heart Rate | |
| 75-94 BPM | 1 |
| ≥95 BPM | 2 |
| Previous Surgery or Fracture | 1 |
| Hemoptysis | 1 |
| Active Cancer | 1 |
| Unilateral Leg Pain | 1 |
| Pain on lower limb palpation and unilateral edema | 1 |
| Age >65 Years | 1 |
| Clinical Probability | |
| Three-point score | |
| Low | 0–1 |
| Intermediate | 2–4 |
| High | ≥5 |
| Two-point Score | |
| PE Unlikely | 0–2 |
| PE Likely | ≥3 |
| Clinical Variable | Points |
|---|---|
| Active Cancer | +1 |
| Paralysis, Paresis, or Plaster Immobilization of Lower Extremities | +1 |
| Bedridden for 3+ days, or major surgery in past 12 weeks involving general or regional anesthesia | +1 |
| Deep Venous System Localized Tenderness | +1 |
| Swelling of Entire Leg | +1 |
| Calf Swelling ≥3 cm larger on other leg | +1 |
| Pitting Edema only on symptomatic leg | +1 |
| Collateral Superficial (Non-varicose) Veins | +1 |
| Previously Documented DVT | +1 |
| Alternative Diagnosis at least as likely as DVT | −2 |
| Three-point Wells Score | |
| Low | <1 |
| Intermediate | 1–2 |
| High | >2 |
| Two-point Wells Score | |
| Unlikely | ≤1 |
| Likely | ≥2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, H.; Sun, H.; Hussain, A.N.; Vakde, T. Advances in the Diagnosis of Venous Thromboembolism: A Literature Review. Diagnostics 2020, 10, 365. https://doi.org/10.3390/diagnostics10060365
Patel H, Sun H, Hussain AN, Vakde T. Advances in the Diagnosis of Venous Thromboembolism: A Literature Review. Diagnostics. 2020; 10(6):365. https://doi.org/10.3390/diagnostics10060365
Chicago/Turabian StylePatel, Harish, Haozhe Sun, Ali N. Hussain, and Trupti Vakde. 2020. "Advances in the Diagnosis of Venous Thromboembolism: A Literature Review" Diagnostics 10, no. 6: 365. https://doi.org/10.3390/diagnostics10060365
APA StylePatel, H., Sun, H., Hussain, A. N., & Vakde, T. (2020). Advances in the Diagnosis of Venous Thromboembolism: A Literature Review. Diagnostics, 10(6), 365. https://doi.org/10.3390/diagnostics10060365




