A Finite Element Analysis Study from 3D CT to Predict Transcatheter Heart Valve Thrombosis
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Oversight
2.2. Patient Selection
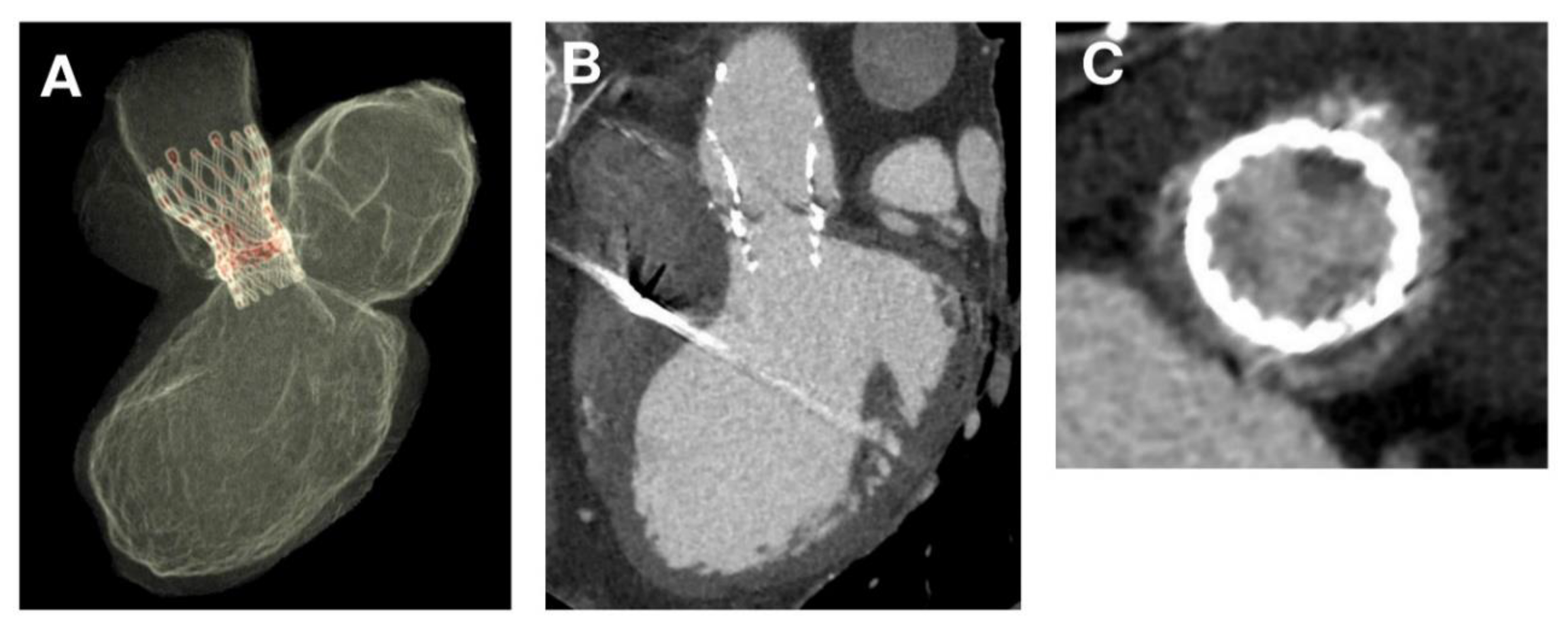
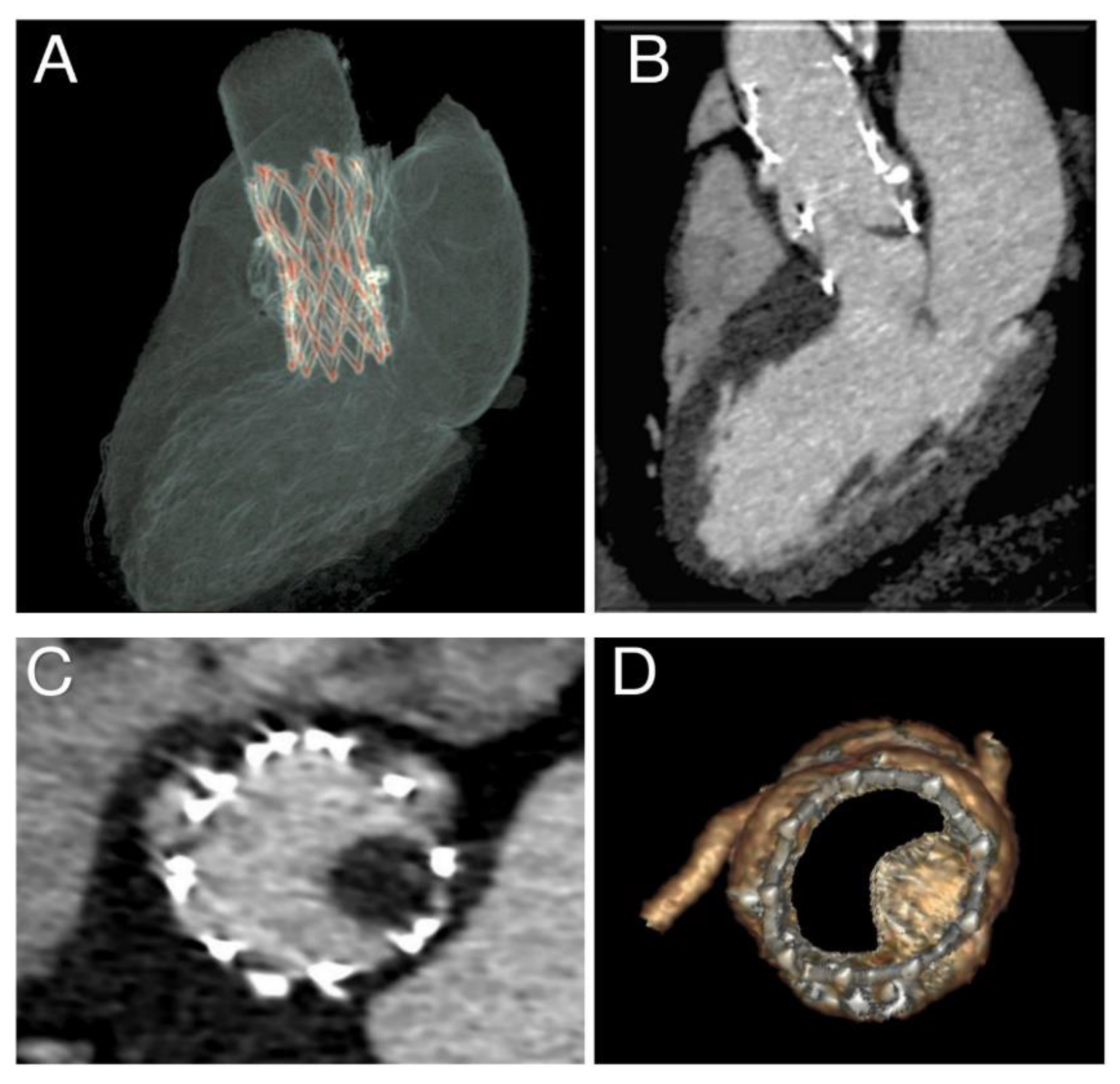
2.3. CT Imaging and Evaluation
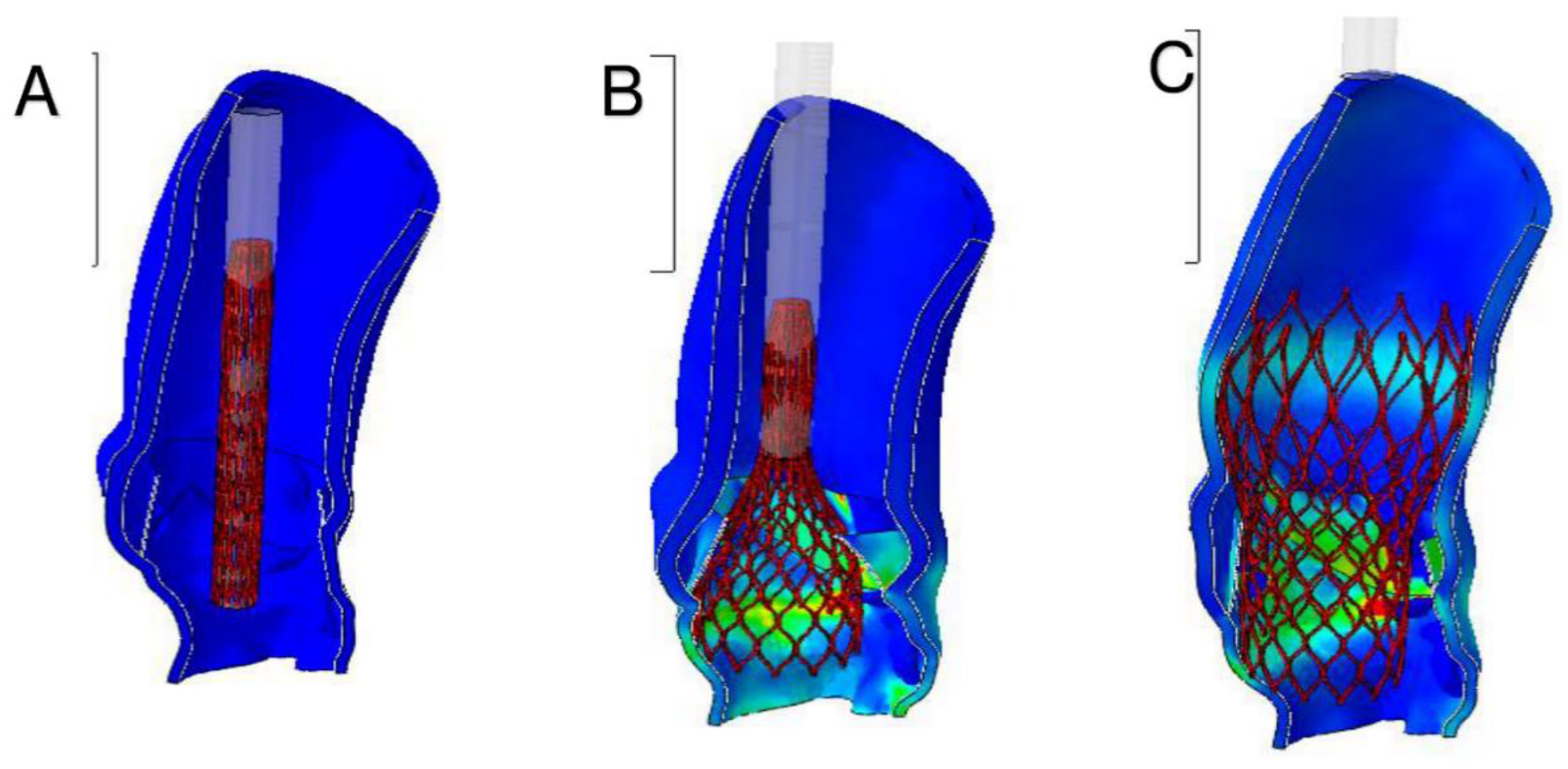
2.4. The Use of Finite Element Analysis Investigation
2.5. Computed Biomodelling of Thrombosis
3. Results
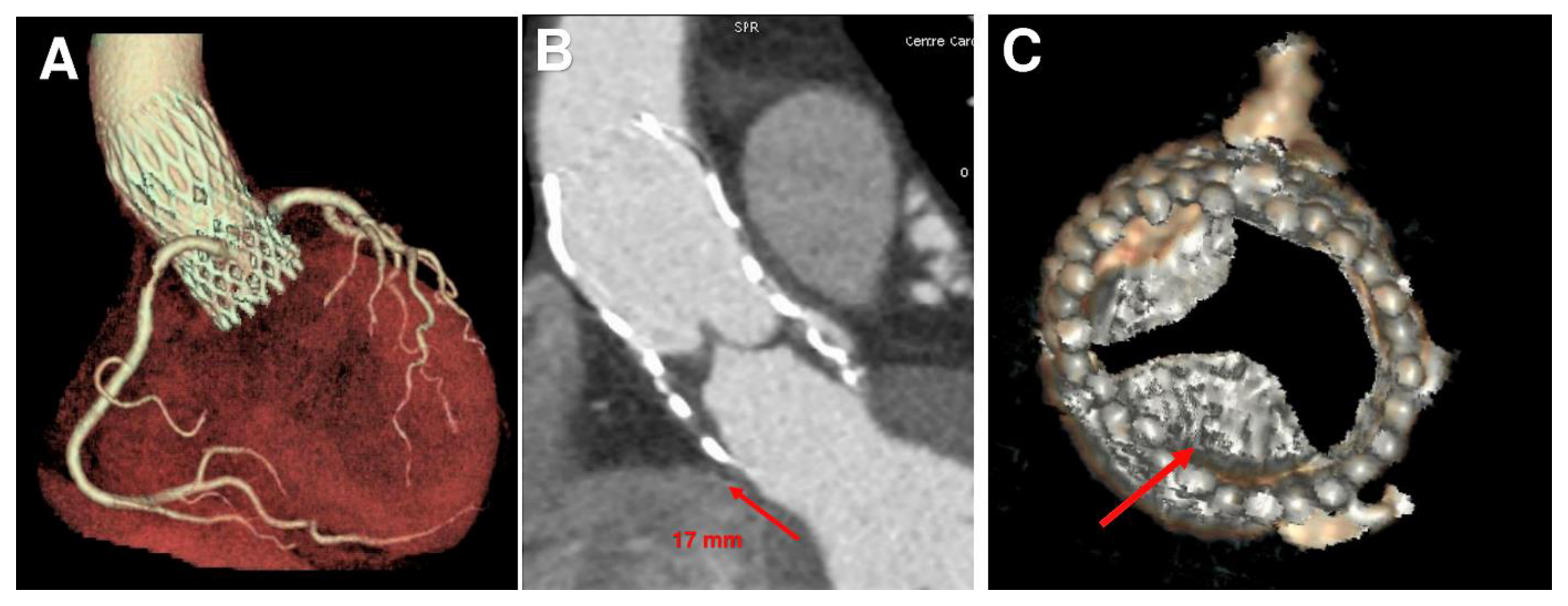
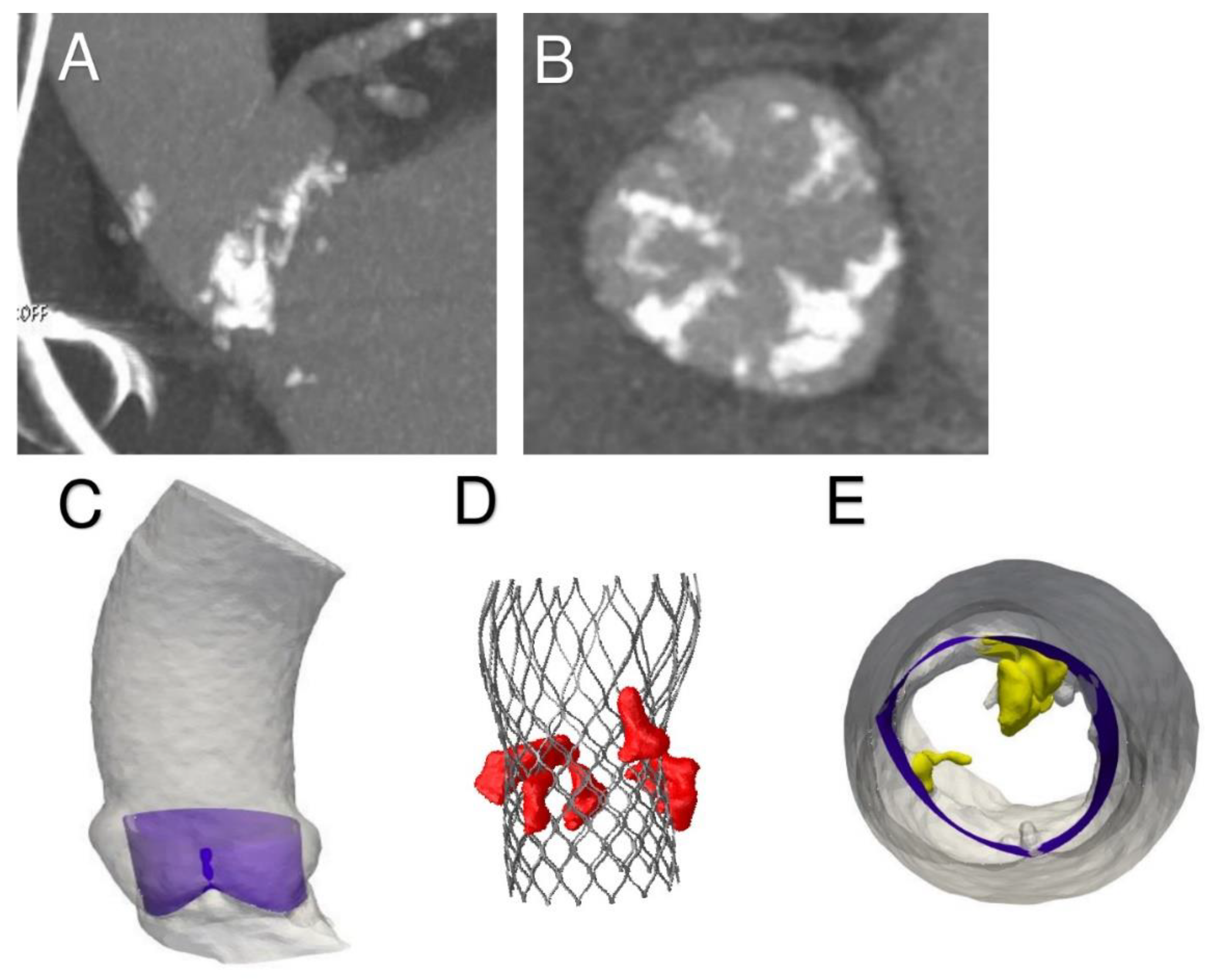
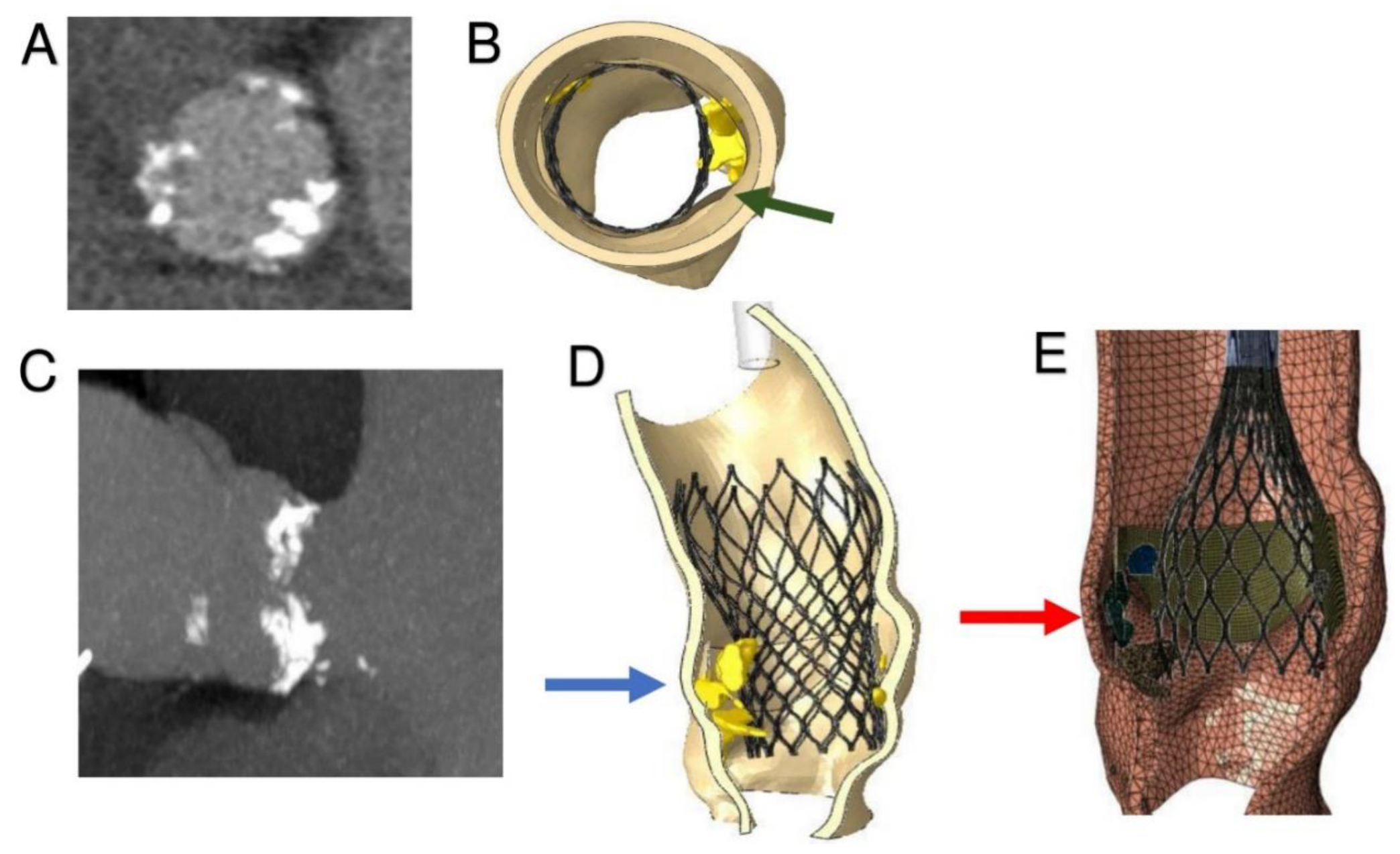
3.1. Phase 1—Processing Medical Images
3.2. Phase 2—Simulation of the Entire Clinical Procedure after Analysis of the Acquired Data
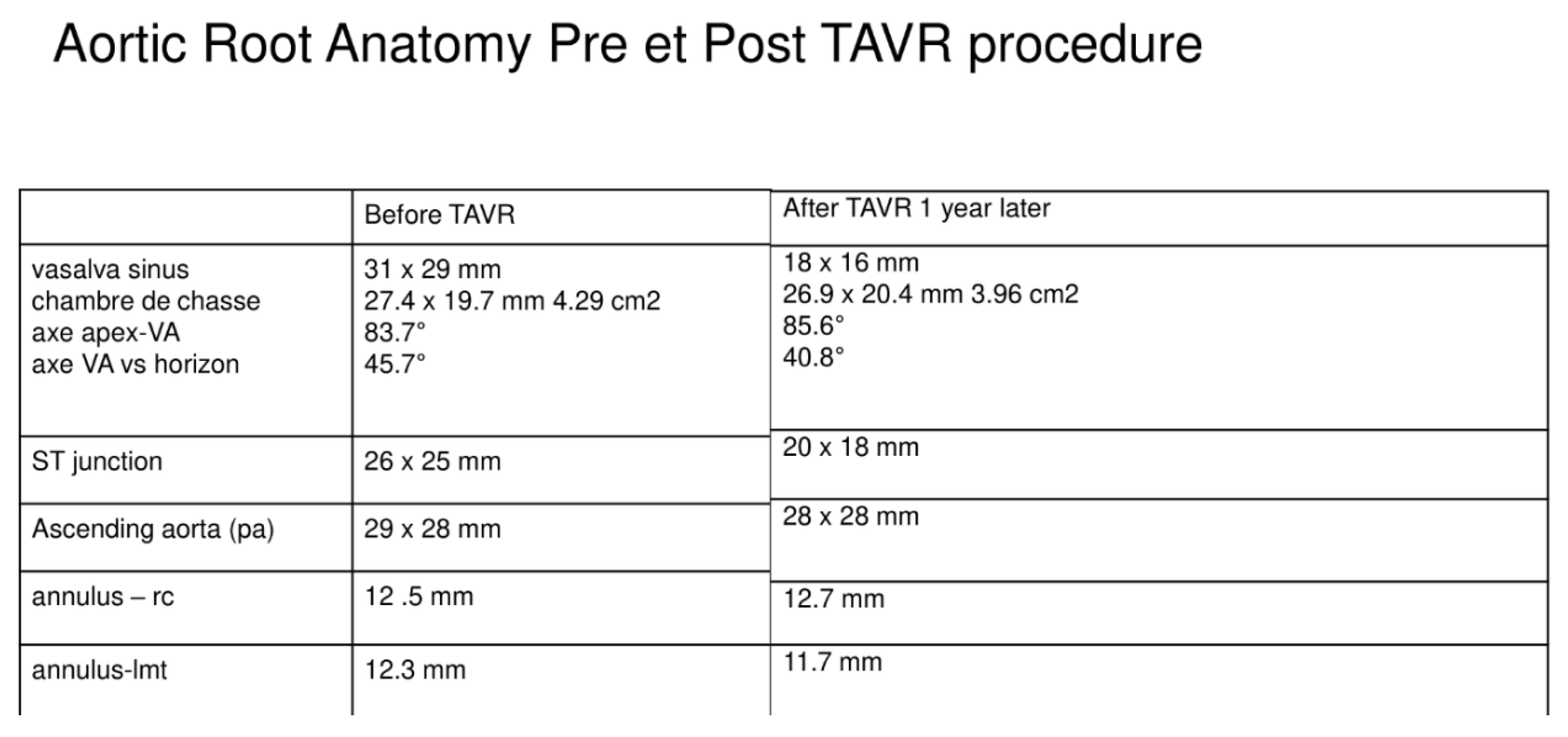
3.3. Phase 3—Post-Processing of the Simulation Results and Comparison with Follow-Up Data
4. Discussion
Dynamic of Aortic Root and Persistent Bulky Calcification: Still Relevant?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J., Jr.; Kleiman, N.S.; et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Böhm, M.; Makkar, R.; Svensson, L.G.; Kodali, S.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. New Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Sondergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. New Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. New Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. New Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Ihlemann, N.; Jorgensen, T.H.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrom, T.; Clemmensen, P.; et al. Five-year clinical and echocardiographic outcomes from the Nordic Aortic Valve Intervention (NOTION) randomized clinical trial in lower surgical risk patients. Circulation 2019, 139, 2714–2723. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef]
- Grover, F.L.; Vemulapalli, S.; Carroll, J.D.; Edwards, F.H.; Mack, M.J.; Thourani, V.H.; Brindis, R.G.; Shahian, D.M.; Ruiz, C.E.; Jacobs, J.P.; et al. 2016 Annual Report of The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J. Am. Coll. Cardiol. 2017, 69, 1215–1230. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 70, 252–289. [Google Scholar] [CrossRef]
- Falk, V. Transcatheter Aortic Valve Replacement Indications Should Not Be Expanded to Lower-Risk and Younger Patients. Circulation 2014, 130, 2332–2342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Del Trigo, M.; Munoz-Garcia, A.J.; Wijeysundera, H.C.; Nombela-Franco, L.; Cheema, A.N.; Gutierrez, E.; Serra, V.; Kefer, J.; Amat-Santos, I.J.; Benitez, L.M.; et al. Faculty of 1000 evaluation for Incidence, Timing, and Predictors of Valve Hemodynamic Deterioration After Transcatheter Aortic Valve Replacement: Multicenter Registry. J. Am. Coll. Cardiol. 2016, 67, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. PARTNER 1 Trial Investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomized controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef]
- Makkar, R.R.; Fontana, G.; Jilaihawi, H.; Chakravarty, T.; Kofoed, K.F.; De Backer, O.; Asch, F.M.; Ruiz, C.E.; Olsen, N.T.; Trento, A.; et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N. Engl. J. Med. 2015, 373, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Hansson, N.C.; Grove, E.L.; Andersen, H.R.; Leipsic, J.; Mathiassen, O.N.; Jensen, J.M.; Jensen, K.T.; Blanke, P.; Leetmaa, T.; Tang, M.; et al. Transcatheter Aortic Valve Thrombosis: Incidence, Predisposing Factors, and Clinical Implications. J. Am. Coll. Cardiol. 2016, 68, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Vollema, E.M.; Kong, W.K.F.; Katsanos, S.; Kamperidis, V.; van Rosendael, P.J.; van der Kley, F.; de Weger, A.; Ajmone, M.N.; Delgado, V.; Bax, J.J. Transcatheter aortic valve thrombosis: The relation between hypo-attenuated leaflet thickening, abnormal valve haemodynamics, and stroke. Eur. Heart J. 2017, 38, 1207–1217. [Google Scholar] [CrossRef]
- Chakravarty, T.; Sondergaard, L.; Friedman, J.; De Backer, O.; Berman, D.; Kofoed, K.F.; Jilaihawi, H.; Shiota, T.; Abramowitz, Y.; Jørgensen, T.H.; et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: An observational study. Lancet 2017, 389, 2383–2392. [Google Scholar] [CrossRef]
- Fuchs, A.; De Backer, O.; Brooks, M.; De Knegt, M.; Bieliauskas, G.; Yamamoto, M.; Yanagisawa, R.; Hayashida, K.; Søndergaard, L.; Kofoed, K.; et al. Subclinical leaflet thickening and stent frame geometry in self-expanding transcatheter heart valves. EuroIntervention 2017, 13, e1067–e1075. [Google Scholar] [CrossRef]
- De Backer, O.; Dangas, G.D.; Jilaihawi, H.; Leipsic, J.A.; Terkelsen, C.J.; Makkar, R.; Kini, A.S.; Veien, K.T.; Abdel-Wahab, M.; Kim, W.-K.; et al. Reduced Leaflet Motion after Transcatheter Aortic-Valve Replacement. New Engl. J. Med. 2019, 382, 130–139. [Google Scholar] [CrossRef]
- Nuehrenberg, T.; Hromek, J.; Kille, A.; Hochholzer, W.; Hein, M.; Trenk, D.; Neumann, F.-J.; Stratz, C.; Ruile, P. Impact of On-Clopidogrel Platelet Reactivity on Incidence of Hypoattenuated Leaflet Thickening After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 12–18. [Google Scholar] [CrossRef]
- Morganti, S.; Brambilla, N.; Petronio, A.; Reali, A.; Bedogni, F.; Auricchio, F.; Information, P.E.K.F.C. Prediction of patient-specific post-operative outcomes of TAVI procedure: The impact of the positioning strategy on valve performance. J. Biomech. 2016, 49, 2513–2519. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; O’Brien, S.M.; Wu, C.; Sikora, J.A.H.; Griffith, B.P.; Gammie, J.S. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: Changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J. Thorac. Cardiovasc. Surg. 2009, 137, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Dangas, G.; Weitz, J.I.; Giustino, G.; Makkar, R.; Mehran, R. Prosthetic Heart Valve Thrombosis. J. Am. Coll. Cardiol. 2016, 68, 2670–2689. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, T.; Bouquiaux-Stablo, A.-L.; Candolfi, P.; Mirza, A.; Loardi, C.; May, M.-A.; El-Khoury, R.; Marchand, M.; Aupart, M. Very Long-Term Outcomes of the Carpentier-Edwards Perimount Valve in Aortic Position. Ann. Thorac. Surg. 2015, 99, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.-P.; Berti, S.; Cequier, A.; Van Belle, E.; Lefèvre, T.; Leprince, P.; Neumann, F.-J.; Vicaut, E.; Montalescot, G. Oral anti-Xa anticoagulation after trans-aortic valve implantation for aortic stenosis: The randomized ATLANTIS trial. Am. Hear. J. 2018, 200, 44–50. [Google Scholar] [CrossRef]
- Nappi, F.; Mazzocchi, L.; Singh, S.S.A.; Morganti, S.; Sablayrolles, J.-L.; Acar, C.; Auricchio, F. Complementary Role of the Computed Biomodelling through Finite Element Analysis and Computed Tomography for Diagnosis of Transcatheter Heart Valve Thrombosis. BioMed Res. Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Morganti, S.; Conti, M.; Aiello, M.; Valentini, A.; Mazzola, A.; Reali, A.; Auricchio, F. Simulation of transcatheter aortic valve implantation through patient-specific finite element analysis: Two clinical cases. J. Biomech. 2014, 47, 2547–2555. [Google Scholar] [CrossRef]
- Bonhoeffer, P.; Boudjemline, Y.; Saliba, Z.; Hausse, A.O.; Aggoun, Y.; Bonnet, D.; Sidi, D.; Kachaner, J. Transcatheter Implantation of a Bovine Valve in Pulmonary Position. Circulation 2000, 102, 813–816. [Google Scholar] [CrossRef]
- Vesely, I.; Casarotto, D.C.; Gerosa, G. Mechanics of cryopreserved aortic and pulmonary homografts. J. Hear. Valve Dis. 2000, 9, 27–37. [Google Scholar]
- Nappi, F.; Spadaccio, C.; Fraldi, M.; Montagnani, S.; Fouret, P.; Chachques, J.C.; Acar, C. A composite semiresorbable armoured scaffold stabilizes pulmonary autograft after the Ross operation: Mr Ross’s dream fulfilled. J. Thorac. Cardiovasc. Surg. 2016, 151, 155–164. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Fouret, P.; Hammoudi, N.; Chachques, J.C.; Chello, M.; Acar, C. An experimental model of the Ross operation: Development of resorbable reinforcements for pulmonary autografts. J. Thorac. Cardiovasc. Surg. 2015, 149, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Carotenuto, A.R.; Di Vito, D.; Spadaccio, C.; Acar, C.; Fraldi, M. Stress-shielding, growth and remodeling of pulmonary artery reinforced with copolymer scaffold and transposed into aortic position. Biomech. Model. Mechanobiol. 2015, 15, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Fraldi, M.; Spadaccio, C.; Carotenuto, A.R.; Arcucci, A.; Castaldo, C.; Chachques, J.C.; Acar, C. Biomechanics drive histological wall remodeling of neoaortic root: A mathematical model to study the expression levels of ki 67, metalloprotease, and apoptosis transition. J. Biomed. Mater. Res. Part A 2016, 104, 2785–2793. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Montagnani, S.; Acar, C.; Nappi, F. Introducing bioresorbable scaffolds into the show. A potential adjunct to resuscitate Ross procedure. Int. J. Cardiol. 2015, 190, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Cavender, M.A.; Kim, S.M. Utility of Dual Antiplatelet Therapy for the Prevention of Subclinical Leaflet Thrombosis. JACC: Cardiovasc. Interv. 2019, 12, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Rodés-Cabau, J.; Masson, J.-B.; Welsh, R.C.; Del Blanco, B.G.; Pelletier, M.; Webb, J.G.; Al-Qoofi, F.; Généreux, P.; Maluenda, G.; Thoenes, M.; et al. Aspirin Versus Aspirin Plus Clopidogrel as Antithrombotic Treatment Following Transcatheter Aortic Valve Replacement With a Balloon-Expandable Valve. JACC: Cardiovasc. Interv. 2017, 10, 1357–1365. [Google Scholar] [CrossRef]
- Al Halabi, S.; Newman, J.; Farkouh, M.E.; Fortuin, D.; Leya, F.; Sweeney, J.; Darki, A.; Lopez, J.; Steen, L.; Lewis, B.; et al. Meta-Analysis of Studies Comparing Dual- Versus Mono-Antiplatelet Therapy Following Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2018, 122, 141–148. [Google Scholar] [CrossRef]
- Li, K.; Sun, W. Simulated Thin Pericardial Bioprosthetic Valve Leaflet Deformation under Static Pressure-Only Loading Conditions: Implications for Percutaneous Valves. Ann. Biomed. Eng. 2010, 38, 2690–2701. [Google Scholar] [CrossRef]
- Xuan, Y.; Krishnan, K.; Ye, J.; Dvir, D.; Guccione, J.M.; Ge, L.; Tseng, E.E. Stent and leaflet stresses in a 26-mm first-generation balloon-expandable transcatheter aortic valve. J. Thorac. Cardiovasc. Surg. 2016, 153, 1065–1073. [Google Scholar] [CrossRef]
- Nappi, F.; Attias, D.; Singh, S.S.A.; Prot, V. Finite element analysis applied to the transcatheter mitral valve therapy: Studying the present, imagining the future. J. Thorac. Cardiovasc. Surg. 2019, 157, e149–e151. [Google Scholar] [CrossRef]
- Kunzelman, K.S.; Cochran, R.P.; Chuong, C.; Ring, W.S.; Verrier, E.D.; Eberhart, R.D. Finite element analysis of the mitral valve. J. Hear. Valve Dis. 1993, 2, 326–340. [Google Scholar]
- Krishnamurthy, G.; Ennis, D.B.; Itoh, A.; Bothe, W.; Swanson, J.C.; Karlsson, M.; Kuhl, E.; Miller, D.C.; Ingels, N.B. Material properties of the ovine mitral valve anterior leaflet in vivo from inverse finite element analysis. Am. J. Physiol. Circ. Physiol. 2008, 295, H1141–H1149. [Google Scholar] [CrossRef] [PubMed]
- Prot, V.; Skallerud, B.; Sommer, G.; Holzapfel, G.A. On modelling and analysis of healthy and pathological human mitral valves: Two case studies. J. Mech. Behav. Biomed. Mater. 2010, 3, 167–177. [Google Scholar] [CrossRef]
- Prot, V.E.; Skallerud, B. Contributions of prestrains, hyperelasticity, and muscle fiber activation on mitral valve systolic performance. Int. J. Numer. Methods Biomed. Eng. 2016, 33, e2806. [Google Scholar] [CrossRef]
- Sun, W.; Abad, A.; Sacks, M.S. Simulated Bioprosthetic Heart Valve Deformation under Quasi-Static Loading. J. Biomech. Eng. 2005, 127, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Sun, W. Simulation of long-term fatigue damage in bioprosthetic heart valves: Effects of leaflet and stent elastic properties. Biomech. Model. Mechanobiol. 2013, 13, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Overtchouk, P.; Guedeney, P.; Rouanet, S.; Verhoye, J.P.; Lefévre, T.; Van Belle, E.; Eltchaninoff, H.; Gilard, M.; Leprince, P.; Iung, B.; et al. Long-Term Mortality and Early Valve Dysfunction According to Anticoagulation Use. J. Am. Coll. Cardiol. 2019, 73, 13–21. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, F.; Mazzocchi, L.; Timofeva, I.; Macron, L.; Morganti, S.; Avtaar Singh, S.S.; Attias, D.; Congedo, A.; Auricchio, F. A Finite Element Analysis Study from 3D CT to Predict Transcatheter Heart Valve Thrombosis. Diagnostics 2020, 10, 183. https://doi.org/10.3390/diagnostics10040183
Nappi F, Mazzocchi L, Timofeva I, Macron L, Morganti S, Avtaar Singh SS, Attias D, Congedo A, Auricchio F. A Finite Element Analysis Study from 3D CT to Predict Transcatheter Heart Valve Thrombosis. Diagnostics. 2020; 10(4):183. https://doi.org/10.3390/diagnostics10040183
Chicago/Turabian StyleNappi, Francesco, Laura Mazzocchi, Irina Timofeva, Laurent Macron, Simone Morganti, Sanjeet Singh Avtaar Singh, David Attias, Antonio Congedo, and Ferdinando Auricchio. 2020. "A Finite Element Analysis Study from 3D CT to Predict Transcatheter Heart Valve Thrombosis" Diagnostics 10, no. 4: 183. https://doi.org/10.3390/diagnostics10040183
APA StyleNappi, F., Mazzocchi, L., Timofeva, I., Macron, L., Morganti, S., Avtaar Singh, S. S., Attias, D., Congedo, A., & Auricchio, F. (2020). A Finite Element Analysis Study from 3D CT to Predict Transcatheter Heart Valve Thrombosis. Diagnostics, 10(4), 183. https://doi.org/10.3390/diagnostics10040183




