Epithelial-Mesenchymal Transition Proteins in Neuroendocrine Neoplasms: Differential Immunohistochemical Expression in Different Sites and Correlation with Clinico-Pathological Features
Abstract
1. Introduction
2. Methods
2.1. Collective
2.2. Immunohistochemistry
2.3. Immunostaining Scoring System
2.4. Statistical Evaluation
2.5. Ethical Approval
2.6. Declarations
3. Results
3.1. Clinico-Pathological Features
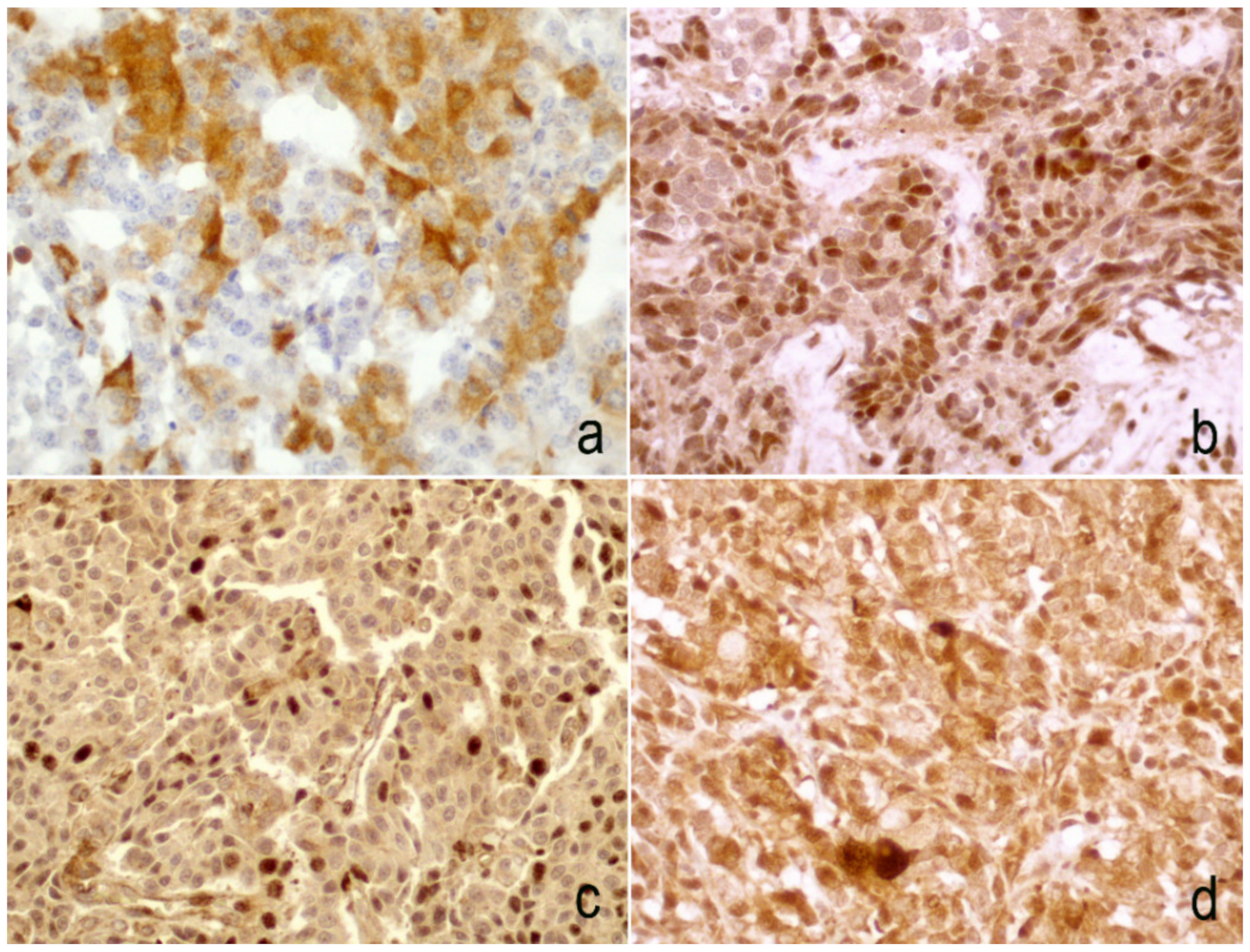
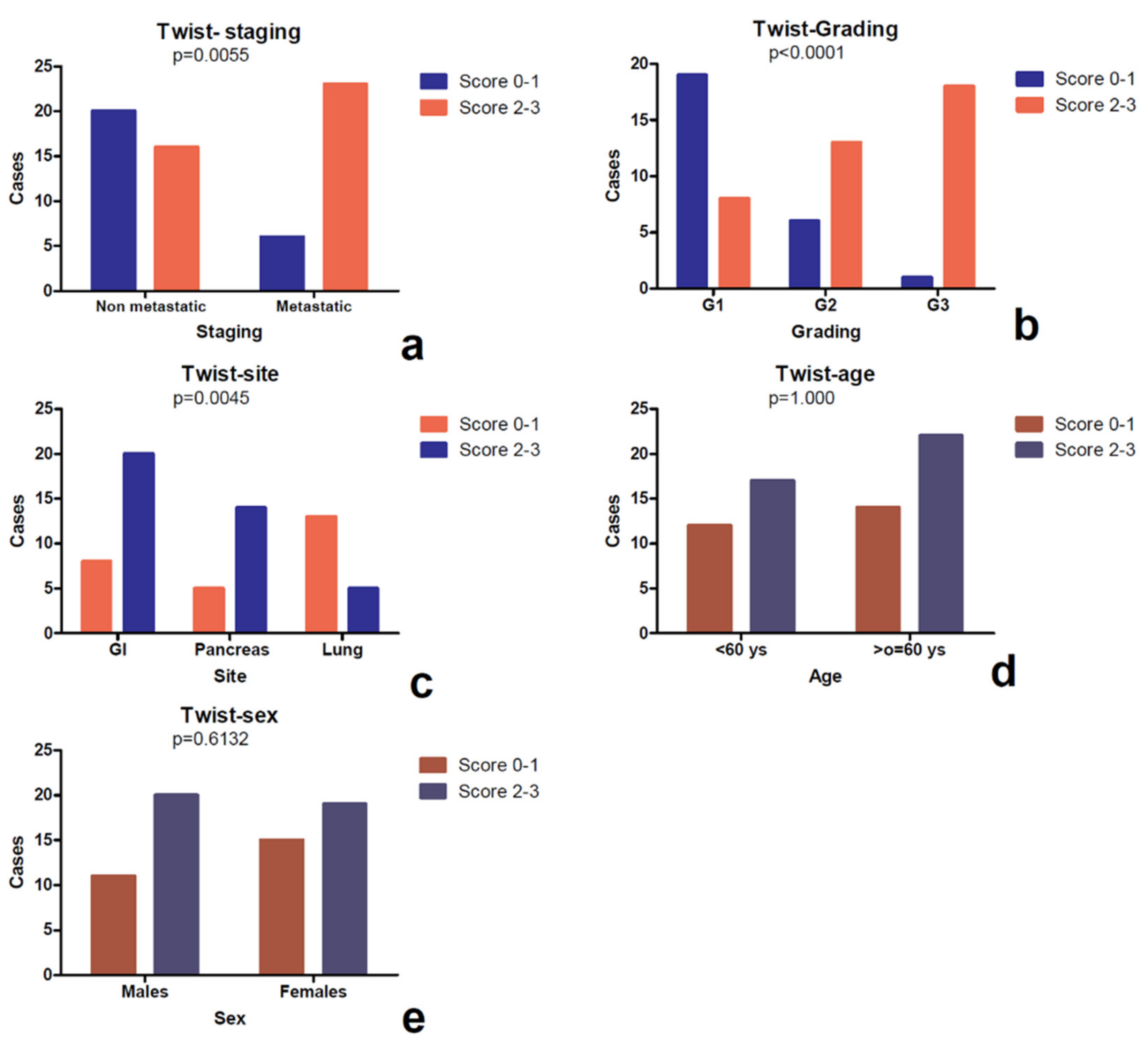
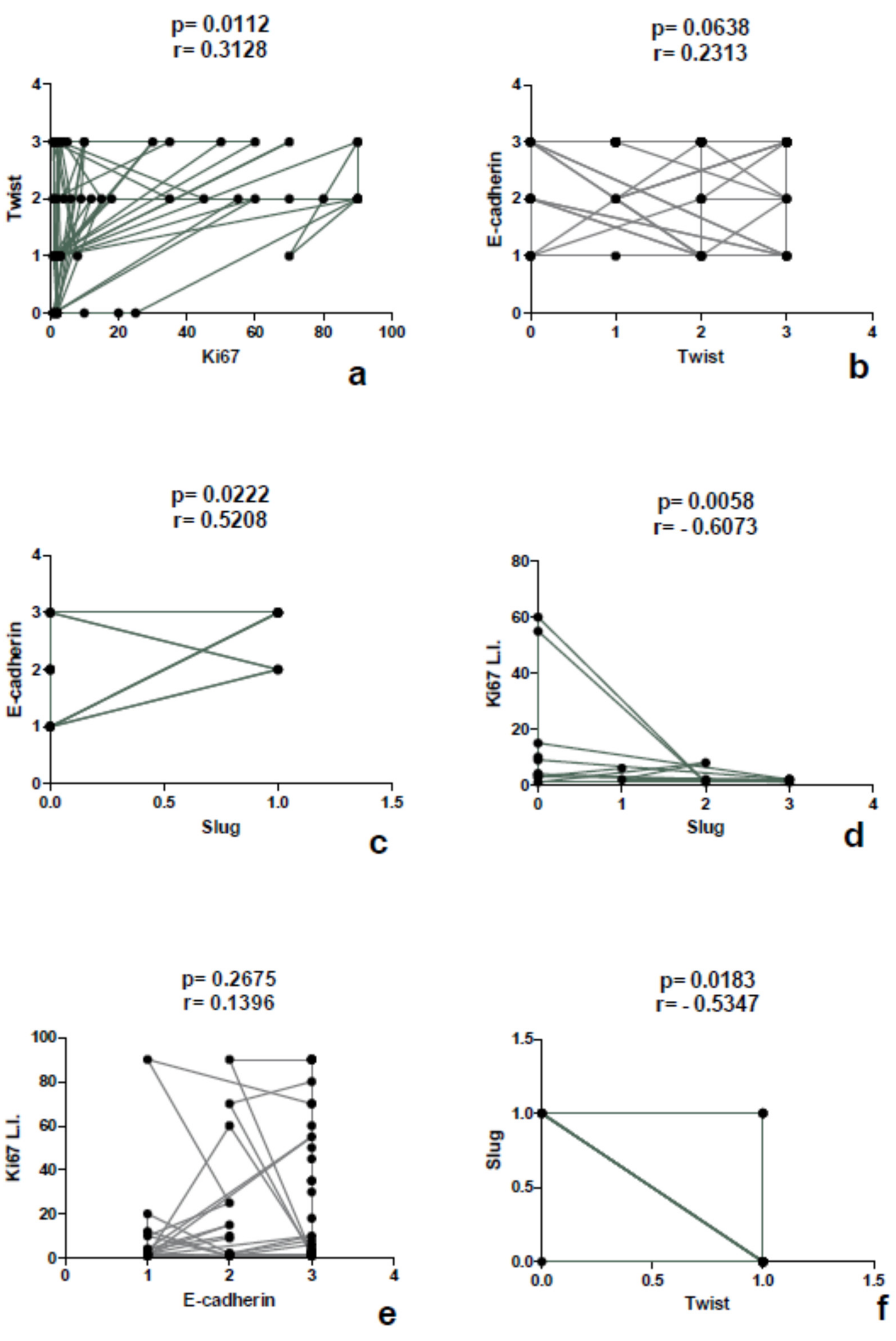
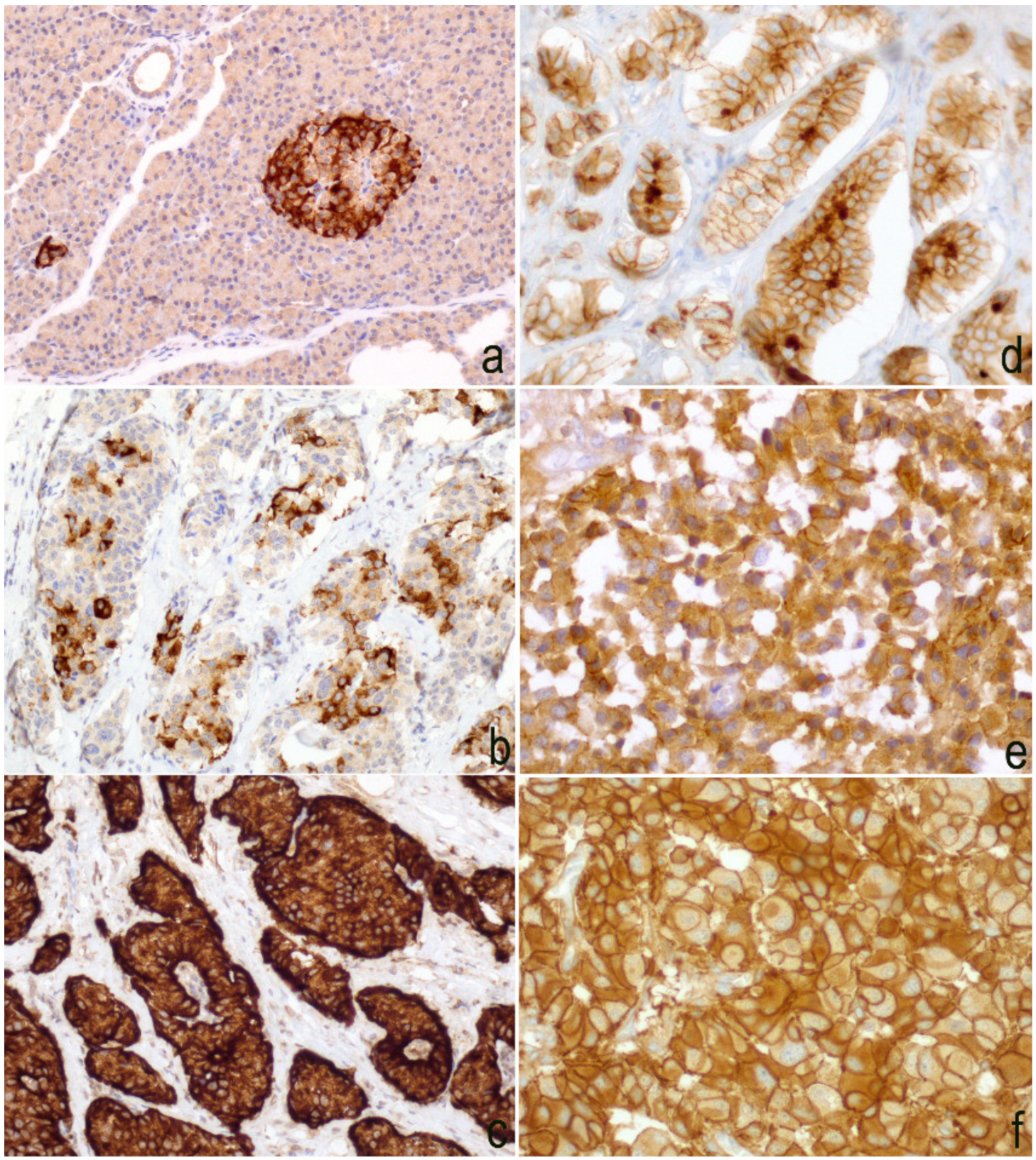
3.2. Differential Expression of Twist in Single Sites
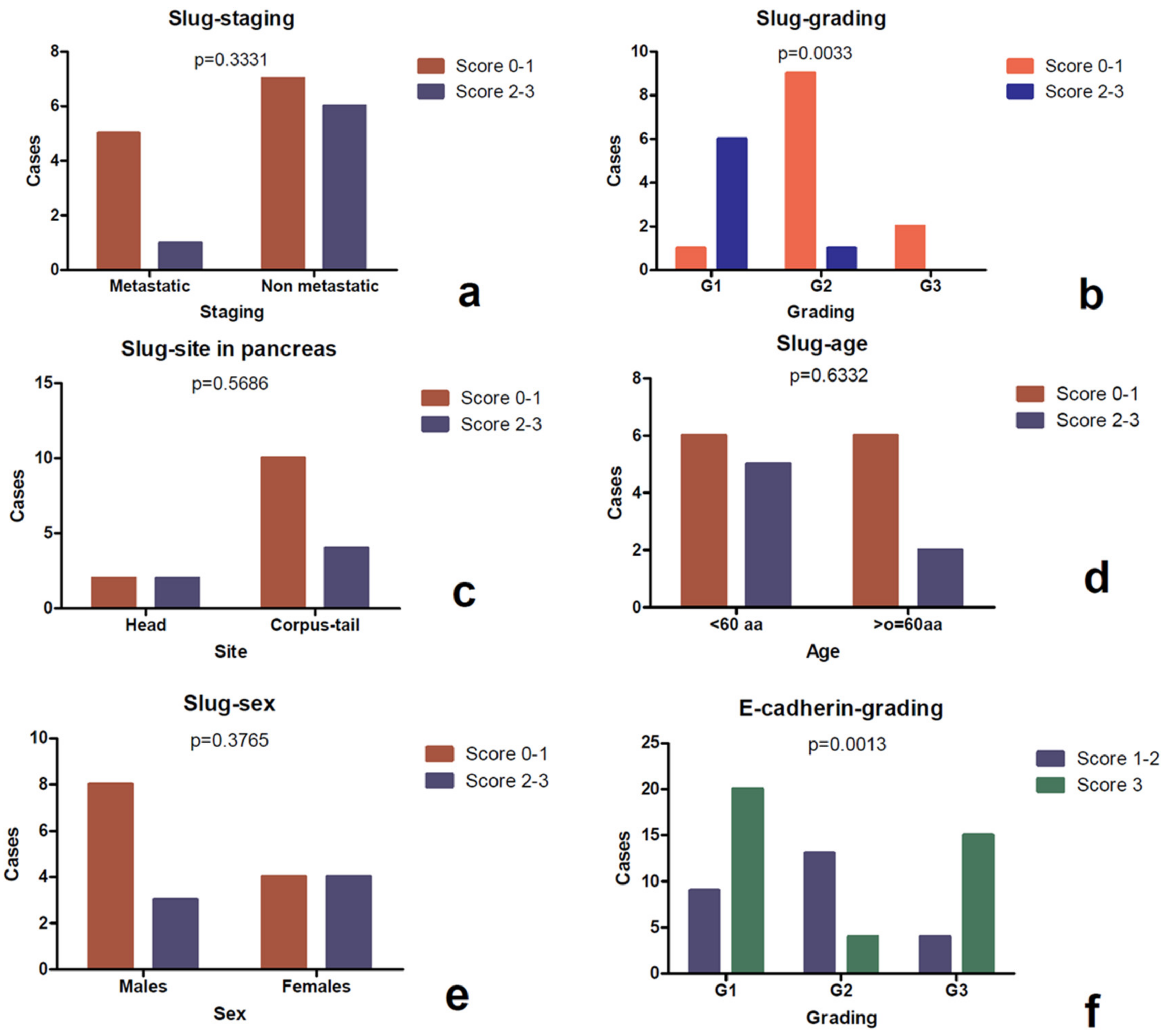
3.3. Expression of Slug
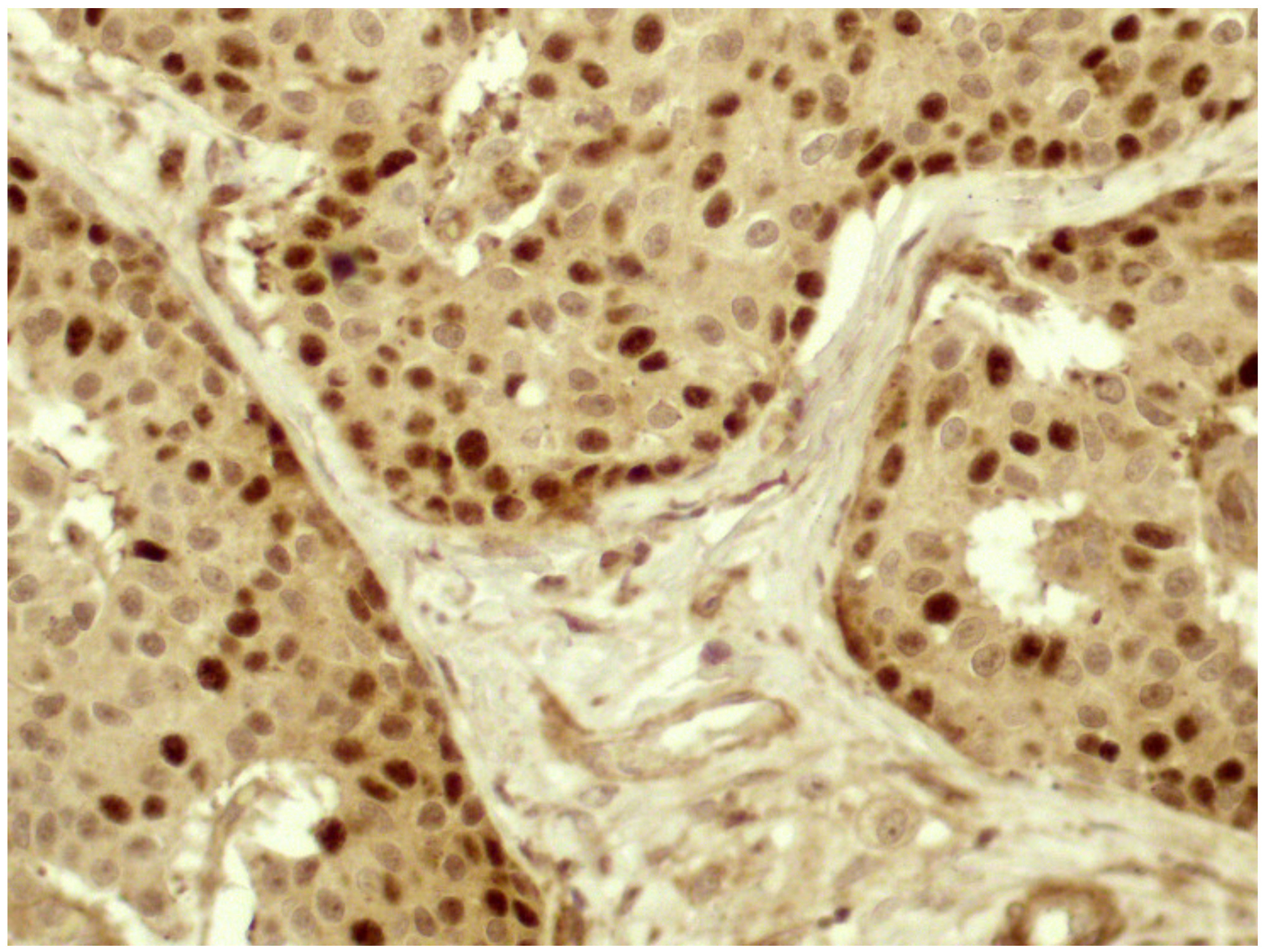
3.4. Expression of E-Cadherin
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Fraenkel, M.; Faggiano, A.; Valk, G.D. Epidemiology of neuroendocrine tumors. Front. Horm. Res. 2015, 44, 1–23. [Google Scholar] [PubMed]
- Klimstra, D.S.; Kloppel, G.; La Rosa, S.; Rindi, G. Classification of Neuroendocrine Neoplasms of the Digestive System. In WHO Classification of Tumors of the Digestive System, 5th ed.; IARC Press: Lyon, France, 2019; pp. 16–19. [Google Scholar]
- Rindi, G.; D’Adda, T.; Froio, E.; Fellegara, G.; Bordi, C. Prognostic factors in gastrointestinal endocrine tumors. Endocr. Pathol. 2007, 18, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Zatelli, M.C.; Guadagno, E.; Messina, E.; Lo Calzo, F.; Faggiano, A.; Colao, A. Open issues on G3 neuroendocrine neoplasms: Back to the future. Endocr. Relat. Cancer 2018, 25, R375–R384. [Google Scholar] [CrossRef] [PubMed]
- Klöppel, G.; Rindi, G.; Anlauf, M.; Perren, A.; Komminoth, P. Site-specificbiology and pathology of gastroenteropancreatic neuroendocrine tumors. Virchows Arch. 2007, 451 (Suppl. 1), S9–S27. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Beasley, M.B.; Austin, J.H.M.; Capelozzi, V.L.; Chirieac, L.R.; Devesa, S.S.; Frank, G.A.; Gazdar, A.; Ishikawa, Y.; Jen, J.; et al. Neuroendocrine tumours. In WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th ed.; IARC Press: Lyon, France, 2015; pp. 63–78. [Google Scholar]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Fernandez-Cuesta, L. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.A. The biology of cancer. In Garland Science, 2nd ed.; Garland Science, Taylor and Francis Group: New York, NY, USA, 2013; Chapter 14. [Google Scholar]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, M.; Kouvaras, E.; Papamichali, R.; Samara, M.; Chiotoglou, I.; Koukoulis, G. Smad4 and epithelial-mesenchymal transition proteins in colorectal carcinoma: An immunohistochemical study. J. Mol. Histol. 2018, 49, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sun, X.; Yu, L.; Zhou, R.; Li, A.; Li, M.; Yang, W. Differential expression and clinical significance of epithelial-mesenchymal transition markers among different histological types of triple-negative breast cancer. J. Cancer 2018, 9, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Jia, Z.; Liu, H.; Liu, Q.; Zhang, T.; Guo, W.; Li, P.; Deng, M.; Li, S. Prognostic and clinicopathologicalvalue of Twist expression in breastcancer: A meta-analysis. PLoS ONE 2017, 12, e018619. [Google Scholar]
- Fendrich, V.; Maschuw, K.; Waldmann, J.; Buchholz, M.; Rehm, J.; Gress, T.M.; Bartsch, D.K.; König, A. Epithelial-mesenchymaltransitionis a critical step in tumorgenesis of pancreatic neuroendocrine tumors. Cancers 2012, 4, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Galván, J.A.; Astudillo, A.; Vallina, A.; Fonseca, P.J.; Gómez-Izquierdo, L.; García-Carbonero, R.; González, M.V. Epithelial-mesenchymaltransition markers in the differentialdiagnosis of gastroenteropancreatic neuroendocrine tumors. Am. J. Clin. Pathol. 2013, 140, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Rizzo, F.; Simone, V.; Bisceglia, F.; Stucci, S.; Seeber, A.; Spizzo, G.; Montrone, T.; Resta, L.; Silvestris, F. Reviewing the osteotropism in neuroendocrine tumors: The role of epithelial-mesenchymal transition. Neuroendocrinology 2016, 103, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Ikezono, Y.; Koga, H.; Akiba, J.; Abe, M.; Yoshida, T.; Wada, F.; Nakamura, T.; Iwamoto, H.; Masuda, A.; Sakaue, T.; et al. Pancreatic neuroendocrine tumors and EMT behavior are driven by the CSC marker DCLK1. Mol. Cancer Res. 2017, 15, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Kurahara, H.; Maemura, K.; Mataki, Y.; Sakoda, M.; Iino, S.; Ueno, S.; Shinchi, H.; Natsugoe, S. Impact of snail and E-cadherinexpression in pancreatic neuroendocrine tumors. Oncol. Lett. 2017, 14, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.; Chiu, Y.F.; Kuo, M.H.; Lee, K.L.; Lee, A.C.; Yu, C.C.; Chang, J.L.; Huang, W.C.; Hsiao, S.H.; Lin, S.E.; et al. Expression of neuroendocrine factor VGF in lung cancer cells confers resistance to EGFR kinase inhibitors and triggers epithelial-to-mesenchymal transition. Cancer Res. 2017, 77, 3013–3026. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Schilsky, R.L.; Laurie, E.G.; Washington, M.K.; Sullivan, D.C.; Brookland, R.K.; Brierley, J.D.; Balch, C.M.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Reid, M.D.; Bagci, P.; Ohike, N.; Saka, B.; Erbarut, S.I.; Dursun, N.; Balci, S.; Gucer, H.; Jang, K.-T.; Tajiri, T.; et al. Calculation of the Ki67 index in pancreatic neuroendocrine tumors: A comparative analysis of four counting methodologies. Mod. Pathol. 2016, 29, 93. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.H.; Park, H.J.; Choi, Y.; Won, Y.J.; Lee, S.J.; Park, D.Y. TWIST mediates resistance to paclitaxel by regulating Akt and Bcl-2 expression in gastric cancer cells. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Righi, L.; Gatti, G.; Volante, M.; Papotti, M. Lung neuroendocrine tumors: Pathological characteristics. J. Thorac. Dis. 2017, 9 (Suppl. 15), S1442–S1447. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Mandhani, A.; Agrawal, V.; Garg, M. Positive correlation between matrix metalloproteinases and epithelial-to-mesenchymal transition and its association with clinical outcome in bladder cancer patients. Cancer Microenviron. 2018, 11, 23–39. [Google Scholar] [CrossRef] [PubMed]
| GEP-NEN 2019 WHO | |||
|---|---|---|---|
| Grade | Differentiation | Proliferation | Nomenclature |
| Low | Well differentiated | <2 mitoses/2 mm2 AND Ki67 < 3% | NET G1 |
| Intermediate | 2–20 mitoses/2 mm2 OR Ki67 3–20% | NET G2 | |
| High | >20 mitoses/2 mm2 OR Ki67 > 20% | NET G3 | |
| Poorly differentiated(small cell or large cell) | NEC | ||
| Lung-NEN 2015 WHO | |||
| Grade | Differentiation | Diagnostic Criteria | Nomenclature |
| Low | Well differentiated | <2 mitoses/2 mm2 AND no necrosis | Typical carcinoid |
| Intermediate | Moderately differentiated | 2–20 mitoses/2 mm2 OR foci of necrosis | Atypical carcinoid |
| High | Poorly differentiated (small cell or large cell) | >10 mitoses/2 mm2 | - Small cell lung cancer - Large cell neuroendocrine cancer |
| Cases | Sex | Age | Site | Diagnosis | Grading | Staging | Ki67 | Twist | Slug | E-Cadherin |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 65 | Vater papilla | NEC | G3 | pT3N1Mx | 50% | 3 | 0 | 3 |
| 2 | M | 79 | Ileum | NET | G1 | pT3N0Mx | 2% | 1 | 0 | 3 |
| 3 | M | 56 | Colon | NET | G2 | pT3N1Mx | 18% | 2 | 0 | 3 |
| 4 | F | 59 | Appendix | NET | G1 | pT3N0Mx | 1% | 1 | 0 | 3 |
| 5 | M | 63 | Colon | NEC | G3 | pT3N1Mx | 60% | 3 | 0 | 3 |
| 6 | M | 54 | Stomach | NET | G2 | pT3N1Mx | 5% | 3 | 0 | 3 |
| 7 | M | 69 | Colon | NEC | G3 | pT4aN1Mx | 35% | 3 | 0 | 3 |
| 8 | M | 61 | Ileum | NET | G1 | pT4N1M1 | 1% | 2 | 0 | 3 |
| 9 | F | 56 | Stomach | NEC | G3 | pT3N0M1 | 80% | 2 | 0 | 3 |
| 10 | F | 43 | Ombelical region ° | NEC | G3 | M1 | 70% | 2 | 0 | 2 |
| 11 | F | 76 | Ileum | NET | G1 | pT4N1Mx | 1% | 2 | 0 | 3 |
| 12 | F | 50 | Appendix | NET | G1 | pT3N0Mx | 1% | 1 | 0 | 3 |
| 13 | M | 60 | Ileum | NET | G1 | (m)pT3N1Mx | 1% | 3 | 0 | 3 |
| 14 | M | 50 | Stomach | NET | G3 | pT4N1Mx | 35% | 2 | 0 | 3 |
| 15 | F | 74 | Omentum °° | NEC | G3 | M1 | 90% | 2 | 0 | 3 |
| 16 | M | 53 | Sigma | NEC | G3 | pT3N0Mx | 90% | 2 | 0 | 3 |
| 17 | M | 71 | Stomach | NEC | G3 | pT4aN1Mx | 45% | 2 | 0 | 3 |
| 18 | M | 62 | Rectum | NET | G2 | pT1aNXMx | 3% | 3 | 0 | 3 |
| 19 | M | 61 | Colon | NET | G1 | pT4N1Mx | 2% | 1 | 0 | 3 |
| 20 | F | 64 | Colon | NET | G2 | (m)pT3N1Mx | 3% | 1 | 0 | 3 |
| 21 | M | 64 | Colon | NEC(MiNEN) | G3 | pT2N1Mx | 70% | 3 | 0 | 3 |
| 22 | M | 70 | Colon | NET | G2 | pT4 N1Mx | 3% | 1 | 0 | 3 |
| 23 | F | 66 | Stomach | NET | G1 | pT4N1Mx | 2% | 3 | 0 | 3 |
| 24 | F | 75 | Appendix | NET | G1 | pT1N0 | 2% | 1 | 0 | 3 |
| 25 | F | 77 | Jejunum | NEC | G3 | pT4bN2Mx | 90% | 3 | 0 | 2 |
| 26 | F | 67 | Rectum | NEC(MiNEN) | G3 | pT3N1aMX | 90% | 2 | 0 | 3 |
| 27 | F | 52 | Ileum | NET | G1 | pT4N0M1 | 2% | 1 | 0 | 3 |
| 28 | M | 55 | Peritoneum °°° | NET | G3 | M1 | 30% | 3 | 0 | 3 |
| 29 | M | 59 | Omentum # | NET | G2 | M1 | 3% | 1 | 0 | 3 |
| 30 | M | 40 | Pancreas (T) | NEC | G3 | pT4aNxMx | 60% | 2 | 0 | 2 |
| 31 | F | 57 | Pancreas (H) | NET | G1 | pT1NxMx | 1% | 0 | 2 | 1 |
| 32 | F | 59 | Pancreas (H) | NEC | G3 | pT3N1Mx | 55% | 2 | 0 | 3 |
| 33 | F | 66 | Pancreas (T) | NET | G2 | pT1NxMx | 4% | 2 | 0 | 1 |
| 34 | F | 66 | Pancreas (H) | NET | G2 | pT2N0Mx | 10% | 3 | 0 | 2 |
| 35 | F | 27 | Pancreas (T) | NET | G2 | pT2NxMx | 9% | 2 | 0 | 2 |
| 36 | F | 62 | Pancreas (H) | NET | G1 | pT1N0Mx | 2% | 0 | 3 | 1 |
| 37 | M | 61 | Pancreas (T) | NET | G2 | pT3NxMx | 15% | 2 | 0 | 2 |
| 38 | M | 76 | Pancreas (C) | NET | G2 | pT3N0Mx | 4% | 3 | 0 | 1 |
| 39 | M | 52 | Pancreas (T) | NET | G1 | pT3N0Mx | 1% | 3 | 3 | 1 |
| 40 | M | 76 | Pancreas (T) | NET | G1 | pT3N1Mx | 1% | 3 | 0 | 3 |
| 41 | F | 53 | Pancreas (C) | NET | G2 | pT1N1Mx | 8% | 1 | 2 | 3 |
| 42 | M | 53 | Pancreas (T) | NET | G1 | pT1N1M1 | 2% | 3 | 1 | 2 |
| 43 | M | 27 | Pancreas (T) | NET | G1 | pT2NXM0 | 2% | 3 | 3 | 3 |
| 44 | F | 41 | Pancreas (C) | NET | G1 | pT1NXM0 | 2% | 0 | 2 | 3 |
| 45 | F | 69 | Pancreas (T) | NET | G1 | pT1NXM0 | 2% | 2 | 3 | 3 |
| 46 | F | 67 | Pancreas (T) | NET | G2 | pT3N0M0 | 3% | 3 | 0 | 3 |
| 47 | F | 29 | Pancreas (C) | NET | G2 | (m)pT2N1MX | 6% | 2 | 1 | 3 |
| 48 | F | 62 | Right lung (Inf) | AC | G2 | pT1aN0Mx | 1% | 0 | 0 | 2 |
| 49 | M | 57 | Right lung (Inf) | AC | G2 | pT1aN0Mx | 12% | 2 | 0 | 1 |
| 50 | M | 74 | Right lung (Sup) | TC | G1 | pT1aN0Mx | 1% | 1 | 0 | 1 |
| 51 | F | 52 | Right lung (Mid) | AC | G2 | pT1aN0Mx | 2% | 0 | 0 | 1 |
| 52 | F | 76 | Right lung (Mid) | TC | G1 | pT2aN0Mx | 20% | 0 | 0 | 1 |
| 53 | M | 61 | Right lung (Inf) | TC | G1 | pT1bN0Mx | 2% | 0 | 0 | 2 |
| 54 | F | 63 | Right lung (Inf) | AC | G2 | pT2aN0Mx | 10% | 3 | 0 | 3 |
| 55 | M | 38 | Left lung (Inf) | TC | G1 | pT2aN0Mx | 1% | 0 | 0 | 1 |
| 56 | F | 68 | Right lung (Inf) | TC | G1 | pT1aN0Mx | 1% | 0 | 0 | 3 |
| 57 | F | 59 | Left lung (Sup) | TC | G1 | pT1aNxMx | 2% | 0 | 0 | 2 |
| 58 | M | 56 | Right lung (Sup) | AC | G2 | pT2aN0Mx | 1% | 3 | 0 | 2 |
| 59 | F | 37 | Right lung (Inf) | TC | G1 | pT2aN0Mx | 2% | 0 | 0 | 1 |
| 60 | M | 60 | Left lung (Inf) | TC | G1 | pT1bN0Mx | 1% | 0 | 0 | 2 |
| 61 | F | 73 | Right lung (Inf) | TC | G1 | pT1aN0Mx | 10% | 0 | 0 | 1 |
| 62 | F | 52 | Right lung (Mid) | TC | G1 | pT1aN0Mx | 25% | 0 | 0 | 2 |
| 63 | F | 53 | Right lung (Inf) | NEC | G3 | pT2bN0MX | 90% | 2 | 0 | 1 |
| 64 | M | 65 | Right lung (Sup) | NEC | G3 | pT1cN0MX | 70% | 1 | 0 | 3 |
| 65 | M | 74 | Right laterocervical lymph node ## | NEC | G3 | M1 | 90% | 3 | 0 | 3 |
| Parameter | Measurement |
|---|---|
| Age (years) | |
| Mean | 59.57 |
| Median | 61 |
| Range | 27–79 |
| Sex | |
| Males | 31 (48%) |
| Females | 34 (52%) |
| Site | |
| Gastrointestinal | 28 (43%) |
| Pancreatic | 19 (29%) |
| Pulmonary | 18 (28%) |
| Diagnosis | |
| NET | 33 (51%) |
| TC | 10 (15%) |
| AC | 5 (8%) |
| NEC | 17 (26%) |
| Grading | |
| G1 | 27 (42%) |
| G2 | 19 (29%) |
| G3 | 19 (29%) |
| Ki67 L.I. | |
| Range | 1–90% |
| Mean | 21.48% |
| Staging | |
| Metastatic | 29 (45%) |
| Non metastatic | 36 (55%) |
| Twist | ||||||
|---|---|---|---|---|---|---|
| Gastrointestinal | Pancreatic | Pulmonary | ||||
| Score 0–1 | Score 2–3 | Score 0–1 | Score 2–3 | Score 0–1 | Score 2–3 | |
| Age | ||||||
| <60 years | 3 | 7 | 4 | 7 | 5 | 3 |
| ≥60 years | 5 | 13 | 1 | 7 | 8 | 3 |
| p value | 1.000 | 0.3378 | 1.000 | |||
| Sex | ||||||
| Males | 3 | 12 | 3 | 4 | 5 | 4 |
| Females | 5 | 8 | 2 | 10 | 8 | 1 |
| p value | 0.4097 | 0.3047 | 0.2941 | |||
| Staging | ||||||
| Metastatic | 5 | 17 | 5 | 1 | 13 | 4 |
| Non metastatic | 4 | 2 | 7 | 6 | 0 | 1 |
| p value | 0.0638 | 0.3331 | 0.2778 | |||
| Grading | ||||||
| G1 | 6 | 4 | 3 | 5 | 10 | 0 |
| G2 | 2 | 2 | 2 | 7 | 2 | 3 |
| G3 | 0 | 14 | 0 | 2 | 1 | 2 |
| p value | 0.0034 | 0.3852 | 0.0129 | |||
| Slug | ||||||
| Score 0–1 | -- | -- | 1 | 4 | -- | -- |
| Score 2–3 | -- | -- | 11 | 3 | -- | -- |
| p value | -- | 0.0379 | -- | |||
| E-Cadherin | ||||||
| Gastrointestinal | Pancreatic | Pulmonary | ||||
| Score 1–2 | Score 3 | Score 1–2 | Score 3 | Score 1–2 | Score 3 | |
| Age | ||||||
| <60 years | 1 | 9 | 6 | 5 | 7 | 1 |
| ≥60 years | 1 | 17 | 5 | 3 | 7 | 3 |
| p value | 1.000 | 1.000 | 0.5882 | |||
| Sex | ||||||
| Males | 0 | 15 | 5 | 2 | 6 | 3 |
| Females | 2 | 11 | 6 | 6 | 8 | 1 |
| p value | 0.2063 | 0.6332 | 0.5765 | |||
| Staging | ||||||
| Metastastic | 2 | 15 | 1 | 5 | 8 | 9 |
| Non metastastic | 0 | 1 | 7 | 6 | 0 | 1 |
| p value | 1.000 | 0.1770 | 1.000 | |||
| Grading | ||||||
| G1 | 0 | 10 | 1 | 6 | 9 | 1 |
| G2 | 0 | 4 | 8 | 2 | 5 | 0 |
| G3 | 2 | 12 | 2 | 0 | 0 | 3 |
| p value | 0.3406 | 0.0116 | 0.0017 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guadagno, E.; Campione, S.; Pignatiello, S.; Borrelli, G.; De Dominicis, G.; De Rosa, N.; Del Basso De Caro, M. Epithelial-Mesenchymal Transition Proteins in Neuroendocrine Neoplasms: Differential Immunohistochemical Expression in Different Sites and Correlation with Clinico-Pathological Features. Diagnostics 2020, 10, 351. https://doi.org/10.3390/diagnostics10060351
Guadagno E, Campione S, Pignatiello S, Borrelli G, De Dominicis G, De Rosa N, Del Basso De Caro M. Epithelial-Mesenchymal Transition Proteins in Neuroendocrine Neoplasms: Differential Immunohistochemical Expression in Different Sites and Correlation with Clinico-Pathological Features. Diagnostics. 2020; 10(6):351. https://doi.org/10.3390/diagnostics10060351
Chicago/Turabian StyleGuadagno, Elia, Severo Campione, Sara Pignatiello, Giorgio Borrelli, Gianfranco De Dominicis, Nicolina De Rosa, and Marialaura Del Basso De Caro. 2020. "Epithelial-Mesenchymal Transition Proteins in Neuroendocrine Neoplasms: Differential Immunohistochemical Expression in Different Sites and Correlation with Clinico-Pathological Features" Diagnostics 10, no. 6: 351. https://doi.org/10.3390/diagnostics10060351
APA StyleGuadagno, E., Campione, S., Pignatiello, S., Borrelli, G., De Dominicis, G., De Rosa, N., & Del Basso De Caro, M. (2020). Epithelial-Mesenchymal Transition Proteins in Neuroendocrine Neoplasms: Differential Immunohistochemical Expression in Different Sites and Correlation with Clinico-Pathological Features. Diagnostics, 10(6), 351. https://doi.org/10.3390/diagnostics10060351





