Comparison between Diffusion-Weighted Sequences with Selective and Non-Selective Fat Suppression in the Evaluation of Crohn’s Disease Activity: Are They Equally Useful?
Abstract
1. Introduction
- (1)
- Measuring ADC values of DWI with SPIR (ADC-DWISPIR) and DWI with STIR (ADC-DWISTIR) in groups of adult and paediatric patients, comparing each individual patient group and assessing their mutual correlation,
- (2)
- comparing ADC- DWISPIR and ADC-DWISTIR values between the groups of adults and pediatric patients,
- (3)
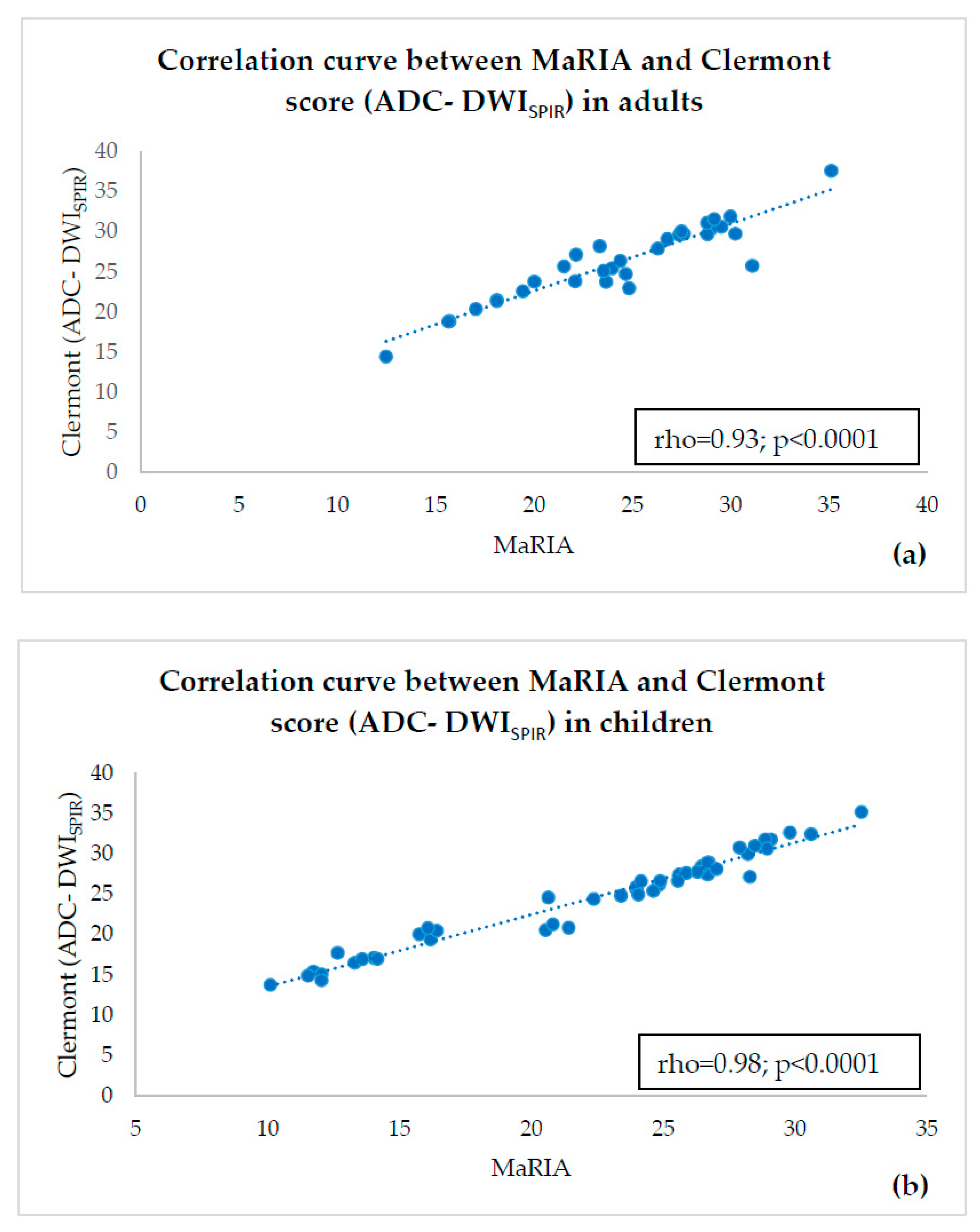
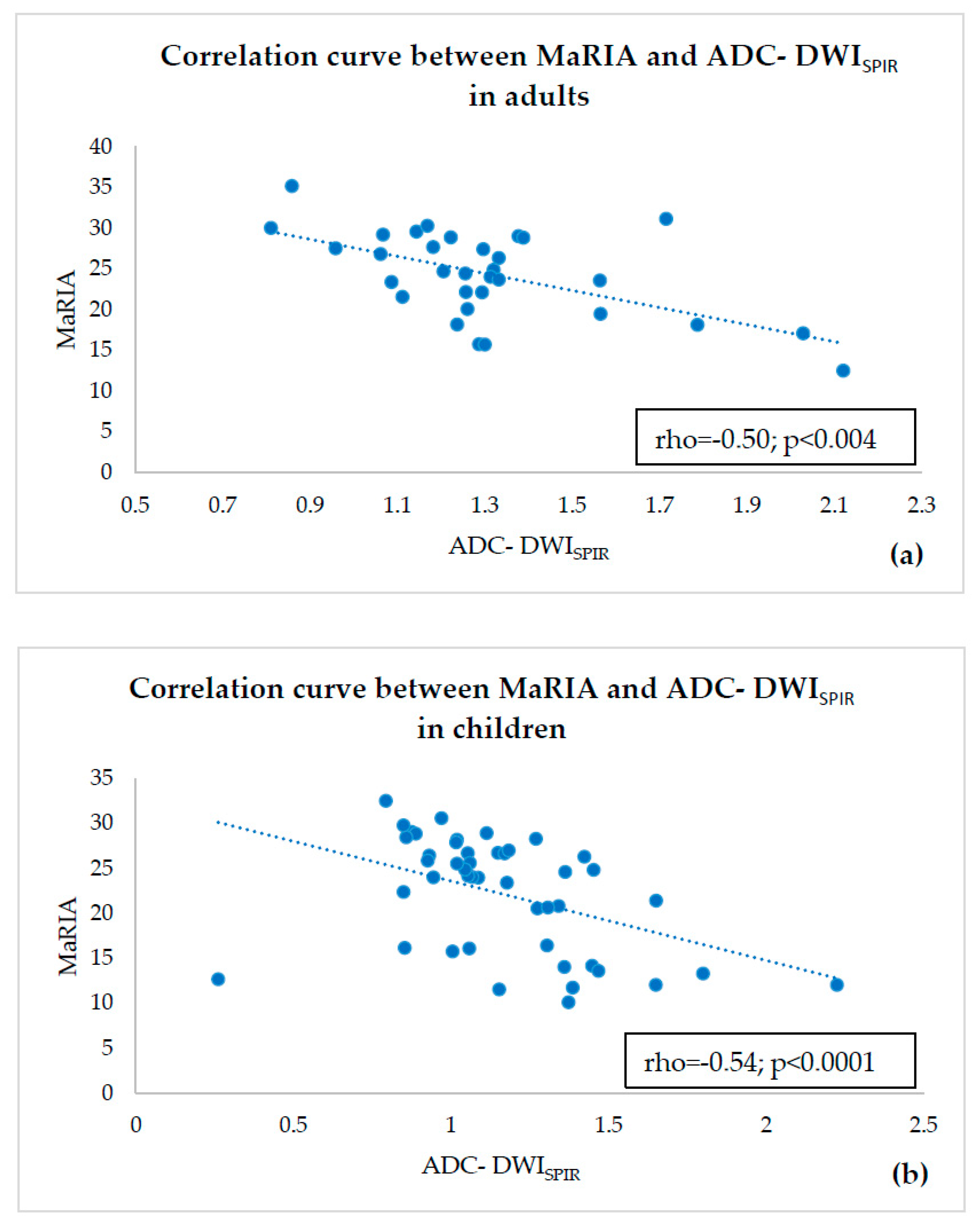
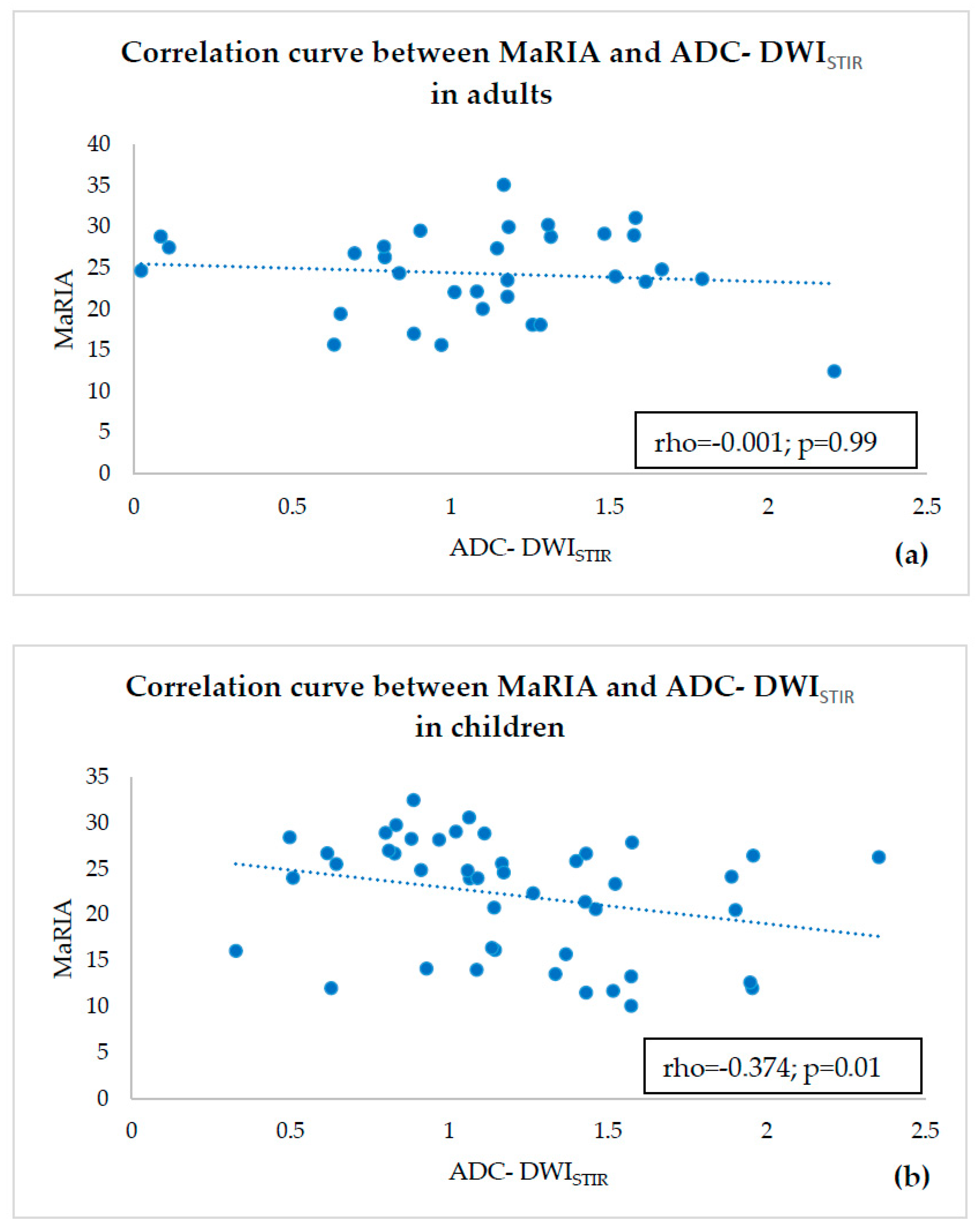
- estimating correlations of ADC-DWISPIR and ADC-DWISTIR values with the corresponding MaRIA, calculated from the contrast–enhanced sequences within the same bowel segments,
- (4)
- calculating Clermont scores values based on ADC-DWISPIR and ADC-DWISTIR and estimating their correlation with MaRIA within the same bowel segments.
2. Materials and Methods
2.1. Patient Population
2.2. MRI Technique
- (1)
- coronal bTFE (Balanced Turbo Field Echo) cine sequence for real-time assessment of the bowel peristalsis,
- (2)
- axial DWI sequence with SPIR, using diffusion factors b fixed at 0, 600 and 800 s/mm2, with corresponding ADC maps,
- (3)
- axial DWIBS sequence (DWI with STIR), using diffusion factors b fixed at 0, 600 and 800 s/mm2, with corresponding ADC maps,
- (4)
- axial and coronal T2-weighted sequences without fat suppression (T2 TSE),
- (5)
- axial and coronal T2-weighted sequences with fat suppression (T2 SPAIR),
- (6)
- coronal T2 fat suppression magnetic resonance cholangiopancreatography (MRCP) sequence with radial 3D reconstructions,
- (7)
- coronal T1-weighted dynamic postcontrast images e-THRIVE (T1 high-resolution isotropic volume excitation), followed by delayed post-contrast axial e-THRIVE images.
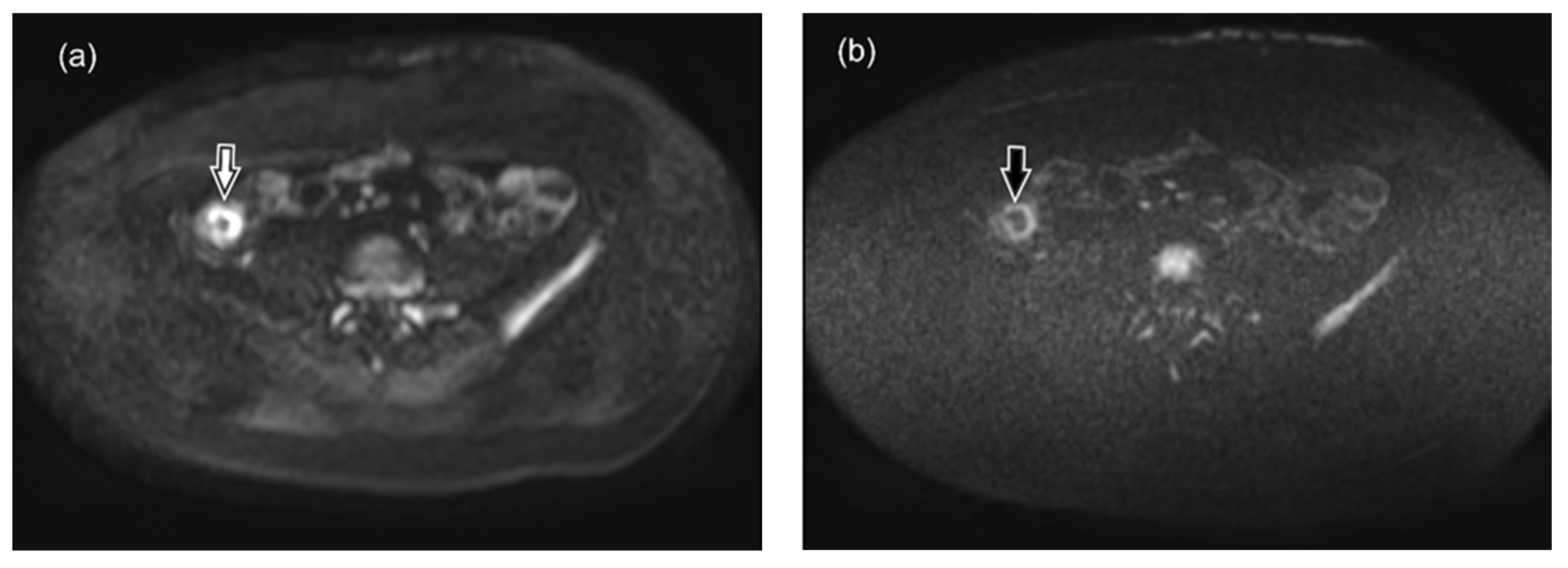
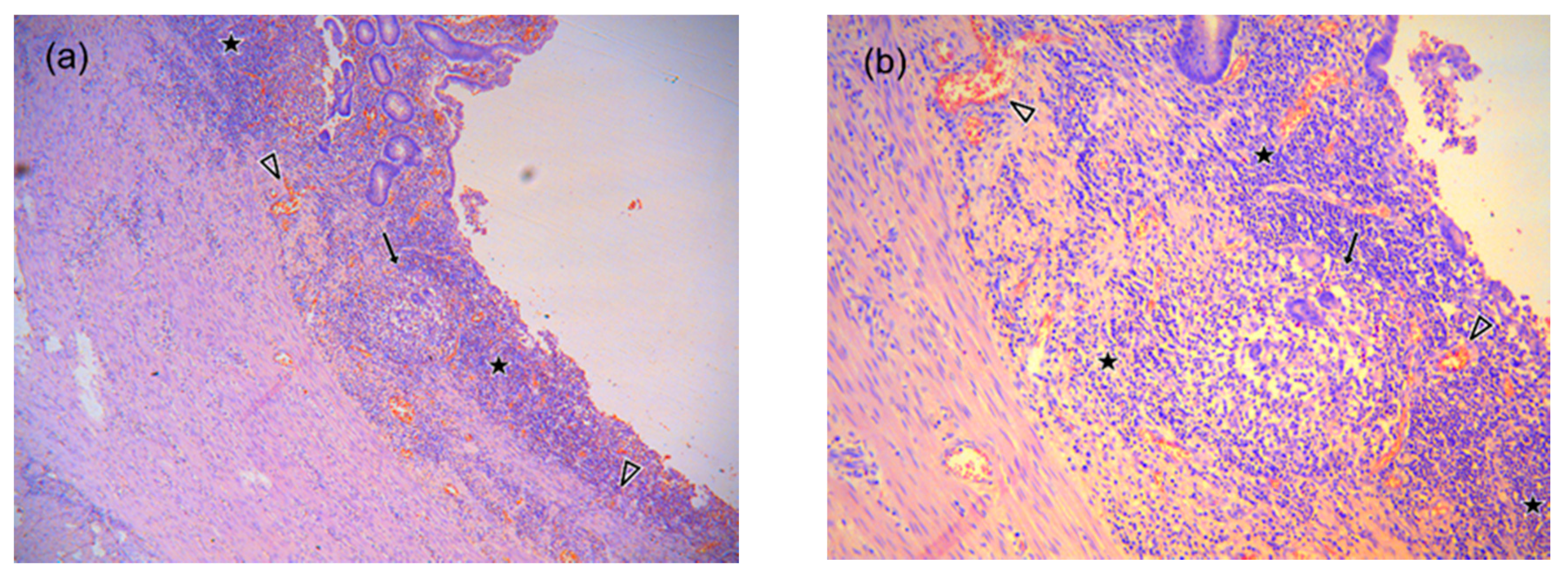
2.3. MR Image Analysis
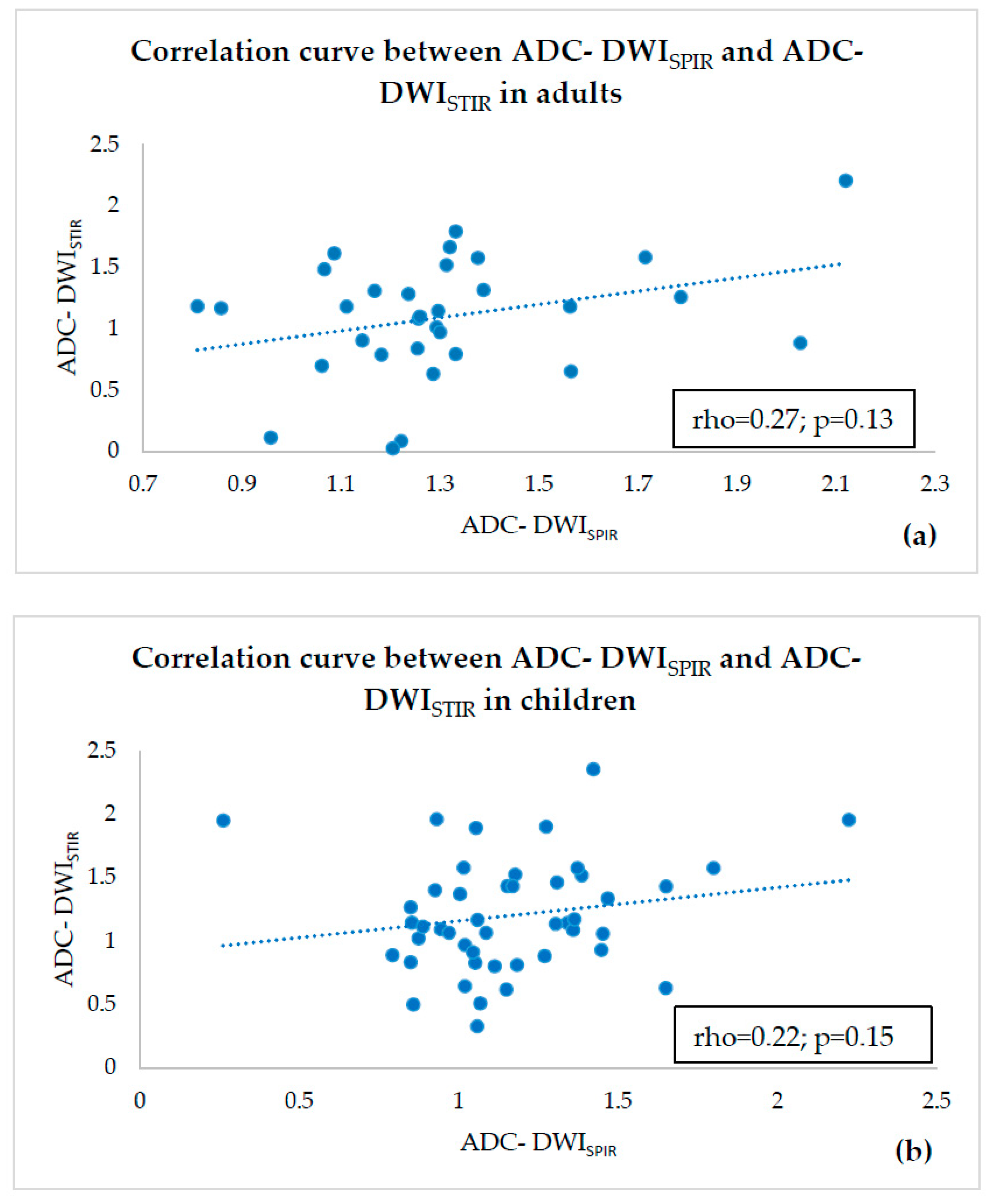
2.4. Statistical Analysis
3. Results
4. Discussion
- (1)
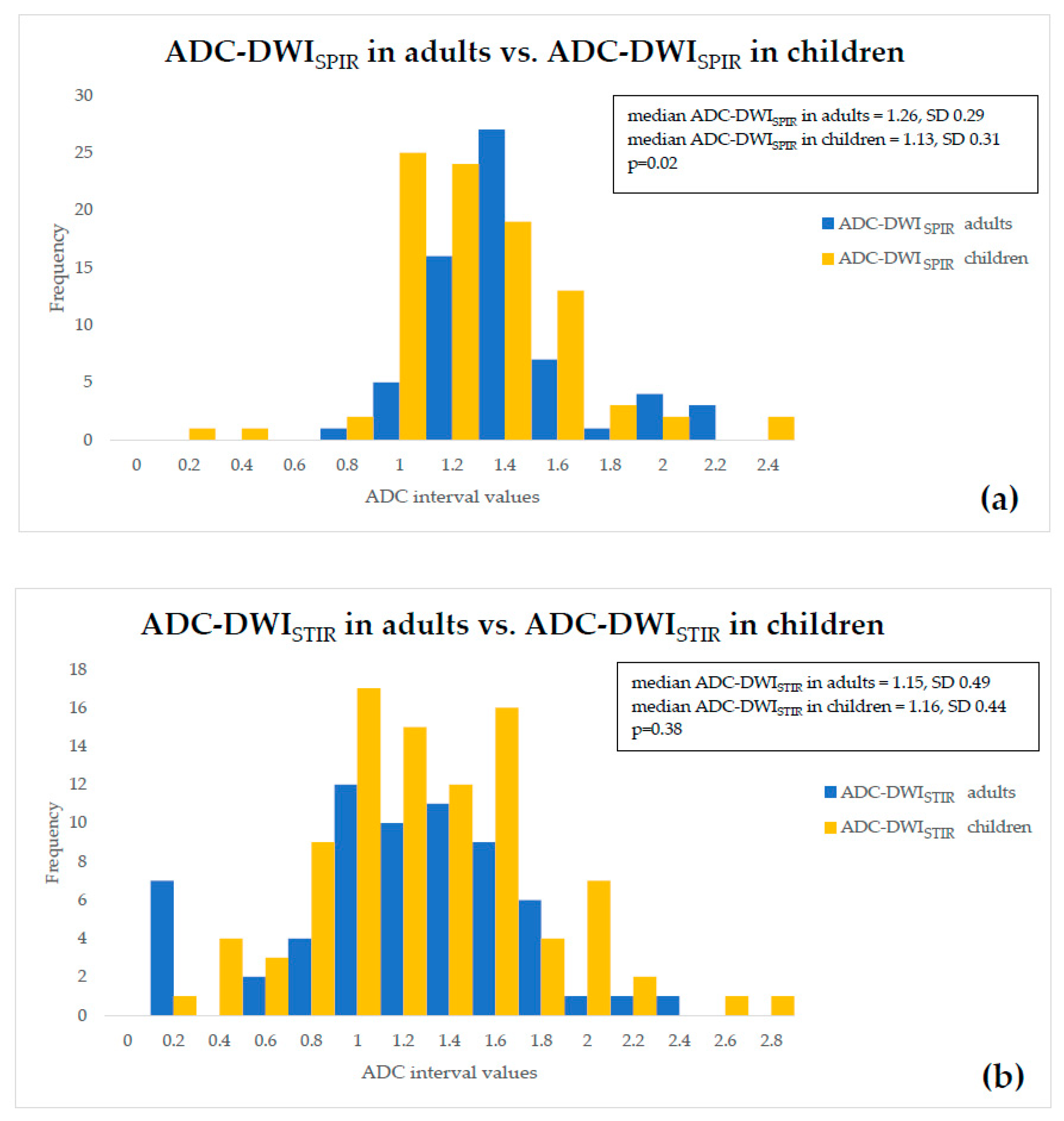
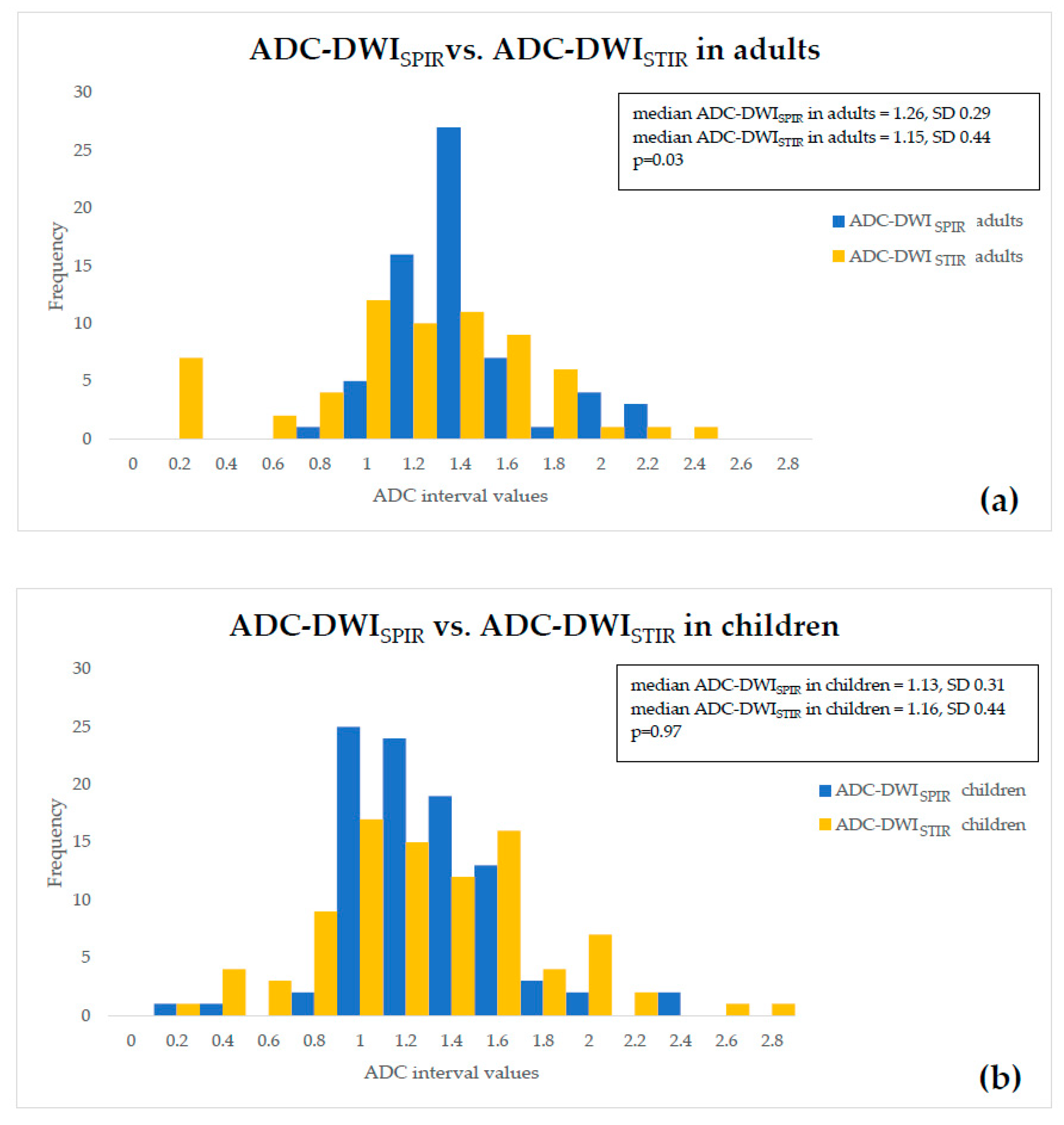
- ADC values of both adults and children were compared within a single fat suppression technique, and we observed statistically significant ADC-DWISPIR difference between adults and children (1.31 × 10−3 mm2/s, SD 0.29, vs. 1.16 × 10−3 mm2/s, SD 0.31; p = 0.02), with 12.12% lower ADC values in children compared to adults, but no statistically significant difference between the ADC-DWISTIR values in adults and children (1.09 × 10−3 mm2/s, SD 0.49, vs. 1.20 mm2/s × 10−3, SD 0.44; p = 0.38);
- (2)
- both DWISPIR and DWISTIR techniques were compared within one patient group, both in adults and children. In this case, the analysis showed difference of 16.73% between ADC-DWISPIR and ADC-DWISTIR values in adults, being lower in DWISTIR (1.31 × 10−3 mm2/s, SD 0.29, vs. 1.09 × 10−3 mm2/s, SD 0.49; p = 0.03), but did not show difference between ADC-DWISPIR and ADC-DWISTIR values in the children’s group (1.16 mm2/s × 10−3, SD 0.31, vs. 1.20 × 10−3 mm2/s, SD 0.44, p = 0.97).
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dulai, P.S.; Singh, S.; Cesarini, M.; Bouguen, G.; Nelson, S.A.; Peyrin-Biroulet, L.; Feagan, B.G.; Ordas, I.; Sandborn, W.J.; Santillan, C.; et al. Cochrane Database of Systematic Reviews MRI scoring indices for evaluation of disease activity and severity in Crohn ’ s disease (Methodology Protocol). Cochrane Database Syst. Rev. 2015, 4, 1–8. [Google Scholar]
- Cosnes, J.; Gowerrousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef]
- Sakuraba, H.; Ishiguro, Y.; Hasui, K.; Hiraga, H.; Fukuda, S.; Shibutani, K.; Takai, Y. Prediction of maintained mucosal healing in patients with Crohn’s disease under treatment with infliximab using diffusion-weighted magnetic resonance imaging. Digestion 2014, 89, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn’s Colitis 2019, 13, 144K–164K. [Google Scholar] [CrossRef] [PubMed]
- Rozendorn, N.; Amitai, M.M.; Eliakim, R.A.; Kopylov, U.; Klang, E. A review of magnetic resonance enterography-based indices for quantification of Crohn’s disease inflammation. Therap. Adv. Gastroenterol. 2018, 11, 1756284818765956. [Google Scholar] [CrossRef] [PubMed]
- Dohan, A.; Taylor, S.; Hoeffel, C.; Barret, M.; Allez, M.; Dautry, R.; Zappa, M.; Savoye-Collet, C.; Dray, X.; Boudiaf, M.; et al. Diffusion-Weighted MRI in Crohn’s Disease: Current Status and Recommendations. J. Magn. Reson. Imaging 2016, 44, 1381–1396. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rodriguez, J.; Lai, S.; Ehst, B.D.; Fine, D.M.; Bluemke, D.A. Nephrogenic Systemic Fibrosis: Incidence, Associations, and Effect of Risk Factor Assessment—Report of 33 Cases. Radiology 2009, 250, 371–377. [Google Scholar] [CrossRef]
- Gulani, V.; Calamante, F.; Shellock, F.G.; Kanal, E.; Reeder, S.B. Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol. 2017, 16, 564–570. [Google Scholar] [CrossRef]
- Quattrocchi, C.C.; van der Molen, A.J. Gadolinium Retention in the Body and Brain: Is It Time for an International Joint Research Effort? Radiology 2017, 282, 12–16. [Google Scholar] [CrossRef]
- Baliyan, V.; Das, C.J.; Sharma, R.; Gupta, A.K. Diffusion weighted imaging: Technique and applications. World J. Radiol. 2016, 8, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Oto, A.; Kayhan, A.; Williams, J.T.B.; Fan, X.; Yun, L.; Arkani, S.; Rubin, D.T. Active Crohn’s disease in the small bowel: Evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J. Magn. Reson. Imaging 2011, 33, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Sirin, S.; Kathemann, S.; Schweiger, B.; Hahnemann, M.L.; Forsting, M.; Lauenstein, T.C.; Kinner, S. Magnetic resonance colonography including diffusion-weighted imaging in children and adolescents with inflammatory bowel disease: Do we really need intravenous contrast? Investig. Radiol. 2015, 50, 32–39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dubron, C.; Avni, F.; Boutry, N.; Turck, D.; Duhamel, A.; Amzallag-Bellenger, E. Prospective evaluation of free-breathing diffusion weighted imaging for the detection of inflammatory bowel disease with MR enterography in childhood population. Br. J. Radiol. 2016, 89, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, H.; Pabst, T.; Dick, A.; MacHann, W.; Evangelista, L.; Wirth, C.; Köstler, H.; Hahn, D.; Beer, M. Small-bowel MRI in children and young adults with Crohn disease: Retrospective head-to-head comparison of contrast-enhanced and diffusion-weighted MRI. Pediatr. Radiol. 2013, 43, 103–114. [Google Scholar] [CrossRef]
- Oto, A.; Zhu, F.; Kulkarni, K.; Karczmar, G.S.; Turner, J.R.; Rubin, D. Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn’s disease. Acad. Radiol. 2009, 16, 597–603. [Google Scholar] [CrossRef]
- Buisson, A.; Joubert, A.; Montoriol, P.F.; Ines, D.D.; Hordonneau, C.; Pereira, B.; Garcier, J.M.; Bommelaer, G.; Petitcolin, V. Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn’s disease. Aliment. Pharmacol. Ther. 2013, 37, 537–545. [Google Scholar] [CrossRef]
- Kim, K.-J.; Lee, Y.; Park, S.H.; Kang, B.-K.; Seo, N.; Yang, S.-K.; Ye, B.D.; Park, S.H.; Kim, S.Y.; Baek, S.; et al. Diffusion-weighted MR Enterography for Evaluating Crohn’s Disease: How Does It Add Diagnostically to Conventional MR Enterography? Inflamm. Bowel Dis. 2015, 21, 101–109. [Google Scholar] [CrossRef]
- Kopylov, U.; Klang, E.; Yablecovitch, D.; Lahat, A.; Avidan, B.; Neuman, S.; Levhar, N.; Greener, T.; Rozendorn, N.; Beytelman, A.; et al. Magnetic resonance enterography versus capsule endoscopy activity indices for quantification of small bowel inflammation in Crohn’s disease. Therap. Adv. Gastroenterol. 2016, 9, 655–663. [Google Scholar] [CrossRef]
- Hordonneau, C.; Buisson, A.; Scanzi, J.; Goutorbe, F.; Pereira, B.; Borderon, C.; Da Ines, D.; Montoriol, P.F.; Garcier, J.M.; Boyer, L.; et al. Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: Validation of quantitative index of activity. Am. J. Gastroenterol. 2014, 109, 89–98. [Google Scholar] [CrossRef]
- Drake-Pérez, M.; Boto, J.; Fitsiori, A.; Lovblad, K.; Vargas, M.I. Clinical applications of diffusion weighted imaging in neuroradiology. Insights Imaging 2018, 9, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Javier Sánchez-González, J.L.-M. Diffusion-Weighted Imaging: Acquisition and Biophysical Basis. In Diffusion MRI Outside the Brain: A Case-Based Review and Clinical Applications; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–15. [Google Scholar]
- Kwee, T.C.; Takahara, T.; Ochiai, R.; Katahira, K.; Van Cauteren, M.; Imai, Y.; Nievelstein, R.A.J.; Luijten, P.R. Whole-body diffusion-weighted magnetic resonance imaging. Eur. J. Radiol. 2009, 70, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Del Grande, F.; Santini, F.; Aro, M.R.; Gold, G.E.; Carrino, J.A. Fat-Suppression Techniques for 3-T MR Imaging of the Musculosceletal system. Radiographics 2014, 34, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Indrati, R. Comparing SPIR and SPAIR Fat Suppression Techniques in Magnetic Resonance Imaging (MRI) of Wrist Joint. J. Med. Sci. Clin. Res. 2017, 05, 23180–23185. [Google Scholar] [CrossRef]
- Horger, W. Fat Suppression in the Abdomen (Siemens). MAGNETOM Flash 2007, 3, 114–119. [Google Scholar]
- Takahara, T.; Imai, Y.; Yamashita, T.; Yasuda, S.; Nasu, S.; Van Cauteren, M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): Technical improvement using free breathing, STIR and high resolution 3D display. Radiat. Med. 2004, 22, 275–282. [Google Scholar] [PubMed]
- Moore, W.A.; Khatri, G.; Madhuranthakam, A.J.; Sims, R.D.; Pedrosa, I. Added value of diffusion-weighted acquisitions in MRI of the abdomen and pelvis. Am. J. Roentgenol. 2014, 202, 995–1006. [Google Scholar] [CrossRef][Green Version]
- Ouyang, Z.; Ouyang, Y.; Zhu, M.; Lu, Y.; Zhang, Z.; Shi, J.; Li, X.; Ren, G. Diffusion-weighted imaging with fat suppression using short-tau inversion recovery: Clinical utility for diagnosis of breast lesions. Clin. Radiol. 2014, 69, e337–e344. [Google Scholar] [CrossRef]
- Kwee, T.C.; Takahara, T.; Ochiai, R.; Nievelstein, R.A.J.; Luijten, P.R. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): Features and potential applications in oncology. Eur. Radiol. 2008, 18, 1937–1952. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Salomonowitz, E.; Bernt, R.; Haller, J.; Gruber, S.; Bogner, W.; Pinker, K.; van der Riet, W. Diffusion-weighted MR imaging with background body signal suppression (DWIBS) for the diagnosis of malignant and benign breast lesions. Eur. Radiol. 2009, 19, 2349–2356. [Google Scholar] [CrossRef]
- “American College of Radiology, WWW.ACR.ORG,” ACR–SAR–SPR Practice Parameter for the Performance of Magnetic Resonance (MR) Enterography. Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/MR-Enterog.pdf (accessed on 1 March 2020).
- Sinha, R.; Rajiah, P.; Ramachandran, I.; Sanders, S.; Murphy, P.D. Diffusion-weighted MR imaging of the gastrointestinal tract: Technique, indications, and imaging findings. Radiographics 2013, 33, 655–676. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, S.H. Bowel Inflammation in Crohn Disease. Am. J. Roentgenol. 2016, 207, 40–48. [Google Scholar] [CrossRef]
- Kiryu, S.; Dodanuki, K.; Takao, H.; Watanabe, M.; Inoue, Y.; Takazoe, M.; Sahara, R.; Unuma, K.; Ohtomo, K. Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn’s disease. J. Magn. Reson. Imaging 2009, 29, 880–886. [Google Scholar] [CrossRef]
- Kwee, T.C.; Takahara, T.; Koh, D.M.; Nievelstein, R.A.J.; Luijten, P.R. Comparison and reproducibility of ADC measurements in breathhold, respiratory triggered, and free-breathing diffusion-weighted MR imaging of the liver. J. Magn. Reson. Imaging 2008, 28, 1141–1148. [Google Scholar] [CrossRef]
- Jeong, K.K.; Kyoung, A.K.; Park, B.W.; Kim, N.; Cho, K.S. Feasibility of diffusion-weighted imaging in the differentiation of metastatic from nonmetastatic lymph nodes: Early experience. J. Magn. Reson. Imaging 2008, 28, 714–719. [Google Scholar]
- Stone, A.J.; Browne, J.E.; Lennon, B.; Meaney, J.F.; Fagan, A.J. Effect of motion on the ADC quantification accuracy of whole-body DWIBS. Magn. Reson. Mater. Phys. Biol. Med. 2012, 25, 263–266. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rimola, J.; Rodriguez, S.; García-Bosch, O.; Ordás, I.; Ayala, E.; Aceituno, M.; Pellisé, M.; Ayuso, C.; Ricart, E.; Donoso, L.; et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut 2009, 58, 1113–1120. [Google Scholar] [CrossRef]
- Moy, M.P.; Sauk, J.; Gee, M.S. The role of MR enterography in assessing Crohn’s disease activity and treatment response. Gastroenterol. Res. Pract. 2016, 2016, 8168695. [Google Scholar] [CrossRef] [PubMed]
- Nardone, O.M.; Iacucci, M.; Cannatelli, R.; Zardo, D.; Ghosh, S. Can advanced endoscopic techniques for assessment of mucosal inflammation and healing approximate histology in inflammatory bowel disease? Ther. Adv. Gastroenterol. 2019, 12, 1756284819863015. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, F.; Stasi, E.; Bevivino, G.; Scarozza, P.; Biancone, L.; Zuzzi, S.; Rossi, C.; Pallone, F.; Calabrese, E. A Sonographic Lesion Index for Crohn’s Disease Helps Monitor Changes in Transmural Bowel Damage During Therapy. Clin. Gastroenterol. Hepatol. 2014, 12, 2071–2077. [Google Scholar] [CrossRef]
- Civitelli, F.; Nuti, F.; Oliva, S.; Messina, L.; La Torre, G.; Viola, F.; Cucchiara, S.; Aloi, M. Looking beyond Mucosal Healing: Effect of Biologic Therapy on Transmural Healing in Pediatric Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 2418–2424. [Google Scholar] [CrossRef] [PubMed]
- Serban, E.D. Treat-to-target in Crohn’s disease: Will transmural healing become a therapeutic endpoint? World J. Clin. Cases 2018, 6, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; Imperatore, N.; Testa, A.; De Palma, G.D.; Nardone, O.M.; Pellegrini, L.; Caporaso, N.; Rispo, A. One-year clinical outcomes with biologics in Crohn’s disease: Transmural healing compared with mucosal or no healing. Aliment. Pharmacol. Ther. 2019, 49, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- Ordás, I.; Rimola, J.; Alfaro, I.; Rodríguez, S.; Castro-Poceiro, J.; Ramírez-Morros, A.; Gallego, M.; Giner, À.; Barastegui, R.; Fernández-Clotet, A.; et al. Development and Validation of a Simplified Magnetic Resonance Index of Activity for Crohn’s Disease. Gastroenterology 2019, 157, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Steward, M.J.; Punwani, S.; Proctor, I.; Adjei-Gyamfi, Y.; Chatterjee, F.; Bloom, S.; Novelli, M.; Halligan, S.; Rodriguez-Justo, M.; Taylor, S.A. Non-perforating small bowel Crohn’s disease assessed by MRI enterography: Derivation and histopathological validation of an MR-based activity index. Eur. J. Radiol. 2012, 81, 2080–2088. [Google Scholar] [CrossRef]
- Buisson, A.; Hordonneau, C.; Goutte, M.; Boyer, L.; Pereira, B.; Bommelaer, G. Diffusion-weighted magnetic resonance imaging is effective to detect ileocolonic ulcerations in Crohn’s disease. Aliment. Pharmacol. Ther. 2015, 42, 452–460. [Google Scholar] [CrossRef]
- Buisson, A.; Hordonneau, C.; Goutorbe, F.; Allimant, C.; Goutte, M.; Reymond, M.; Pereira, B.; Bommelaer, G. Bowel wall healing assessed using magnetic resonance imaging predicts sustained clinical remission and decreased risk of surgery in Crohn’s disease. J. Gastroenterol. 2018, 54, 312–320. [Google Scholar] [CrossRef]
- Chilla, G.S.; Tan, C.H.; Xu, C.; Poh, C.L. Diffusion weighted magnetic resonance imaging and its recent trend-a survey. Quant. Imaging Med. Surg. 2015, 5, 407–422. [Google Scholar] [PubMed]
- Novacek, G.; Gröchenig, H.P.; Haas, T.; Wenzl, H.; Steiner, P.; Koch, R.; Feichtenschlager, T.; Eckhardt, G.; Mayer, A.; Kirchgatterer, A.; et al. Diagnostic delay in patients with inflammatory bowel disease in Austria. Wien. Klin. Wochenschr. 2019, 131, 104–112. [Google Scholar] [CrossRef]
- Caruso, A.; D’Incà, R.; Scarpa, M.; Manfrin, P.; Rudatis, M.; Pozza, A.; Angriman, I.; Buda, A.; Sturniolo, G.C.; Lacognata, C. Diffusion-weighted magnetic resonance for assessing ileal Crohn’s disease activity. Inflamm. Bowel Dis. 2014, 20, 1575–1583. [Google Scholar] [CrossRef]
- Kinner, S.; Blex, S.; Maderwald, S.; Forsting, M.; Gerken, G.; Lauenstein, T.C. Addition of diffusion-weighted imaging can improve diagnostic confidence in bowel MRI. Clin. Radiol. 2014, 69, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Ninivaggi, V.; Missere, M.; Restaino, G.; Gangemi, E.; Di Matteo, M.; Pierro, A.; Sallustio, G.; Bonomo, L. MR-enterography with diffusion weighted imaging: ADC values in normal and pathological bowel loops, a possible threshold ADC value to differentiate active from inactive Crohn’s disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4540–4546. [Google Scholar] [PubMed]
- Tielbeek, J.A.W.; Ziech, M.L.W.; Li, Z.; Lavini, C.; Bipat, S.; Bemelman, W.A.; Roelofs, J.J.T.H.; Ponsioen, C.Y.; Vos, F.M.; Stoker, J. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur. Radiol. 2014, 24, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, F.; Liu, F.; He, W.; Tian, J.; Han, H.; Cao, P. Identifying the inflammatory and fibrotic bowel stricture: MRI diffusion-weighted imaging in Crohn’s disease. Radiol. Infect. Dis. 2015, 2, 128–133. [Google Scholar] [CrossRef]
- Morani, A.C.; Smith, E.A.; Ganeshan, D.; Dillman, J.R. Diffusion-Weighted MRI in Pediatric Inflammatory Bowel Disease. Am. J. Roentgenol. 2015, 204, 1269–1277. [Google Scholar] [CrossRef]
- Geboes, K. Histopathology of Crohn’s Disease and Ulcerative Colitis. In Inflammatory Bowel Diseases; Satsangi, J.S.L., Ed.; Churchill-Livingstone Elsevier: Edinburgh, Scotland; London, UK; Melbourne, Australia, 2003; Volume 18, pp. 255–276. [Google Scholar]
- Feakins, R.M. Inflammatory bowel disease biopsies: Updated British Society of Gastroenterology reporting guidelines. J. Clin. Pathol. 2013, 66, 1005–1026. [Google Scholar] [CrossRef]
- Magro, F.; Langner, C.; Driessen, A.; Ensari, A.; Geboes, K.; Mantzaris, G.J.; Villanacci, V.; Becheanu, G.; Nunes, P.B.; Cathomas, G.; et al. European consensus on the histopathology of inflammatory bowel disease. J. Crohn’s Colitis 2013, 7, 827–851. [Google Scholar] [CrossRef]
- Roger, M.; Feakins, N.A.S. An update on the pathology of chronic inflammatory bowel disease. In Recent Advances in Histopathology 23; Massimo Pignatelli, P.G., Ed.; JP Medical Ltd.: London, UK, 2014; pp. 117–134. ISBN 9781907816857. [Google Scholar]
- Robert Riddell, D.J. Gastrointestinal Pathology and its Clinical Implications, 2nd ed.; Lippincott Williams & Wilkins, Ed.; Wolters Kluwer: Philadelphia, PA, USA, 2014; Volume II, ISBN 9780781722162. [Google Scholar]
- Li, X.A.; Zhou, Y.; Zhou, S.X.; Liu, H.R.; Xu, J.M.; Gao, L.; Yu, X.J.; Li, X.H. Histopathology of melanosis coli and determination of its associated genes by comparative analysis of expression microarrays. Mol. Med. Rep. 2015, 12, 5807–5815. [Google Scholar] [CrossRef]
- Lambert, J.R.; Luk, S.C.; Pritzker, K.P. Brown bowel syndrome in Crohn’ disease. Arch. Pathol. Lab. Med. 1980, 104, 201–205. [Google Scholar]
- Liu, Z.H.; Fooo, D.C.C.; Law, W.L.; Chan, F.S.Y.; Fan, J.K.M.; Peng, J.S. Melanosis coli: Harmless pigmentation? A case-control retrospective study of 657 cases. PLoS ONE 2017, 12, e0186668. [Google Scholar] [CrossRef]
- González Ballester, M.Á.; Zisserman, A.P.; Brady, M. Estimation of the partial volume effect in MRI. Med. Image Anal. 2002, 6, 389–405. [Google Scholar] [CrossRef]
- Scherrer, B.; Gholipour, A.; Warfield, S.K. Super-Resolution in Diffusion-Weighted Imaging Benoit. Med. Image Comput. Comput. Assist. Interv. 2011, 14, 124–132. [Google Scholar] [PubMed]
- Choi, S.H.; Kim, K.W.; Lee, J.Y.; Kim, K.J.; Park, S.H. Diffusion-weighted Magnetic Resonance Enterography for Evaluating Bowel Inflammation in Crohn’s Disease: A Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2016, 22, 669–679. [Google Scholar] [CrossRef]
- Aggarwal, R.; Ranganathan, P. Common pitfalls in statistical analysis: The use of correlation techniques. Perspect. Clin. Res. 2016, 7, 187–190. [Google Scholar]
- Apine, I.; Pirksta, I.; Pitura, R.; Pokrotnieks, J.; Puíîte, I.; Krûmiòa, G. Repeatability of Magnetic Resonance Measurements Used for Estimating Crohn’s Disease Activity. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2020, 74, 75–82. [Google Scholar] [CrossRef]

| Scanning Protocol | DWISPIR 1 | DWISTIR (DWIBS)_ 2 |
|---|---|---|
| Sequence | SE-EPI 3 | STIR-EPI 4 |
| Mode | Single shot | Single shot |
| Coil | SENSE 5 body | SENSE body |
| Slice orientation | Axial | Axial |
| FOV 6 | RL 7 400 mm, AP 8 350 mm, FH 9 303 mm | RL 400 mm, AP 350 mm, FH 303 mm |
| ACQ 10 voxel size | RL 3.03 mm × AP 3.57 mm × slice thickness 6 mm | RL 2.50 mm × AP 2.98 mm × slice thickness 6 mm |
| Reconstruction voxel size | RL 1.79 mm × AP 1.79 mm × slice thickness 6 mm | RL 1.39 mm × AP 1.39 mm × slice thickness 6 mm |
| Fold-over suppression | No | No |
| Reconstruction matrix | 224 | 288 |
| SENSE | Yes | Yes |
| P reduction (AP) | 2 | 2.5 |
| Number of stacks | 1 | 1 |
| Type | Parallel | Parallel |
| Slices | 46 | 46 |
| Slice gap (mm) | 0.6 | 0.6 |
| Slice orientation | Transverse | Transverse |
| Fold-over direction | AP | AP |
| Fat shift direction | A | P |
| TE 11 | 66 ms | 78 ms |
| TR 12 | 1426 ms | 7055 ms |
| TI 13 | - | 180 ms |
| Fast imaging mode | EPI 14 | EPI |
| Flip angle | 90° | |
| Fat suppression | SPIR | STIR |
| b factors | 0, 600, 800 s/mm2 | 0, 600, 800 s/mm2 |
| Respiratory compensation | Trigger | No |
| Number of signal averages | 3 | 5 |
| Total scan time | 4 min. 12 s | 5 min. 56 s |
| Measurement | N | Minimum Value | Maximum Value | Median Value | SD |
|---|---|---|---|---|---|
| ADC-DWISPIR (mm2/s), adults | 32 | 0.66 × 10−3 | 2.16 × 10−3 | 1.26 × 10−3 | 0.29 |
| ADC-DWISPIR (mm2/s), children | 46 | 0.18 × 10−3 | 2.23 × 10−3 | 1.13 × 10−3 | 0.31 |
| ADC-DWISTIR (mm2/s), adults | 32 | 0.01 × 10−3 | 2.37 × 10−3 | 1.15 × 10−3 | 0.49 |
| ADC-DWISTIR (mm2/s), children | 46 | 0.20 × 10−3 | 2.74 × 10−3 | 1.16 × 10−3 | 0.44 |
| MaRIA, adults | 32 | 10.65 | 36.65 | 24.43 | 5.31 |
| MaRIA, children | 46 | 9.96 | 37.67 | 22.08 | 6.67 |
| DWISPIR-based Clermont score, adults | 32 | 12.85 | 39.23 | 26.23 | 4.76 |
| DWISPIR-based Clermont score, children | 46 | 13.59 | 40.74 | 23.53 | 5.42 |
| DWISTIR-based Clermont score, adults | 32 | 5.92 | 38.78 | 24.28 | 4.65 |
| DWISTIR-based Clermont score, children | 46 | 8.25 | 39.52 | 24.39 | 5.77 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apine, I.; Pitura, R.; Franckevica, I.; Pokrotnieks, J.; Krumina, G. Comparison between Diffusion-Weighted Sequences with Selective and Non-Selective Fat Suppression in the Evaluation of Crohn’s Disease Activity: Are They Equally Useful? Diagnostics 2020, 10, 347. https://doi.org/10.3390/diagnostics10060347
Apine I, Pitura R, Franckevica I, Pokrotnieks J, Krumina G. Comparison between Diffusion-Weighted Sequences with Selective and Non-Selective Fat Suppression in the Evaluation of Crohn’s Disease Activity: Are They Equally Useful? Diagnostics. 2020; 10(6):347. https://doi.org/10.3390/diagnostics10060347
Chicago/Turabian StyleApine, Ilze, Reinis Pitura, Ivanda Franckevica, Juris Pokrotnieks, and Gaida Krumina. 2020. "Comparison between Diffusion-Weighted Sequences with Selective and Non-Selective Fat Suppression in the Evaluation of Crohn’s Disease Activity: Are They Equally Useful?" Diagnostics 10, no. 6: 347. https://doi.org/10.3390/diagnostics10060347
APA StyleApine, I., Pitura, R., Franckevica, I., Pokrotnieks, J., & Krumina, G. (2020). Comparison between Diffusion-Weighted Sequences with Selective and Non-Selective Fat Suppression in the Evaluation of Crohn’s Disease Activity: Are They Equally Useful? Diagnostics, 10(6), 347. https://doi.org/10.3390/diagnostics10060347





