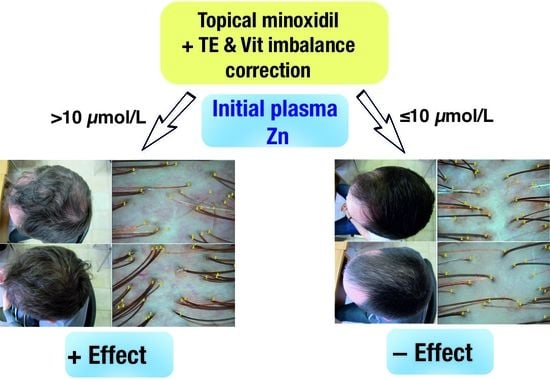
Plasma Zinc Levels in Males with Androgenetic Alopecia as Possible Predictors of the Subsequent Conservative Therapy’s Effectiveness
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Trichograms Analysis
2.3. Conservative Treatment algorithm for AGA
2.4. Studying of the Genetic and Non-Genetic Factors Considered as Possible Predictors of the Effectiveness of Conservative Treatment
2.5. Statistical Analysis
3. Results
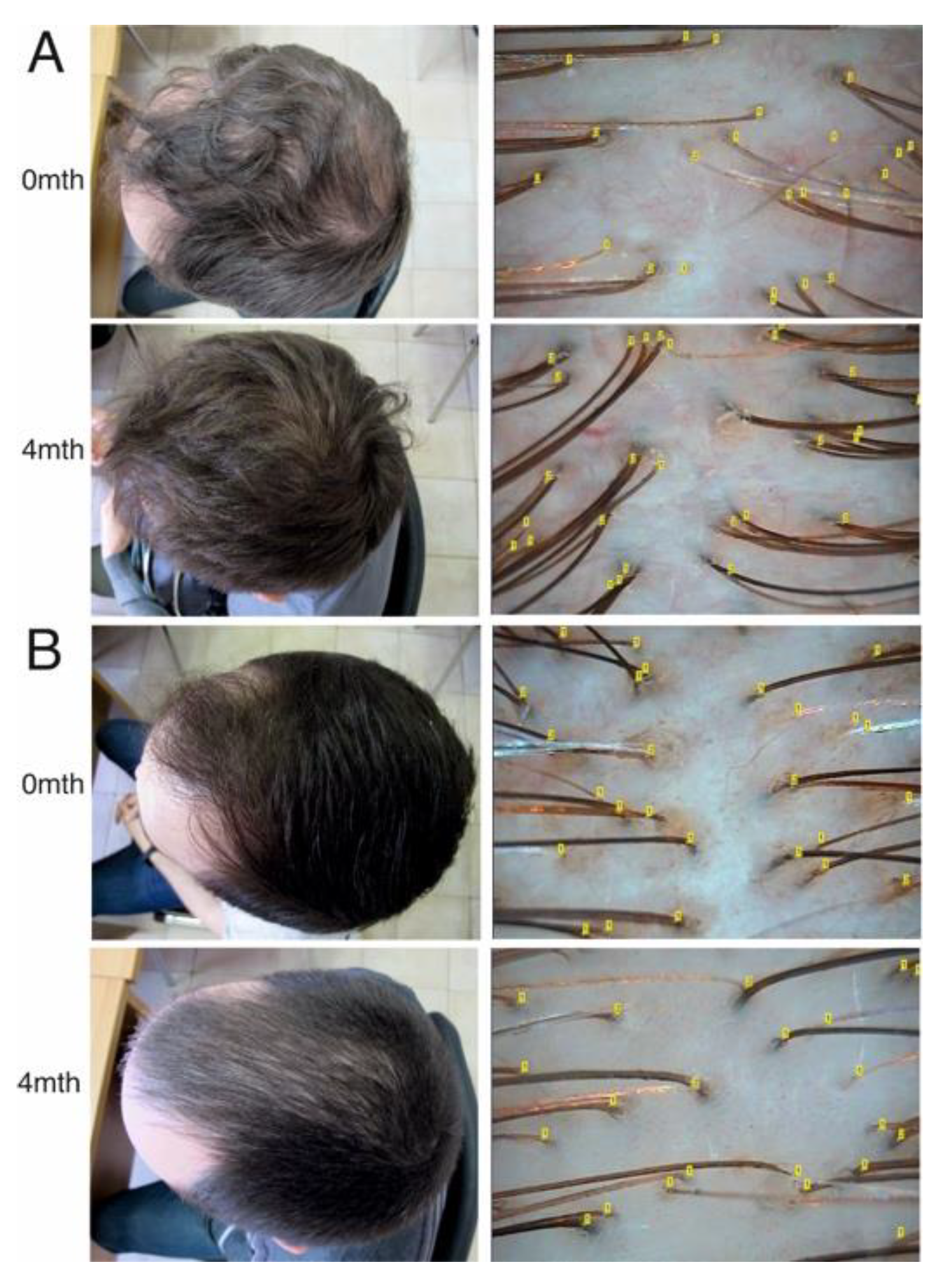
3.1. Clinical Characteristics of AGA and an Assessment of the Effectiveness of Conservative Therapy
3.2. Analysis of Genetic Values in the Positive Effect and Absence of Effect Groups of Patients with AGA
3.3. Analysis of Non-Genetic Values in Positive Effect and Absence of Effect Groups of Patients with AGA
3.4. Assessing the Predictive Effectiveness of Zn in Determining the Response to Conservative AGA Therapy
3.5. Zn Correlation with Other Trace Elements and Vitamins
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGA | Androgenic alopecia |
| MLP | Multilayer perceptron |
| SNPs | Single-nucleotide polymorphisms |
| TE | Trace elements |
References
- Severi, G.; Sinclair, R.; Hopper, J.L.; English, D.R.; McCredie, M.R.E.; Boyle, P.; Giles, G.G. Androgenetic alopecia in men aged 40–69 years: Prevalence and risk factors. Br. J. Dermatol. 2003, 149, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Kondrakhina, I.N.; Verbenko, D.A.; Zatevalov, A.M.; Kubanov, A.A.; Deryabin, D.G. The value of genetic and non-genetic factors in the occurrence and development of androgenetic alopecia in men: Multifactor analysis. Ann. Russ. Acad. Med. Sci. 2019, 74, 167–175. [Google Scholar] [CrossRef]
- Marcińska, M.; Pośpiech, E.; Abidi, S.; Andersen, J.D.; van den Berge, M.; Carracedo, Á. Evaluation of DNA Variants Associated with Androgenetic Alopecia and Their Potentialto Predict Male Pattern Baldness. PLoS ONE 2015, 10, e0127852. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, A.; Kousar, S.; Ali, A.; Hussain, T.; Babar, M.E.; Ali, Y. Androgen Receptor (AR) gene polymorphism rs6152 is associated with androgenetic alopecia. Biomed. Lett. 2019, 5, 120–125. [Google Scholar]
- Rhie, A.; Son, H.-Y.; Kwak, S.J.; Lee, S.; Kim, D.Y.; Lew, B.-L.; Jo, S.J. Genetic variations associated with response to dutasteride in the treatment of male subjects with androgenetic alopecia. PLoS ONE 2019, 14, e0222533. [Google Scholar] [CrossRef]
- Kaufman, K.D. Androgens and alopecia. Mol. Cell. Endocrinol. 2002, 198, 89–95. [Google Scholar] [CrossRef]
- Goldberg, L.J.; Lenzy, Y.L. Nutrition and Hair. Clin. Dermatol. 2010, 28, 412–419. [Google Scholar] [CrossRef]
- Guo, E.L.; Katta, R. Diet and hair loss: Effects of nutrient deficiency and supplement use. Dermatol. Pract. Concept. 2017, 7, 1. [Google Scholar] [CrossRef]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The Role of Vitamins and Minerals in Hair Loss: A Review. Dermatol. Ther. (Heidelb.) 2019, 9, 51–70. [Google Scholar] [CrossRef]
- Kondrakhina, I.N.; Verbenko, D.A.; Zatevalov, A.M.; Kubanov, A.A.; Deryabin, D.G. SNP variation in male pattern hair loss in Russians with different dihydrotestosterone levels. Meta Gene 2019, 19, 219–224. [Google Scholar] [CrossRef]
- Rose, P.T. Advances in Hair Restoration. Dermatol Clin. 2018, 36, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zito, P.M.; Raggio, B.S. Hair Transplantation. [Updated 2020 Apr 14]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547740/ (accessed on 22 May 2020).
- Yepuri, V.; Mysore, V. Effect of Hormones on Hair. In Hair Transplantation, 1th ed.; Mysore, V., Ed.; JP Medical Ltd: New Delhi, India, 2016; Chapter 4; pp. 20–23. [Google Scholar]
- Wolff, H.; Fischer, T.W.; Blume-Peytavi, U. The diagnosis and treatment of hair and scalp diseases. Deutsch. Ärztebl. Int. 2016, 113, 377. [Google Scholar] [CrossRef] [PubMed]
- Bayer, M.; Gahrtz, M.; Voss, W.; Schlippe, G.; Whitfield, T. The Effect of a Food Supplement and a Hair Lotion on the Progression of Androgenetic Alopecia. J. Cosmet. Dermatol. Sci. Appl. 2019, 9, 292–304. [Google Scholar] [CrossRef]
- Mysore, V.; Parthasaradhi, A.; Kharkar, R.D.; Ghoshal, A.K.; Ganjoo, A.; Ravichandran, G.; Saraswat, A.; Shah, Y.; Singh, M.; Remadevi, T.J.; et al. Expert consensus on the management of Telogen Effluvium in India. Int. J. Trichol. 2019, 11, 107–112. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Dev. Ther. 2019, 13, 2777. [Google Scholar] [CrossRef]
- Alhaj, E.; Alhaj, N.; Alhaj, N.E. Diffuse Alopecia in a Child due to Dietary Zinc Deficiency. SKINmed Dermatol. Clin. 2007, 6, 199–200. [Google Scholar] [CrossRef]
- Park, H.; Kim, C.W.; Kim, S.S.; Park, C.W. The therapeutic effect and the changed serum zinc level after zinc supplementation in alopecia areata patients who had a low serum zinc level. Ann. Dermatol. 2009, 21, 142–146. [Google Scholar] [CrossRef]
- Beoy, L.A.; Woei, W.J.; Hay, Y.K. Effects of tocotrienol supplementation on hair growth in human volunteers. Trop. Life Sci. Res. 2010, 21, 91. [Google Scholar]
- Jacquet, A.; Coolen, V.; Vandermander, J. Effect of dietary supplementation with INVERSION® femme on slimming, hair loss, and skin and nail parameters in women. Adv. Ther. 2007, 24, 1154–1171. [Google Scholar] [CrossRef]
- Rajput, R.J. Controlled clinical trial for evaluation of hair growth with low dose cyclical nutrition therapy in men and women without the use of finasteride. Plast. Aesthet. Res. 2017, 4, 161–173. [Google Scholar] [CrossRef]
- Declaration of Helsinki—WMA—The World Medical Association. Available online: https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/ (accessed on 22 April 2020).
- Subramanian, R.; Gayathri, S.; Rathnavel, C.; Raj, V. Analysis of mineral and heavy metals in some medicinal plants collected from local market. Asian Pac. J. Trop. Biomed. 2012, 2, S74–S78. [Google Scholar] [CrossRef]
- STATISTICA TIBCO Software v. 13. Available online: https://docs.tibco.com/products/tibco-statistica-13-3-0 (accessed on 13 May 2020).
- Wang, H.; Zheng, H. Positive Predictive Value. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Hara, T.; Takeda, T.A.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Kil, M.S.; Kim, C.W.; Kim, S.S. Analysis of Serum Zinc and Copper Concentrations in Hair Loss. Ann. Dermatol. 2013, 25, 405–409. [Google Scholar] [CrossRef] [PubMed]
- El-Esawy, F.M.; Hussein, M.S.; Mansour, A.I. Serum Biotin and Zinc in Male Androgenetic Alopecia. J. Cosmet. Dermatol. 2019, 18, 1546–1549. [Google Scholar] [CrossRef]
- Rahman, F.; Akhter, Q.S. Serum Zinc and Copper Levels in Alopecia. J. Bangladesh Soc. Physiol. 2019, 14, 21–25. [Google Scholar] [CrossRef]
- Surit, M.; Bhabani, S.S.; Ajaya, J.K.; Bikash, K.R. Evaluation of Serum Zinc, Iron Profile and Vitamin D in Females of Reproductive Age Group with Diffuse Hair Loss: A Case Control Study. Indian J. Clin. Dermatol. 2019, 2, 47–50. [Google Scholar]
- Ozturk, P.; Kurutas, E.; Ataseven, A.; Dokur, N.; Gumusalan, Y.; Gorur, A.; Tamer, L.; Inaloz, S. BMI and Levels of Zinc, Copper in Hair, Serum and Urine of Turkish Male Patients with Androgenetic Alopecia. J. Trace Elem. Med. Biol. 2014, 28, 266–270. [Google Scholar] [CrossRef]
- Tsai, F.M.; Wang, L.K.; Chen, M.L.; Lee, M.C.; Linand, Y.Y.; Wang, C.H. Induction of Cell Proliferation and Cell Death in Human Follicle Dermal Papilla Cells by Zinc Chloride. Indian J. Pharm. Sci. 2019, 81, 781–785. [Google Scholar] [CrossRef]
- Skalnaya, M.G.; Skalny, A.V. Essential Trace Elements in Human Health: A Physician’s View; House of Tomsk State University: Tomsk, Russia, 2018. [Google Scholar]
- Rossander-Hultén, L.; Brune, M.; Sandström, B.; Lönnerdal, B.; Hallberg, L. Competitive inhibition of iron absorption by manganese and zinc in humans. Am. J. Clin. Nutr. 1991, 54, 152–156. [Google Scholar] [CrossRef]
- Bunk, M.J.; Dnistrian, A.M.; Schwartz, M.K.; Rivlin, R.S. Dietary zinc deficiency decreases plasma concentrations of vitamin E. Proc. Soc. Exp. Biol. Med. 1989, 190, 379–384. [Google Scholar] [CrossRef]
- Gatiatulina, E.R.; Sheina, E.A.; Nemereshina, O.N.; Popova, E.V.; Polyakova, V.S.; Agletdinov, E.F.; Tinkov, A.A. Effect of Zn Supplementation on Trace Element Status in Rats with Diet-Induced Non-alcoholic Fatty Liver Disease. Biol. Trace Elem. Res. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Himoto, T.; Masaki, T. Associations between zinc deficiency and metabolic abnormalities in patients with chronic liver disease. Nutrients 2018, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The antioxidant role of selenium and selenocompounds. Biomed. Pharmacother. 2003, 57, 134–144. [Google Scholar] [CrossRef]
- Beckett, G.J.; Arthur, J.R. Selenium and Endocrine Systems. J. Endocrinol. 2005, 184, 455–465. [Google Scholar] [CrossRef]
- Prie, B.E.; Iosif, L.; Tivig, I.; Stoian, I.; Giurcaneanu, C. Oxidative Stress in Androgenetic Alopecia. J. Med. Life 2016, 9, 79–83. [Google Scholar]
- Upton, J.H.; Hannen, R.F.; Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Oxidative Stress-Associated Senescence in Dermal Papilla Cells of Men with Androgenetic Alopecia. J. Investig. Dermatol. 2015, 135, 1244–1252. [Google Scholar] [CrossRef]
- Bosch-Morell, F.; Mérida, S.; Navea, A. Oxidative stress in myopia. Oxidative Med. Cell. Longev. 2015, 2015, 750637. [Google Scholar]
- Nesari, A.; Mansouri, M.T.; Khodayar, M.J.; Rezaei, M. Preadministration of high-dose alpha-tocopherol improved memory impairment and mitochondrial dysfunction induced by proteasome inhibition in rat hippocampus. Nutr. Neurosci. 2019. [Google Scholar] [CrossRef]
- Sadiq, M.; Akram, N.A.; Ashraf, M. Alpha-Tocopherol-Induced Regulation of Growth and Metabolism in Plants under Non-stress and Stress Conditions. J. Plant Growth Regul. 2019, 38, 1325–1340. [Google Scholar] [CrossRef]
- Song, Z.; Lv, J.; Sheikhahmadi, A.; Uerlings, J.; Everaert, N. Attenuating effect of zinc and vitamin E on the intestinal oxidative stress induced by silver nanoparticles in broiler chickens. Biol. Trace Elem. Res. 2017, 180, 306–313. [Google Scholar] [CrossRef]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef] [PubMed]

| Localization | Parameter | Before Treatment | 4 Months after the Treatment | ||||
|---|---|---|---|---|---|---|---|
| Positive Effect | Absence of Effect | p-Value | Positive Effect | Absence of Effect | p-Value | ||
| Androgen-dependent (parietal) region | Hair density (number of hairs per square cm) | 190 (170–201) | 188 (131–199.5) | 0.285 | 220 (200–242.5) | 170 (125–197) | <0.001 |
| Anagen hairs, % | 70 (62–80) | 69.5 (60–76.5) | 0.115 | 85 (80–88) | 70 (62.5–80) | <0.001 | |
| Telogen hairs, % | 30 (20–37) | 30 (23.5–40) | 0.544 | 15 (12–20) | 30 (20–37.5) | <0.001 | |
| Average hair diameter (μm) | 43 (40–45) | 41.5 (35–43) | 0.568 | 48.5 (45–51) | 40 (35–45) | <0.001 | |
| Androgen-independent (occipital) region | Hair density (number of hairs per square cm) | 250 (204–263) | 261 (217.5–306.5) | 0.336 | 256 (230–287.5) | 265(215–305) | 0.614 |
| Anagen hairs, % | 89 (81–93) | 90 (84–96) | 0.483 | 91.5 (87.5–95) | 90 (85–95) | 0.713 | |
| Telogen hairs, % | 11 (6–18,5) | 10 (4–16) | 0.229 | 8.5 (5–12.5) | 10 (5–15) | 0.721 | |
| Average hair diameter (μm) | 55 (54–58) | 57 (54–58.5) | 0.482 | 57 (55.5–59.5) | 57 (55–60) | 0.991 | |
| Parameter | Group | p-Value | |
|---|---|---|---|
| Positive Effect | Absence of Effect | ||
| Testesteron, nmpl/L | 16.4 (10.95–24.95) | 20.3 (12.75–36.5) | 0.361 |
| Testesteron free, pg/mL | 16.76 (12.02–25.56) | 16 (9.5–26.5) | 0.696 |
| Dihydrotestosterone, pg/mL | 703.68 (571.87–1250.54) | 850.14 (516.37–1406.70) | 0.831 |
| Sex hormone binding globulin, nmol/L | 29.05 (21.0–45.0) | 34.25 (23.0–47.8) | 0.887 |
| 17-OH-progesterone, ng/mL | 1.5 (1.02–2.0) | 1.38 (1.0–2.0) | 0.859 |
| Androstendion, ng/mL | 2.06 (1.06–3.11) | 1.81 (0.94–3.30) | 0.477 |
| Thyroid stimulating hormon, µIU/mL | 2.42 (2.0–3.0) | 2 (1.7–2.7) | 0.245 |
| Insulin, µIU/mL | 7.0 (3.0–12.9) | 4.97 (3.0–10) | 0.627 |
| Prostate specific antigen, ng/mL | 0.61 (0.33–1.0) | 0.84 (0.64–1.00) | 0.222 |
| Cholesterol, mmol/L | 4.35 (3.72–5.07) | 4 (3.98–5.00) | 0.803 |
| Glucose, mmol/L | 4.63 (4.25–5.0) | 4.99 (4.0–5.0) | 0.878 |
| Ferretin, ng/mL | 148.0 (70.95.0–204.0) | 200 (118.5–287.0) | 0.162 |
| Zn, µmol/L | 12.32 (10.0–14.4) | 9.1 (8.5–10.6) | 0.034 * |
| Cu, µmol/L | 10.7 (9.7–13.2) | 11.5 (9.5–17.5) | 0.749 |
| Mg, mmol/L | 0.85 (0.76–0.97) | 0.82 (0.75–1.00) | 0.785 |
| Ca, mmol/L | 2.4 (2.3–2.5) | 2.33 (2.3–2.4) | 0.173 |
| Fe, µmol/L | 19.4 (15.0–27.3) | 24.5 (21.0–29.0) | 0.081 |
| Se, µg/L | 0.77 (0.55–1.0) | 0.67 (0.51–1.00) | 0.484 |
| B12, pg/mL | 319.5 (189.5–414.0) | 275.0 (200.0–362.0) | 0.74 |
| E, µg/mL | 6.7 (4.2–11.0) | 4.6 (4.0–7.5) | 0.255 |
| D, ng/mL | 24.15 (19.0–34.9) | 20.5 (18.0–32.5) | 0.286 |
| Folic acid, ng/mL | 4.9 (3.0–9.0) | 3.69 (3.0–9.8) | 0.545 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondrakhina, I.N.; Verbenko, D.A.; Zatevalov, A.M.; Gatiatulina, E.R.; Nikonorov, A.A.; Deryabin, D.G.; Kubanov, A.A. Plasma Zinc Levels in Males with Androgenetic Alopecia as Possible Predictors of the Subsequent Conservative Therapy’s Effectiveness. Diagnostics 2020, 10, 336. https://doi.org/10.3390/diagnostics10050336
Kondrakhina IN, Verbenko DA, Zatevalov AM, Gatiatulina ER, Nikonorov AA, Deryabin DG, Kubanov AA. Plasma Zinc Levels in Males with Androgenetic Alopecia as Possible Predictors of the Subsequent Conservative Therapy’s Effectiveness. Diagnostics. 2020; 10(5):336. https://doi.org/10.3390/diagnostics10050336
Chicago/Turabian StyleKondrakhina, Irina N., Dmitry A. Verbenko, Alexander M. Zatevalov, Eugenia R. Gatiatulina, Alexandr A. Nikonorov, Dmitrij G. Deryabin, and Alexey A. Kubanov. 2020. "Plasma Zinc Levels in Males with Androgenetic Alopecia as Possible Predictors of the Subsequent Conservative Therapy’s Effectiveness" Diagnostics 10, no. 5: 336. https://doi.org/10.3390/diagnostics10050336
APA StyleKondrakhina, I. N., Verbenko, D. A., Zatevalov, A. M., Gatiatulina, E. R., Nikonorov, A. A., Deryabin, D. G., & Kubanov, A. A. (2020). Plasma Zinc Levels in Males with Androgenetic Alopecia as Possible Predictors of the Subsequent Conservative Therapy’s Effectiveness. Diagnostics, 10(5), 336. https://doi.org/10.3390/diagnostics10050336