Comparison between Conventional Smear and Liquid-Based Preparation in Endoscopic Ultrasonography-Fine Needle Aspiration Cytology of Pancreatic Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Published Study Search and Selection Criteria
2.2. Data Extraction
2.3. Statistical Analysis
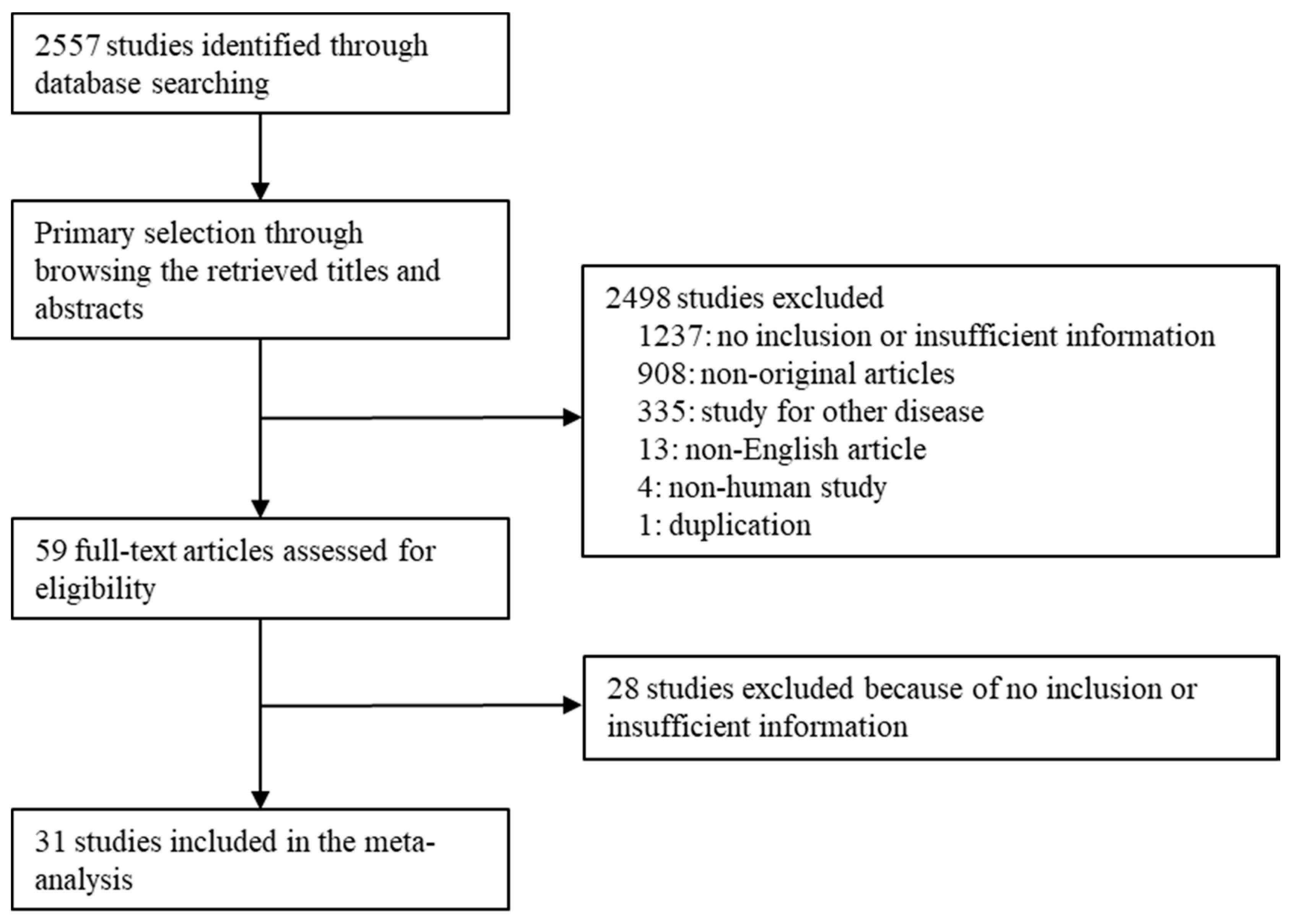
3. Results
3.1. Selection and Characteristics
3.2. Comparison of Sample Adequacy between Conventional Smear and Liquid-Based Preparation
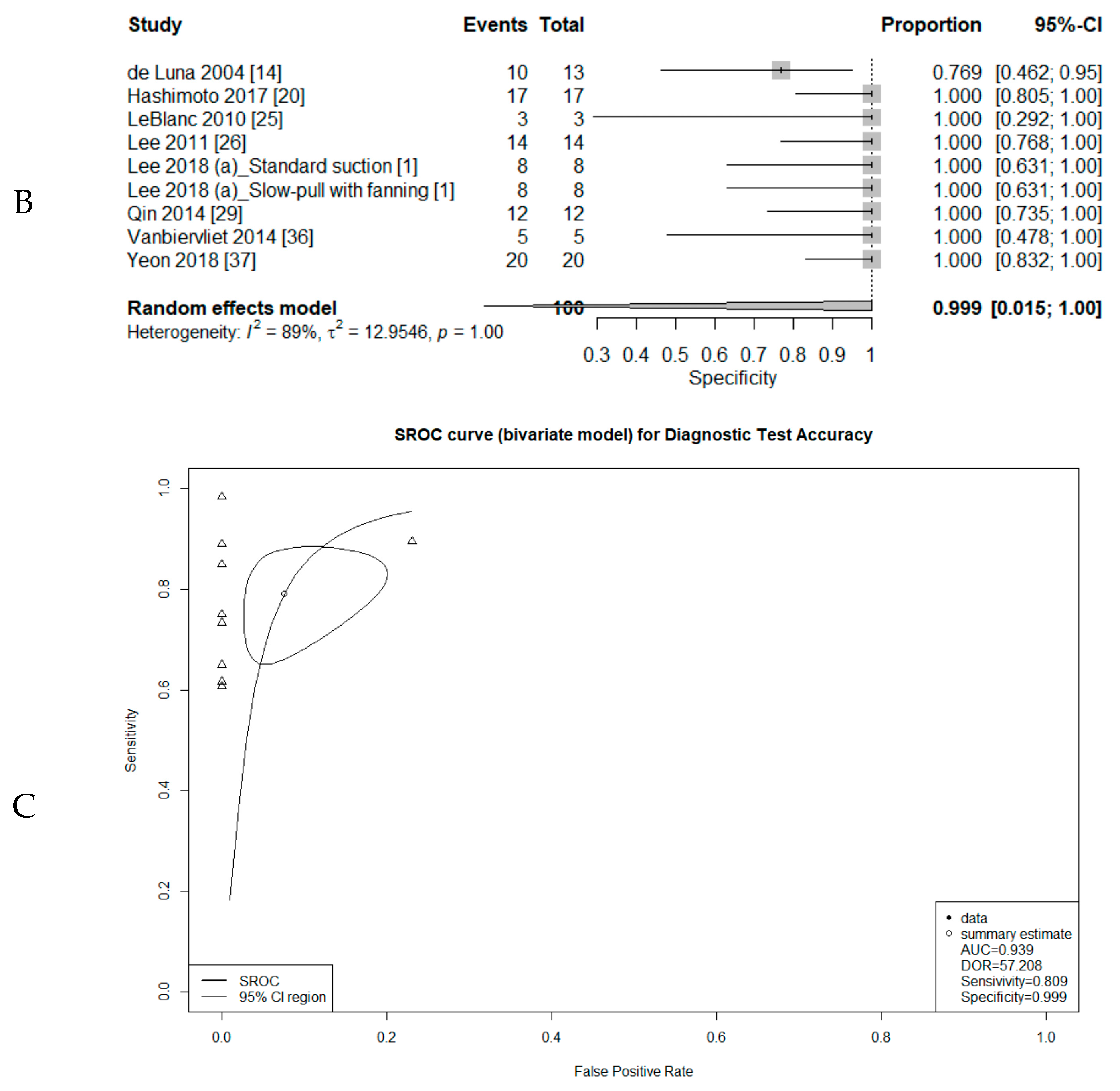
3.3. Comparison of Diagnostic Accuracy between Conventional Smear and Liquid-Based Preparation
3.4. Diagnostic Test Accuracy Review of Cytology
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, J.C.; Kim, H.; Kim, H.W.; Lee, J.; Paik, K.H.; Kang, J.; Hwang, J.H.; Kim, J. It is Necessary to Exam Bottom and Top Slide Smears of EUS-FNA for Pancreatic Cancer. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 553–558. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, H.S.; Hyun, J.J.; Lee, J.M.; Yoo, I.K.; Kim, S.H.; Choi, H.S.; Kim, E.S.; Keum, B.; Seo, Y.S.; et al. Slow-Pull Using a Fanning Technique Is More Useful Than the Standard Suction Technique in EUS-Guided Fine Needle Aspiration in Pancreatic Masses. Gut Liver 2018, 12, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, H.; Sasaki, K.; Ono, S.; Abe, M.; Ishiwatari, H.; Fukutomi, A.; Uesaka, K.; Ono, H. Pathological and Molecular Aspects to Improve Endoscopic Ultrasonography-Guided Fine-Needle Aspiration from Solid Pancreatic Lesions. Pancreas 2018, 47, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.S.; Kang, G.; Yoon, H.K.; Kim, H.J. Diagnostic Test Accuracy Review of Cytology for Squamous Intraepithelial Lesion and Squamous Cell Carcinoma of Uterine Cervix. J. Korean Med. Sci. 2019, 34, e16. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, R.; Schmitt, F.C. Liquid-Based Cytology in Fine-Needle Aspiration of Breast Lesions: A Review. Acta Cytol. 2014, 58, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; She, D.L.; Xiong, H.; Yang, L.; Fu, S.J. Diagnostic Value of Liquid-Based Cytology in Urothelial Carcinoma Diagnosis: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0134940. [Google Scholar] [CrossRef]
- Rarick, J.M.; Wasman, J.; Michael, C.W. The Utility of Liquid-Based Cytology in Salivary Gland Fine-Needle Aspirates: Experience of an Academic Institution. Acta Cytol. 2014, 58, 552–562. [Google Scholar] [CrossRef]
- Rossi, E.D.; Mulè, A.; Russo, R.M.; Pierconti, F.; Fadda, G. Application of Liquid-Based Preparation to Non-Gynaecologic Exfoliative Cytology. Pathologica 2008, 100, 461–465. [Google Scholar]
- Baek, H.W.; Park, M.J.; Rhee, Y.Y.; Lee, K.B.; Kim, M.A.; Park, I.A. Diagnostic Accuracy of Endoscopic Ultrasound-Guided Fine Needle Aspiration Cytology of Pancreatic Lesions. J. Pathol. Transl. Med. 2015, 49, 52–60. [Google Scholar] [CrossRef]
- Baghbanian, M.; Shabazkhani, B.; Ghofrani, H.; Forutan, H.; Dariani, N.; Farahvash, M.; Aletaha, N. Efficacy of Endoscopic Ultrasound Guided Fine Needle Aspiration in Patients with Solid Pancreatic Neoplasms. Saudi J. Gastroenterol. 2012, 18, 358–363. [Google Scholar] [CrossRef]
- Bentz, J.S.; Kochman, M.L.; Faigel, D.O.; Ginsberg, G.G.; Smith, D.B.; Gupta, P.K. Endoscopic Ultrasound-Guided Real-Time Fine-Needle Aspiration: Clinicopathologic Features of 60 Patients. Diagn. Cytopathol. 1998, 18, 98–109. [Google Scholar] [CrossRef]
- Bergeron, J.P.; Perry, K.D.; Houser, P.M.; Yang, J. Endoscopic Ultrasound-Guided Pancreatic Fine-Needle Aspiration: Potential Pitfalls in One Institution’s Experience of 1212 Procedures. Cancer Cytopathol. 2015, 123, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Ding, Q.Y.; Lv, Y.; Guo, W.; Zhi, F.C.; Liu, S.D.; Cheng, T.M. Slow-Pull and Different Conventional Suction Techniques in Endoscopic Ultrasound-Guided Fine-Needle Aspiration of Pancreatic Solid Lesions Using 22-Gauge Needles. World J. Gastroenterol. 2016, 22, 8790–8797. [Google Scholar] [CrossRef] [PubMed]
- de Luna, R.; Eloubeidi, M.A.; Sheffield, M.V.; Eltoum, I.; Jhala, N.; Jhala, D.; Chen, V.K.; Chhieng, D.C. Comparison of ThinPrep and Conventional Preparations in Pancreatic Fine-Needle Aspiration Biopsy. Diagn. Cytopathol. 2004, 30, 71–76. [Google Scholar] [CrossRef]
- Eloubeidi, M.A.; Tamhane, A. EUS-Guided FNA of Solid Pancreatic Masses: A Learning Curve with 300 Consecutive Procedures. Gastrointest. Endosc. 2005, 61, 700–708. [Google Scholar] [CrossRef]
- Eloubeidi, M.A.; Tamhane, A.; Jhala, N.; Chhieng, D.; Jhala, D.; Crowe, D.R.; Eltoum, I.A. Agreement between Rapid Onsite and Final Cytologic Interpretations of EUS-Guided FNA Specimens: Implications for the Endosonographer and Patient Management. Am. J. Gastroenterol. 2006, 101, 2841–2847. [Google Scholar] [CrossRef]
- Eloubeidi, M.A.; Varadarajulu, S.; Desai, S.; Shirley, R.; Heslin, M.J.; Mehra, M.; Arnoletti, J.P.; Eltoum, I.; Wilcox, C.M.; Vickers, S.M. A Prospective Evaluation of an Algorithm Incorporating Routine Preoperative Endoscopic Ultrasound-Guided Fine Needle Aspiration in Suspected Pancreatic Cancer. J. Gastrointest. Surg. 2007, 11, 813–819. [Google Scholar] [CrossRef]
- Furuhata, A.; Minamiguchi, S.; Shirahase, H.; Kodama, Y.; Adachi, S.; Sakurai, T.; Haga, H. Immunohistochemical Antibody Panel for the Differential Diagnosis of Pancreatic Ductal Carcinoma from Gastrointestinal Contamination and Benign Pancreatic Duct Epithelium in Endoscopic Ultrasound-Guided Fine-Needle Aspiration. Pancreas 2017, 46, 531–538. [Google Scholar] [CrossRef]
- Haba, S.; Yamao, K.; Bhatia, V.; Mizuno, N.; Hara, K.; Hijioka, S.; Imaoka, H.; Niwa, Y.; Tajika, M.; Kondo, S.; et al. Diagnostic Ability and Factors Affecting Accuracy of Endoscopic Ultrasound-Guided Fine Needle Aspiration for Pancreatic Solid Lesions: Japanese Large Single Center Experience. J. Gastroenterol. 2013, 48, 973–981. [Google Scholar] [CrossRef]
- Hashimoto, S.; Taguchi, H.; Higashi, M.; Hatanaka, K.; Fujita, T.; Iwaya, H.; Nakazawa, J.; Arima, S.; Iwashita, Y.; Sasaki, F.; et al. Diagnostic Efficacy of Liquid-Based Cytology For Solid Pancreatic Lesion Samples Obtained with Endoscopic Ultrasound-Guided Fine-Needle Aspiration: Propensity Score-Matched Analysis. Dig. Endosc. 2017, 29, 608–616. [Google Scholar] [CrossRef]
- Hikichi, T.; Irisawa, A.; Bhutani, M.S.; Takagi, T.; Shibukawa, G.; Yamamoto, G.; Wakatsuki, T.; Imamura, H.; Takahashi, Y.; Sato, A.; et al. Endoscopic Ultrasound-Guided Fine-Needle Aspiration of Solid Pancreatic Masses with Rapid On-Site Cytological Evaluation by Endosonographers without Attendance of Cytopathologists. J. Gastroenterol. 2009, 44, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Ieni, A.; Todaro, P.; Crino, S.F.; Barresi, V.; Tuccari, G. Endoscopic Ultrasound-Guided Fine-Needle Aspiration Cytology in Pancreaticobiliary Carcinomas: Diagnostic Efficacy of Cell-Block Immunocytochemistry. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 305–312. [Google Scholar] [CrossRef]
- Jang, D.K.; Lee, S.H.; Lee, J.K.; Paik, W.H.; Chung, K.H.; Lee, B.S.; Son, J.H.; Lee, J.W.; Ryu, J.K.; Kim, Y.T.; et al. Comparison of Cytological and Histological Preparations in the Diagnosis of Pancreatic Malignancies Using Endoscopic Ultrasound-Guided Fine Needle Aspiration. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 418–423. [Google Scholar] [CrossRef]
- Jeong, H.; Park, C.S.; Kim, K.B.; Han, J.H.; Yoon, S.M.; Chae, H.B.; Youn, S.J.; Park, S.M. Predictors of Malignancies in Patients with Inconclusive or Negative Results of Endoscopic Ultrasound-guided Fine-needle Aspiration for Solid Pancreatic Masses. Korean J. Gastroenterol. 2018, 71, 153–161. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.K.; Emerson, R.E.; Dewitt, J.; Symms, M.; Cramer, H.M.; McHenry, L.; Wade, C.L.; Wang, X.; Musto, P.; Eichelberger, L.; et al. A Prospective Study Comparing Rapid Assessment of Smears and ThinPrep for Endoscopic Ultrasound–Guided Fine-Needle Aspirates. Endoscopy 2010, 42, 389–394. [Google Scholar] [CrossRef]
- Lee, J.K.; Choi, E.R.; Jang, T.H.; Chung, Y.H.; Jang, K.T.; Park, S.M.; Lee, J.K.; Lee, K.T.; Lee, K.H. A Prospective Comparison of Liquid-Based Cytology and Traditional Smear Cytology in Pancreatic Endoscopic Ultrasound–Guided Fine Needle Aspiration. Acta Cytol. 2011, 55, 401–407. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, Y.J.; Lee, J.K.; Lee, K.T.; Choi, Y.L.; Lee, K.H. KRAS Mutation Analysis of Washing Fluid from Endoscopic Ultrasound-Guided Fine Needle Aspiration Improves Cytologic Diagnosis of Pancreatic Ductal Adenocarcinoma. Oncotarget 2017, 8, 3519–3527. [Google Scholar] [CrossRef][Green Version]
- Pellisé, M.; Castells, A.; Ginès, A.; Solé, M.; Mora, J.; Castellví-Bel, S.; Rodríguez-Moranta, F.; Fernàndez-Esparrach, G.; Llach, J.; Bordas, J.M.; et al. Clinical Usefulness of KRAS Mutational Analysis in the Diagnosis of Pancreatic Adenocarcinoma by Means of Endosonography-Guided Fine-Needle Aspiration Biopsy. Aliment. Pharmacol. Ther. 2003, 17, 1299–1307. [Google Scholar] [CrossRef]
- Qin, S.Y.; Zhou, Y.; Li, P.; Jiang, H.X. Diagnostic Efficacy of Cell Block Immunohistochemistry, Smear Cytology, and Liquid-Based Cytology in Endoscopic Ultrasound–Guided Fine-Needle Aspiration of Pancreatic Lesions: A Single-Institution Experience. PLoS ONE 2014, 9, e108762. [Google Scholar] [CrossRef]
- Ramesh, J.; Kim, H.; Reddy, K.; Eltoum, I.E. Performance Characteristic of Endoscopic Ultrasound-Guided Fine Needle Aspiration Is Unaffected by Pancreatic Mass Size. Endosc. Int. Open 2016, 4, E434–E438. [Google Scholar] [CrossRef][Green Version]
- Saxena, P.; El Zein, M.; Stevens, T.; Abdelgelil, A.; Besharati, S.; Messallam, A.; Kumbhari, V.; Azola, A.; Brainard, J.; Shin, E.J.; et al. Stylet Slow-Pull Versus Standard Suction for Endoscopic Ultrasound-Guided Fine-Needle Aspiration of Solid Pancreatic Lesions: A Multicenter Randomized Trial. Endoscopy 2018, 50, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.R.; Nerlich, A.; Topalidis, T.; Schepp, W. Specialized Clinical Cytology May Improve the Results of EUS (Endoscopic Ultrasound)-Guided Fine-Needle Aspiration (FNA) from Pancreatic Tumors. Endosc. Int. Open 2015, 3, E134–E137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tada, M.; Komatsu, Y.; Kawabe, T.; Sasahira, N.; Isayama, H.; Toda, N.; Shiratori, Y.; Omata., M. Quantitative Analysis of K-ras Gene Mutation in Pancreatic Tissue Obtained by Endoscopic Ultrasonography-Guided Fine Needle Aspiration: Clinical Utility for Diagnosis of Pancreatic Tumor. Am. J. Gastroenterol. 2002, 97, 2263–2270. [Google Scholar] [CrossRef]
- Trisolini, E.; Armellini, E.; Paganotti, A.; Veggiani, C.; Bozzola, C.; Frattini, M.; Pizio, C.; Mancuso, G.; Andorno, S.; Boldorini, R. KRAS Mutation Testing on All Non-Malignant Diagnosis of Pancreatic Endoscopic Ultrasound-Guided Fine-Needle Aspiration Biopsies Improves Diagnostic Accuracy. Pathology 2017, 49, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Ikezawa, K.; Kawada, N.; Fukutake, N.; Katayama, K.; Takakura, R.; Takano, Y.; Ishikawa, O.; Takenaka, A. Diagnostic Accuracy of Endoscopic Ultrasound-Guided Fine Needle Aspiration for Suspected Pancreatic Malignancy in Relation to the Size of Lesions. J. Gastroenterol. Hepatol. 2011, 26, 1256–1261. [Google Scholar] [CrossRef]
- Vanbiervliet, G.; Napoléon, B.; Saint Paul, M.C.; Sakarovitch, C.; Wangermez, M.; Bichard, P.; Subtil, C.; Koch, S.; Grandval, P.; Gincul, R.; et al. Core Needle Versus Standard Needle for Endoscopic Ultrasound-Guided Biopsy of Solid Pancreatic Masses: A Randomized Crossover Study. Endoscopy 2014, 46, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Yeon, M.H.; Jeong, H.S.; Lee, H.S.; Jang, J.S.; Lee, S.; Yoon, S.M.; Chae, H.B.; Park, S.M.; Youn, S.J.; Han, J.H.; et al. Comparison of Liquid-Based Cytology (CellPrepPlus) and Conventional Smears in Pancreaticobiliary Disease. Korean J. Intern. Med. 2018, 33, 883–892. [Google Scholar] [CrossRef]
- Matsubayashi, H.; Matsui, T.; Yabuuchi, Y.; Imai, K.; Tanaka, M.; Kakushima, N.; Sasaki, K.; Ono, H. Endoscopic Ultrasonography Guided-Fine Needle Aspiration for the Diagnosis of Solid Pancreaticobiliary Lesions: Clinical Aspects to Improve the Diagnosis. World J. Gastroenterol. 2016, 22, 628–640. [Google Scholar] [CrossRef]
- Hébert-Magee, S.; Bae, S.; Varadarajulu, S.; Ramesh, J.; Frost, A.R.; Eloubeidi, M.A.; Eltoum, I.A. The Presence of a Cytopathologist Increases the Diagnostic Accuracy of Endoscopic Ultrasound–Guided Fine Needle Aspiration Cytology for Pancreatic Adenocarcinoma: A Meta-Analysis. Cytopathology 2013, 24, 159–171. [Google Scholar] [CrossRef]
- Chatterjee, S.; Oppong, K.W. Endobronchial Ultrasonic Videoscope for Transgastric/Transesophageal Fine-Needle Aspiration in Special Situations: Another Tool for the Gastrointestinal Endosonographer. Endoscopy 2012, 44, E298–F299. [Google Scholar] [CrossRef]
- Nakai, Y.; Isayama, H.; Chang, K.J.; Yamamoto, N.; Hamada, T.; Uchino, R.; Mizuno, S.; Miyabayashi, K.; Yamamoto, K.; Kawakubo, K.; et al. Slow Pull Versus Suction in Endoscopic Ultrasound–Guided Fine-Needle Aspiration of Pancreatic Solid Masses. Dig. Dis. Sci. 2014, 59, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Witt, B.L.; Adler, D.G.; Hilden, K.; Layfield, L.J. A Comparative Needle Study: EUS-FNA Procedures Using the HD ProCore(™) and EchoTip(®) 22-Gauge Needle Types. Diagn. Cytopathol. 2013, 41, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Puli, S.R.; Bechtold, M.L.; Buxbaum, J.L.; Eloubeidi, M.A. How Good Is Endoscopic Ultrasound-Guided Fine-Needle Aspiration in Diagnosing the Correct Etiology for a Solid Pancreatic Mass? A Meta-Analysis and Systematic Review. Pancreas 2013, 42, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.T.; Gokaslan, S.T.; Saboorian, M.H.; Ashfaq, R. Split Sample Comparison of ThinPrep and Conventional Smears in Endoscopic Retrograde Cholangiopancreatography-Guided Pancreatic Fine-Needle Aspirations. Diagn. Cytopathol. 2005, 32, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.X.; Xiao, J.; Orange, M.; Zhang, H.; Zhu, Y.Q. EUS-Guided FNA for Diagnosis of Pancreatic Cystic Lesions: A Meta-Analysis. Cell. Physiol. Biochem. 2015, 36, 1197–1209. [Google Scholar] [CrossRef]
- Thornton, G.D.; McPhail, M.J.; Nayagam, S.; Hewitt, M.J.; Vlavianos, P.; Monahan, K.J. Endoscopic Ultrasound Guided Fine Needle Aspiration for the Diagnosis of Pancreatic Cystic Neoplasms: A Meta-Analysis. Pancreatology 2013, 13, 48–57. [Google Scholar] [CrossRef]



| Study | Cell Preparation | Type of Lesion | No of Patients or Cases | Needle Size | ROSE | |
|---|---|---|---|---|---|---|
| Baek, 2015 [9] | CS | PSM | 191 | ND | ND | |
| Baghbanian, 2012 [10] | CS | PSM | 53 | 22G | ND | |
| Bentz, 1998 [11] | CS | PSM | 60 | 22G | Yes | |
| Bergeron, 2015 [12] | CS | PSM | 1104 | ND | Yes | |
| Chen, 2016 [13] | CS | PSM | 102 | 22G | No | |
| de Luna, 2004 [14] | CS | LBP | PSM | 67 | ND | Yes |
| Eloubeidi, 2005 [15] | CS | PSM | 300 | ND | Yes | |
| Eloubeidi, 2006 [16] | CS | PSM | 158 | 22G | ND | |
| Eloubeidi, 2007 [17] | CS | PSM | 547 | 22G | ND | |
| Furuhata, 2017 [18] | CS | PSM | 75 | 22G | Yes | |
| Haba, 2013 [19] | CS | PSM | 996 | Mixed | Partial | |
| Hashimoto, 2017 [20] | CS | LBP | PSM | 126 | 25G | ND |
| Hikichi, 2009 [21] | CS | PSM | 73 | 22G | Yes | |
| Ieni, 2015 [22] | CS | PSM | 46 | 22G | ND | |
| Jang, 2017 [23] | CS | PSM | 118 | 22G | ND | |
| Jeong, 2018 [24] | CS | PSM | 97 | Mixed | No | |
| LeBlanc, 2010 [25] | CS | LBP | PSM | 130 | 22G | Yes |
| Lee, 2011 [26] | CS | LBP | Mixed | 58 | Mixed | No |
| Lee, 2018 (a) [1] | LBP | PSM | 48 | 22G | ND | |
| Lee, 2018 (b) [2] | CS | PSM | 73 | 22G/25G | No | |
| Park, 2017 [27] | CS | PSM | 43 | Mixed | ND | |
| Pellisé, 2003 [28] | CS | PSM | 33 | 22G | Yes | |
| Qin, 2014 [29] | CS | LBP | PSM | 72 | 22G | No |
| Ramesh, 2016 [30] | CS | PSM | 612 | Mixed | Yes | |
| Saxena, 2018 [31] | CS | PSM | 147 | 22G | Yes | |
| Schneider, 2015 [32] | CS | PSM | 63 | 22G | ND | |
| Tada, 2002 [33] | CS | PSM | 34 | 22G | ND | |
| Trisolini, 2017 [34] | CS | PSM | 107 | 25G | No | |
| Uehara, 2011 [35] | CS | PSM | 120 | Mixed | Yes | |
| Vanbiervliet, 2014 [36] | LBP | PSM | 80 | 22G | ND | |
| Yeon, 2018 [37] | CS | LBP | ND | 43 | 22G | ND |
| Number of Subsets | Fixed Effect (95% CI) | Heterogeneity Test [p-Value] | Random Effect (95% CI) | Egger’s Test [p-Value] | |
|---|---|---|---|---|---|
| Conventional smear | 39 | 0.812 (0.798, 0.825) | <0.001 | 0.828 (0.798, 0.855) | 0.143 |
| Type | |||||
| Solid mass | 36 | 0.810 (0.795, 0.823) | <0.001 | 0.824 (0.792, 0.852) | 0.232 |
| Cystic lesion | 1 | 0.800 (0.572, 0.923) | 1.000 | 0.800 (0.572, 0.923) | - |
| ROSE | |||||
| with ROSE | 7 | 0.921 (0.892, 0.943) | 0.010 | 0.928 (0.879, 0.959) | 0.079 |
| without ROSE | 13 | 0.777 (0.749, 0.803) | <0.001 | 0.809 (0.748, 0.858) | 0.032 |
| Needle size | |||||
| 22 gauge | 5 | 0.798 (0.736, 0.848) | 0.006 | 0.808 (0.682, 0.892) | 0.557 |
| 25 gauge | 5 | 0.779 (0.735, 0.817) | 0.008 | 0.808 (0.720, 0.873) | 0.138 |
| Conventional smear | |||||
| Slow-pull technique | 2 | 0.729 (0.651, 0.795) | 0.047 | 0.762 (0.577, 0.882) | - |
| Fanning technique | 1 | 0.588 (0.487, 0.681) | 1.000 | 0.588 (0.487, 0.681) | - |
| Liquid-based preparation | 5 | 0.867 (0.823, 0.902) | <0.001 | 0.940 (0.844, 0.978) | 0.065 |
| ROSE | |||||
| with ROSE | 1 | 0.980 (0.871, 0.997) | 1.000 | 0.980 (0.871, 0.997) | - |
| without ROSE | 1 | 0.983 (0.888, 0.998) | 1.000 | 0.983 (0.888, 0.998) | - |
| Needle size | |||||
| 22 gauge | 2 | 0.983 (0.935, 0.996) | 0.810 | 0.983 (0.935, 0.996) | - |
| 25 gauge | 1 | 0.902 (0.844, 0.940) | 1.000 | 0.902 (0.844, 0.940) | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, S.H.; Pyo, J.-S.; Son, B.K.; Lee, H.Y.; Oh, I.W.; Chung, K.H. Comparison between Conventional Smear and Liquid-Based Preparation in Endoscopic Ultrasonography-Fine Needle Aspiration Cytology of Pancreatic Lesions. Diagnostics 2020, 10, 293. https://doi.org/10.3390/diagnostics10050293
Ko SH, Pyo J-S, Son BK, Lee HY, Oh IW, Chung KH. Comparison between Conventional Smear and Liquid-Based Preparation in Endoscopic Ultrasonography-Fine Needle Aspiration Cytology of Pancreatic Lesions. Diagnostics. 2020; 10(5):293. https://doi.org/10.3390/diagnostics10050293
Chicago/Turabian StyleKo, Soo Hee, Jung-Soo Pyo, Byoung Kwan Son, Hyo Young Lee, Il Whan Oh, and Kwang Hyun Chung. 2020. "Comparison between Conventional Smear and Liquid-Based Preparation in Endoscopic Ultrasonography-Fine Needle Aspiration Cytology of Pancreatic Lesions" Diagnostics 10, no. 5: 293. https://doi.org/10.3390/diagnostics10050293
APA StyleKo, S. H., Pyo, J.-S., Son, B. K., Lee, H. Y., Oh, I. W., & Chung, K. H. (2020). Comparison between Conventional Smear and Liquid-Based Preparation in Endoscopic Ultrasonography-Fine Needle Aspiration Cytology of Pancreatic Lesions. Diagnostics, 10(5), 293. https://doi.org/10.3390/diagnostics10050293






