Left Supraclavicular Lymph Node Metastasis from Ovarian Cancer Associated with Papillary Thyroid Microcarcinoma, a Confusing Pathology-Essential Role of Functional Imaging
Abstract
:1. Introduction
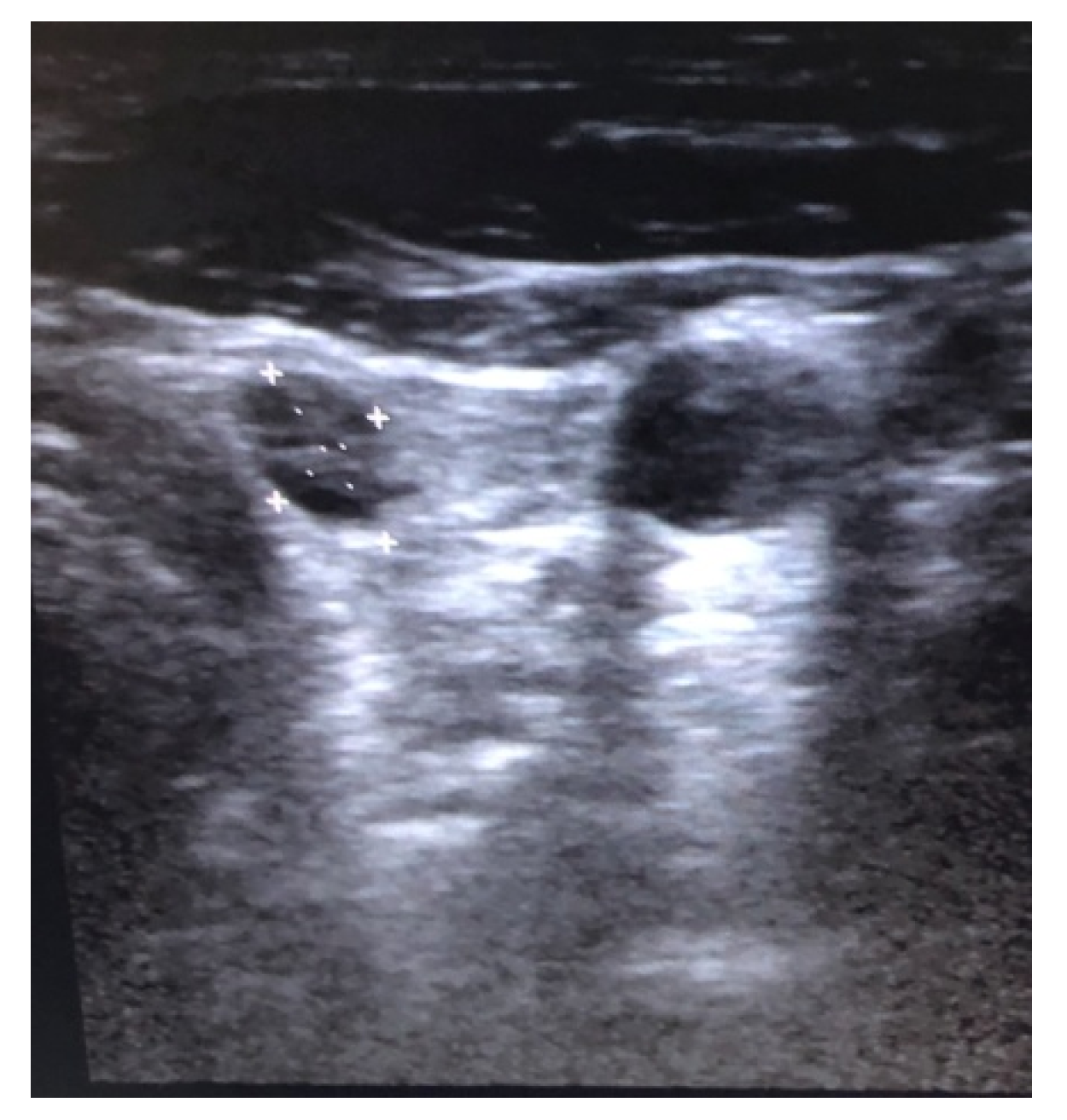
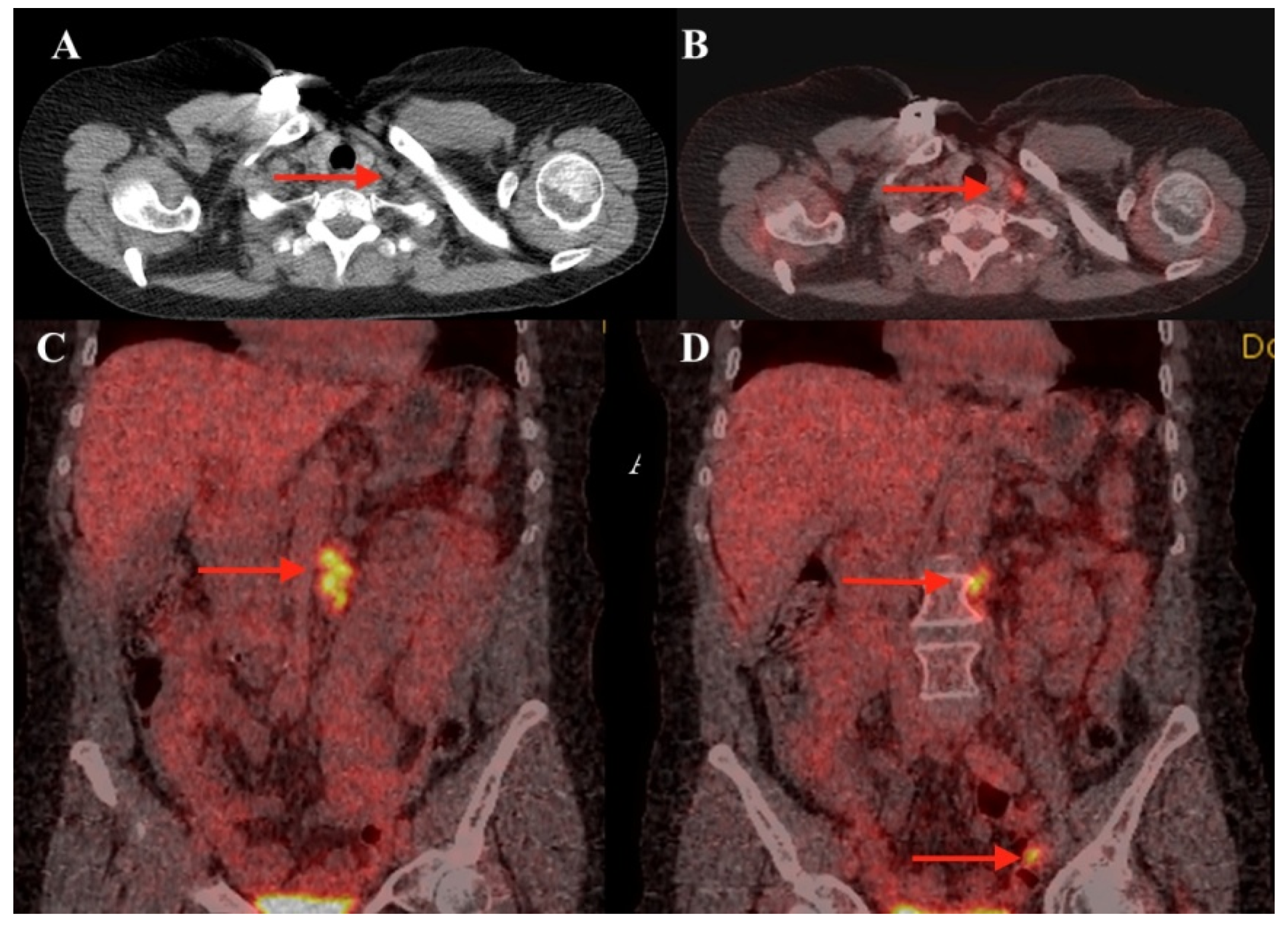
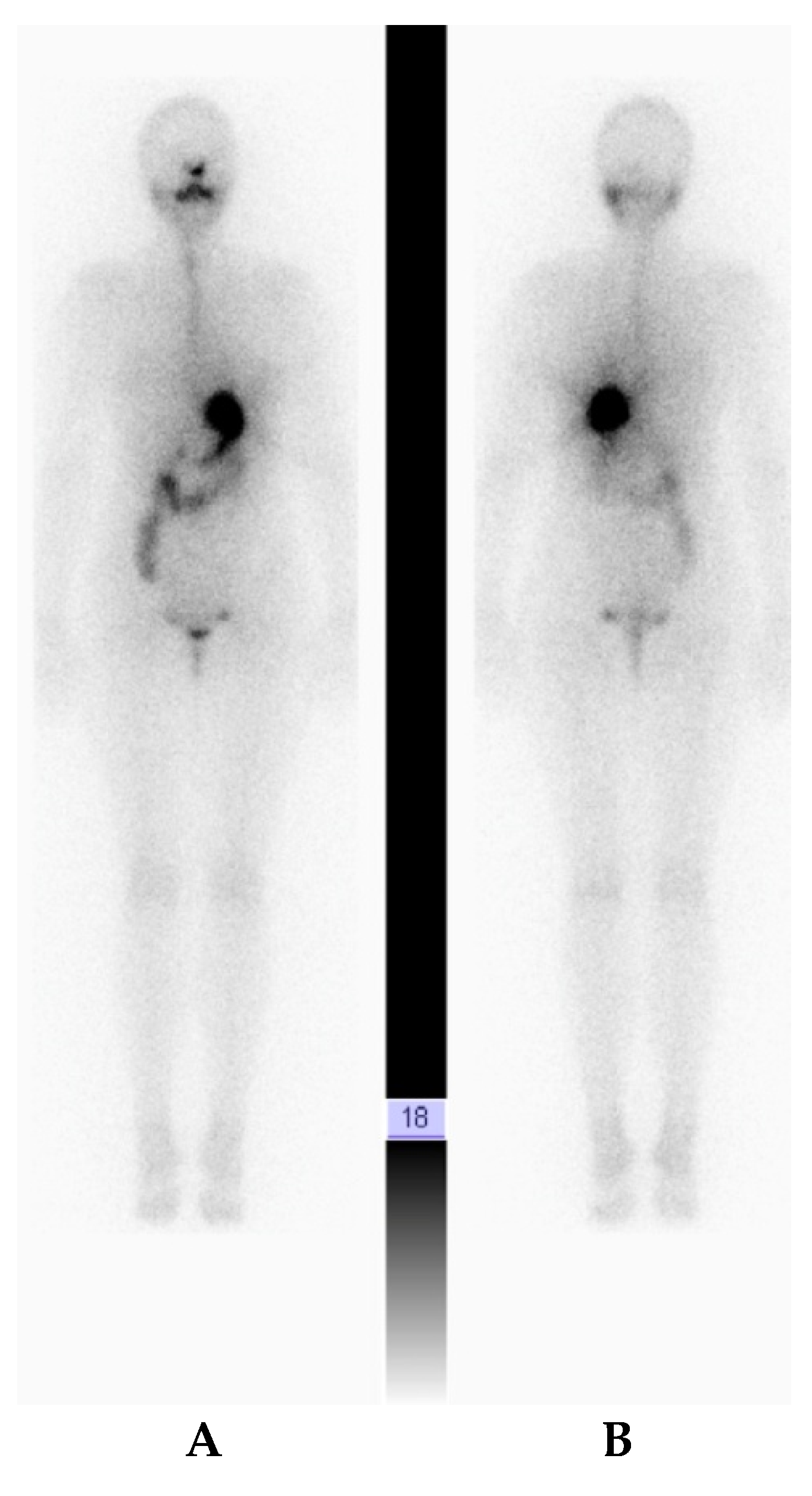
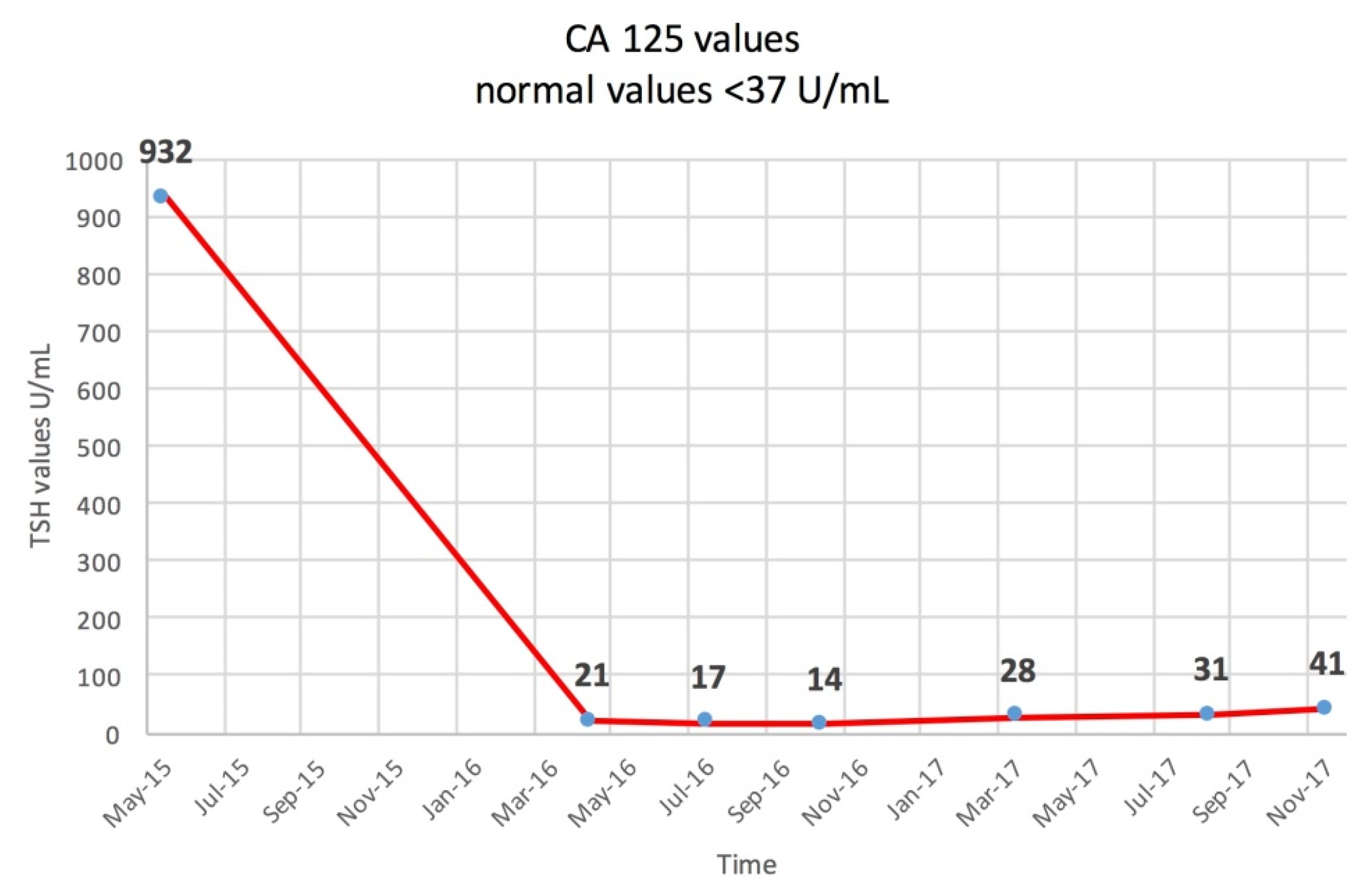
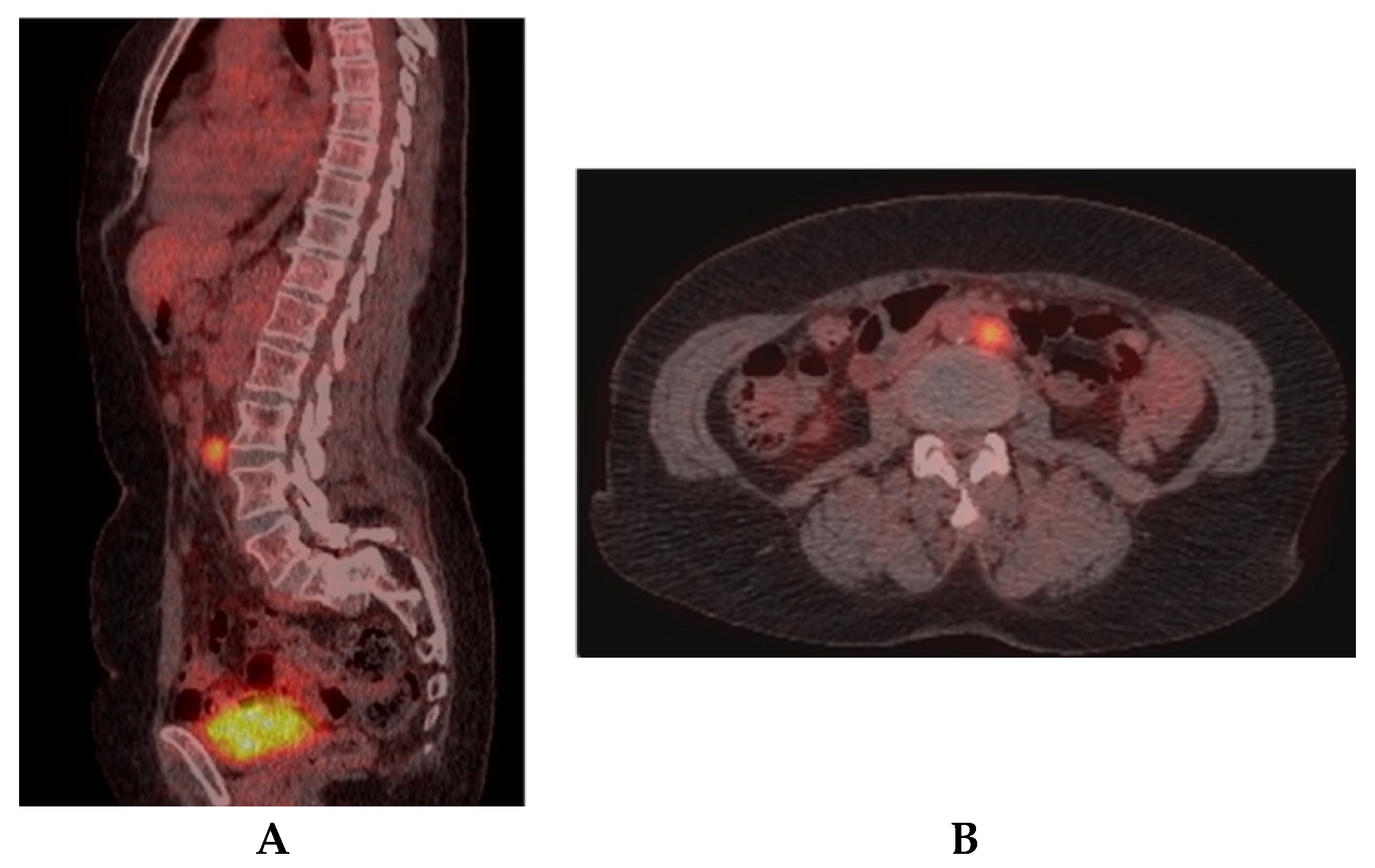
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aarts, J.W.M.; Nieboer, T.E.; Johnson, N.; Tavender, E.; Garry, R.; Mol, B.W.J.; Kluivers, K.B. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst. Rev. 2015, 2015, CD003677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekanayake, C.; Pathmeswaran, A.; Kularatna, S.; Herath, R.; Wijesinghe, P. Cost evaluation, quality of life and pelvic organ function of three approaches to hysterectomy for benign uterine conditions: Study protocol for a randomized controlled trial. Trials 2017, 18, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA. Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Coburn, S.B.; Bray, F.; Sherman, M.E.; Trabert, B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int. J. Cancer 2017, 140, 2451–2460. [Google Scholar] [CrossRef] [Green Version]
- Weidle, U.H.; Birzele, F.; Kollmorgen, G.; Rueger, R. Mechanisms and Targets Involved in Dissemination of Ovarian Cancer. Cancer Genom. Proteom. 2016, 13, 407–423. [Google Scholar] [CrossRef] [Green Version]
- Hirose, S.; Tanabe, H.; Nagayoshi, Y.; Hirata, Y.; Narui, C.; Ochiai, K.; Isonishi, S.; Takano, H.; Okamoto, A. Retrospective analysis of sites of recurrence in stage I epithelial ovarian cancer. J. Gynecol. Oncol. 2018, 29, e37. [Google Scholar] [CrossRef] [Green Version]
- Chekerov, R.; Braicu, I.; Castillo-Tong, D.C.; Richter, R.; Cadron, I.; Mahner, S.; Woelber, L.; Marth, C.; Van Gorp, T.; Speiser, P.; et al. Outcome and clinical management of 275 patients with advanced ovarian cancer International Federation of Obstetrics and Gynecology II to IV inside the European Ovarian Cancer Translational Research Consortium-OVCAD. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2013, 23, 268–275. [Google Scholar] [CrossRef]
- Kira, N.; Takai, N.; Ishii, T.; Kai, K.; Nishida, M.; Nasu, K.; Kashima, K.; Narahara, H. Ovarian small cell carcinoma complicated by carcinomatous meningitis. Rare Tumors 2012, 4, e26. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid Am. Thyroid Assoc. 2016, 26, 26. [Google Scholar]
- Fugazzola, L.; Elisei, R.; Fuhrer, D.; Jarzab, B.; Leboulleux, S.; Newbold, K.; Smit, J. 2019 European Thyroid Association Guidelines for the Treatment and Follow-Up of Advanced Radioiodine-Refractory Thyroid Cancer. Eur. Thyroid J. 2019, 8, 227–245. [Google Scholar] [CrossRef]
- Muntean, V.; Domsa, I.; Zolog, A.; Piciu, D.; Fabian, O.; Bosu, R.; Simescu, R.; Petre, G.; Muntean, M. V Incidental papillary thyroid microcarcinoma: Is completion surgery required? Chirurgia 2013, 108, 490–497. [Google Scholar] [PubMed]
- Trapanese, E.; De Bartolomeis, C.; Angrisani, B.; Tarro, G. Papillary thyroid microcarcinoma (Black Ink). Oncotarget 2018, 9, 29275–29283. [Google Scholar] [CrossRef] [PubMed]
- Piciu, D.; Piciu, A.; Irimie, A. Papillary thyroid microcarcinoma and ectopic papillary thyroid carcinoma in mediastinum: A case report. Clin. Nucl. Med. 2012, 37, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.G.; Tessler, F.N.; Hoang, J.K.; Langer, J.E.; Beland, M.D.; Berland, L.L.; Cronan, J.J.; Desser, T.S.; Frates, M.C.; Hamper, U.M.; et al. Thyroid Ultrasound Reporting Lexicon: White Paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) Committee. J. Am. Coll. Radiol. 2015, 12, 1272–1279. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017, 27, 1341–1346. [Google Scholar] [CrossRef]
- Bãrbuş, E.; Peştean, C.; Larg, M.I.; Piciu, D. Quality of life in thyroid cancer patients: A literature review. Clujul Med. 2017, 90, 147–153. [Google Scholar] [CrossRef] [Green Version]
- National Comprehensive Cancer Network Ovarian Cancer (Version 1.2020). Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (accessed on 2 April 2020).
- Hong, L.; Qiu, H.; Mei, Z.; Zhang, H.; Liu, S.; Cao, H. Ovarian cancer initially presenting with supra-clavicular lymph node metastasis: A case report. Oncol. Lett. 2018, 16, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Pérez-Medina, T.; Magrina, J.F.; Magtibay, P.M.; Rodríguez-Tapia, A.; Pérez-Milán, F.; Ortiz-Quintana, L. The impact of pelvic retroperitoneal invasion and distant nodal metastases in epithelial ovarian cancer. Surg. Oncol. 2014, 23, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Kim, T.H.; Kim, J.-W.; Kim, S.Y.; Kim, H.S.; Lee, T.S.; Chung, H.H.; Kim, Y.-B.; Park, N.H.; Song, Y.S. Improvements to the FIGO staging for ovarian cancer: Reconsideration of lymphatic spread and intraoperative tumor rupture. J. Gynecol. Oncol. 2013, 24, 352–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Ohmichi, M. Recurrent ovarian cancer presenting in the right supraclavicular lymph node with isolated metastasis: A case report. J. Med. Case Rep. 2012, 6, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djordjevic, B.; Malpica, A. Ovarian serous tumors of low malignant potential with nodal low-grade serous carcinoma. Am. J. Surg. Pathol. 2012, 36, 955–963. [Google Scholar] [CrossRef]
- Agarwal, R.; Radhakrishnan, G.; Radhika, A.G.; Jain, J.; Sharma, S.; Srivastava, H. Pregnancy concomitant with metastatic adult granulosa cell tumor. Arch. Gynecol. Obstet. 2011, 284, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-J.; Ding, D.-C.; Chao, T.-K.; Liu, Y.-L.; Liu, Y.-L.; Hwang, K.-S. Metastatic adenocarcinoma of left supraclavicular fossa from occult primary ovarian cancer. Taiwan J. Obstet. Gynecol. 2011, 50, 98–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avril, N.; Gourtsoyianni, S.; Reznek, R. Gynecological cancers. Methods Mol. Biol. 2011, 727, 171–189. [Google Scholar]
- Castellucci, P.; Perrone, A.M.; Picchio, M.; Ghi, T.; Farsad, M.; Nanni, C.; Messa, C.; Meriggiola, M.C.; Pelusi, G.; Al-Nahhas, A.; et al. Diagnostic accuracy of 18F-FDG PET/CT in characterizing ovarian lesions and staging ovarian cancer: Correlation with transvaginal ultrasonography, computed tomography, and histology. Nucl. Med. Commun. 2007, 28, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Fujiwaki, R.; Sawada, K. Spontaneous regression in recurrent epithelial ovarian cancer. Arch. Gynecol. Obstet. 2007, 275, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.; Plantade, A.; Pagès, C.; Afchain, P.; Louvet, C.; Tournigand, C.; de Gramont, A. Isolated lymph node relapse of epithelial ovarian carcinoma: Outcomes and prognostic factors. Gynecol. Oncol. 2007, 104, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Fanti, S.; Nanni, C.; Castellucci, P.; Farsad, M.; Rampin, L.; Gross, M.D.; Mariani, G.; Rubello, D. Supra-clavicular lymph node metastatic spread in patients with ovarian cancer disclosed at 18F-FDG-PET/CT: An unusual finding. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2006, 6, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Hess, K.R.; Varadhachary, G.R.; Taylor, S.H.; Wei, W.; Raber, M.N.; Lenzi, R.; Abbruzzese, J.L. Metastatic patterns in adenocarcinoma. Cancer 2006, 106, 1624–1633. [Google Scholar] [CrossRef]
- Mayadevi, S.; Nagarajan, S.; Van Der Voet, J.C.M.; Nevin, J.; Cruickshank, D.J. Metastatic adenocarcinoma of right supraclavicular fossa--delayed presentation of ovarian primary. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2005, 25, 528–529. [Google Scholar] [CrossRef]
- Euscher, E.D.; Silva, E.G.; Deavers, M.T.; Elishaev, E.; Gershenson, D.M.; Malpica, A. Serous carcinoma of the ovary, fallopian tube, or peritoneum presenting as lymphadenopathy. Am. J. Surg. Pathol. 2004, 28, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Uzan, C.; Morice, P.; Rey, A.; Pautier, P.; Camatte, S.; Lhommé, C.; Haie-Meder, C.; Duvillard, P.; Castaigne, D. Outcomes after combined therapy including surgical resection in patients with epithelial ovarian cancer recurrence(s) exclusively in lymph nodes. Ann. Surg. Oncol. 2004, 11, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Zang, R.Y.; Zhang, Z.Y.; Cai, S.M.; Tang, M.Q.; Chen, J.; Li, Z.T. Epithelial ovarian cancer presenting initially with extraabdominal or intrahepatic metastases: A preliminary report of 25 cases and literature review. Am. J. Clin. Oncol. 2000, 23, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Piciu, D.; Irimie, A.; Duncea, I.; Popita, V.; Straciuc, O.; Pestean, C.; Piciu, A.; Bara, A. Positron emission tomography - computer tomography fusion image, with 18-fluoro-2-deoxyd- glucose in the follow-up of patients with differentiated thyroid carcinoma. Acta Endocrinol. 2010, 6, 15–26. [Google Scholar]
- Larg, M.I.; Barbus, E.; Gabora, K.; Pestean, C.; Cheptea, M.; Piciu, D. 18F-FDG PET/CT in Differentiated Thyroid Carcinoma. Acta Endocrinol. 2019, 15, 203–208. [Google Scholar] [CrossRef]
- Larg, M.-I.; Apostu, D.; Peștean, C.; Gabora, K.; Bădulescu, I.C.; Olariu, E.; Piciu, D. Evaluation of Malignancy Risk in 18F-FDG PET/CT Thyroid Incidentalomas. Diagnostics 2019, 9, 92. [Google Scholar] [CrossRef] [Green Version]
- Bilici, A.; Ustaalioglu, B.B.O.; Seker, M.; Canpolat, N.; Tekinsoy, B.; Salepci, T.; Gumus, M. Clinical value of FDG PET/CT in the diagnosis of suspected recurrent ovarian cancer: Is there an impact of FDG PET/CT on patient management? Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1259–1269. [Google Scholar] [CrossRef]
- Gu, P.; Pan, L.-L.; Wu, S.-Q.; Sun, L.; Huang, G. CA 125, PET alone, PET-CT, CT and MRI in diagnosing recurrent ovarian carcinoma: A systematic review and meta-analysis. Eur. J. Radiol. 2009, 71, 164–174. [Google Scholar] [CrossRef]
- Caobelli, F.; Alongi, P.; Evangelista, L.; Picchio, M.; Saladini, G.; Rensi, M.; Geatti, O.; Castello, A.; Laghai, I.; Popescu, C.E.; et al. Predictive value of (18)F-FDG PET/CT in restaging patients affected by ovarian carcinoma: A multicentre study. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 404–413. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piciu, D.; Meșter, A.; Căinap, C.; Bărbuș, E.; Morariu, D.-S.; Piciu, A. Left Supraclavicular Lymph Node Metastasis from Ovarian Cancer Associated with Papillary Thyroid Microcarcinoma, a Confusing Pathology-Essential Role of Functional Imaging. Diagnostics 2020, 10, 270. https://doi.org/10.3390/diagnostics10050270
Piciu D, Meșter A, Căinap C, Bărbuș E, Morariu D-S, Piciu A. Left Supraclavicular Lymph Node Metastasis from Ovarian Cancer Associated with Papillary Thyroid Microcarcinoma, a Confusing Pathology-Essential Role of Functional Imaging. Diagnostics. 2020; 10(5):270. https://doi.org/10.3390/diagnostics10050270
Chicago/Turabian StylePiciu, Doina, Alexandru Meșter, Calin Căinap, Elena Bărbuș, Dragos-Stefan Morariu, and Andra Piciu. 2020. "Left Supraclavicular Lymph Node Metastasis from Ovarian Cancer Associated with Papillary Thyroid Microcarcinoma, a Confusing Pathology-Essential Role of Functional Imaging" Diagnostics 10, no. 5: 270. https://doi.org/10.3390/diagnostics10050270
APA StylePiciu, D., Meșter, A., Căinap, C., Bărbuș, E., Morariu, D.-S., & Piciu, A. (2020). Left Supraclavicular Lymph Node Metastasis from Ovarian Cancer Associated with Papillary Thyroid Microcarcinoma, a Confusing Pathology-Essential Role of Functional Imaging. Diagnostics, 10(5), 270. https://doi.org/10.3390/diagnostics10050270





