Evaluation of Uterine Artery Doppler and Estrogen Milieu in Oocyte Donation Pregnancies—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Methodology
2.3. Statistical Analysis
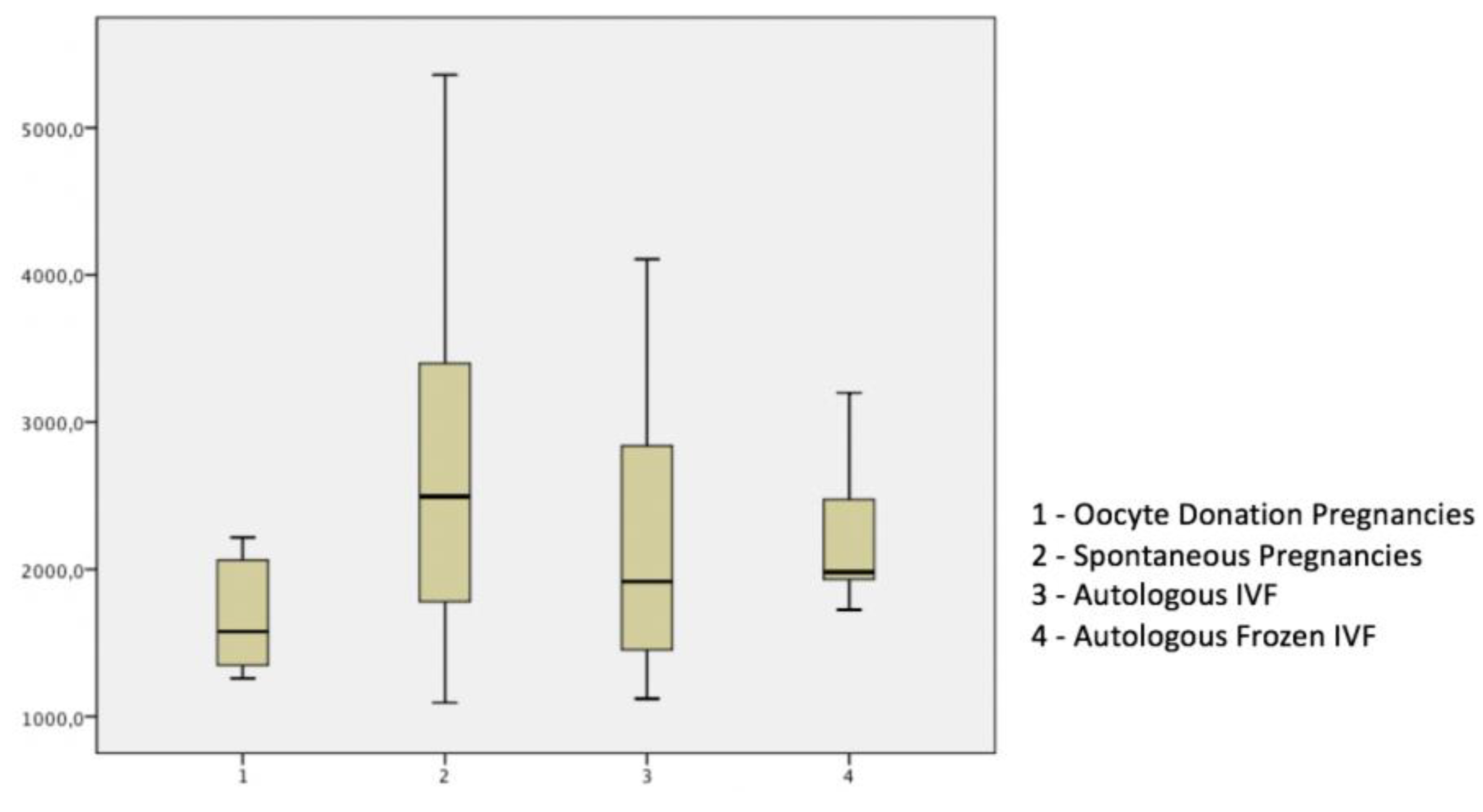
3. Results
4. Discussion
4.1. Summary of Main Findings
4.2. Interpretation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Assisted Reproductive Technology (ART). Available online: https://www.cdc.gov/art/artdata/index.html (accessed on 23 March 2020).
- Kupka, M.S.; Ferraretti, A.P.; de Mouzon, J.; Erb, K.; D’Hooghe, T.; Castilla, J.A. European IVF-Monitoring Consortium, for the European Society of Human Reproduction and Embryology. Assisted reproductive technology in Europe, 2010: Results generated from European registers by ESHRE. Hum. Reprod. 2014, 29, 2099–2113. [Google Scholar] [CrossRef]
- Van Der Hoorn, M.L.; Lashley, E.E.; Bianchi, D.W.; Claas, F.H.; Schonkeren, C.M.; Scherjon, S.A. Clinical and immunologic aspects of oocyte donation pregnancies: A systematic review. Hum. Reprod. Update 2010, 16, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Savasi, V.M.; Mandia, L.; Laoreti, A.; Cetin, I. Maternal and fetal outcomes in oocyte donation pregnancies. Hum. Reprod. Update 2016, 22, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.D.; Pepe, G.J. Estrogen regulation of placental angiogenesis and fetal ovarian development during primate pregnancy. Int. J. Dev. Biol. 2010, 54, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.; Elias, R.T.; Christos, P.J.; Petrini, A.C.; Hancock, K.; Lekovich, J.P. Supraphysiologic estradiol is an independent predictor of low birth weight in full-term singletons born after fresh embryo transfer. Hum. Reprod. 2017, 32, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Inversetti, A.; Mandia, L.; Candiani, M.; Cetin, I.; Larcher, A.; Savasi, V.M.; Papaleo, E.; Cavoretto, P. Uterine artery Doppler pulsatility index at 11–38 weeks in ICSI pregnancies with egg donation. J. Perinat. Med. 2018, 46, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Velauthar, L.; Plana, M.N.; Kalidindi, M.; Zamora, J.; Thilaganathan, B.; Illanes, S.E. First-trimester uterine artery Doppler and adverse pregnancy outcome: A meta-analysis involving 55,974 women. Ultrasound Obstet. Gynecol. 2014, 43, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.; Syngelaki, A.; de Paco Matallana, C. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef]
- Cozzolino, M.; Vitagliano, A.; Di Giovanni, M.V.; Laganà, A.S.; Vitale, S.G.; Blaganje, M.; Starič, K.D.; Borut, K.; Patrelli, T.S.; Noventa, M.; et al. Ultrasound-guided embryo transfer: Summary of the evidence and new perspectives. A systematic review and meta-analysis. Reprod. Biomed. Online 2018, 36, 524–542. [Google Scholar] [CrossRef]
- Larue, L.; Keromnes, G.; Massari, A.; Roche, C.; Moulin, J.; Gronier, H.; Bouret, D.; Cassuto, N.G.; Ayel, J.P. Transvaginal ultrasound-guided embryo transfer in IVF. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 411–416. [Google Scholar] [CrossRef]
- Gómez, O.; Figueras, F.; Fernández, S.; Bennasar, M.; Martínez, J.M.; Puerto, B. Reference ranges for uterine artery mean pulsatility index at 11–41 weeks of gestation. Ultrasound Obstet. Gynecol. 2008, 32, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Cavoretto, P.; Farina, A.; Gaeta, G.; Sigismondi, C.; Spinillo, S.; Casiero, D.; Pozzoni, M.; Vigano, P.; Papaleo, E.; Candiani, M. Longitudinal cohort study of uterine artery Doppler in singleton pregnancies obtained by IVF/ICSI with fresh or frozen blastocyst transfers in relation to pregnancy outcomes. Ultrasound Obstet. Gynecol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Aiello, E.; Pietrolucci, M.E.; Arduini, D. Placental volume and uterine artery doppler evaluation at 11 + 0 to 13 + 6 weeks of gestation in pregnancies conceived with in vitro fertilization: Comparison between autologous and donor oocyte recipients. Ultrasound Obstet. Gynecol. 2016, 47, 726–731. [Google Scholar] [CrossRef]
- Jauniaux, E.; Jurkovic, D.; Delogne-Desnoek, J.; Meuris, S. Influence of human chorionic gonadotrophin, estradiol and progesterone on uteroplacental and corpus luteum blood flow in normal early pregnancy. Hum. Reprod. 1992, 7, 1467–1473. [Google Scholar] [CrossRef]
- Conrad, K.P.; Petersen, J.W.; Chi, Y.Y.; Zhai, X.; Li, M.; Chiu, K.H.; Liu, J.; Lingis, M.D.; Williams, R.S.; Rhoton-Vlasak, A.; et al. Maternal Cardiovascular Dysregulation During Early Pregnancy After In Vitro Fertilization Cycles in the Absence of a Corpus Luteum. Hypertension 2019, 74, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Savasi, V.M.; Mandia, L.; Laoreti, A.; Ghisoni, L.; Duca, P.; Cetin, I. First trimester placental markers in oocyte donation pregnancies. Placenta 2015, 36, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Cavoretto, P.; Giorgione, V.; Cipriani, S.; Viganò, P.; Candiani, M.; Inversetti, A.; Ricci, E.; Parazzini, F. Nuchal translucency measurement, free β-hCG and PAPP-A concentrations in IVF/ICSI pregnancies: Systematic review and meta-analysis. Prenat. Diagn. 2017, 37, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J. Human and animal models: Limitations and comparisons. In The First Twelve Weeks of Gestation; Barnea, E., Hustin, J., Jauniaux, E., Eds.; Springer: Heidelberg, Germany, 1992; pp. 469–585. [Google Scholar]
- Jurkovic, D.; Bourne, T.H.; Jauniaux, E.; Campbell, S.; Collins, W.P. Transvaginal color Doppler study of blood flow in ectopic pregnancies. Fertil. Steril. 1992, 57, 68–73. [Google Scholar] [CrossRef]
- Aberdeen, G.W.; Bonagura, T.W.; Harman, C.R.; Pepe, G.J.; Albrecht, E.D. Suppression of trophoblast uterine spiral artery remodeling by estrogen during baboon pregnancy: Impact on uterine and fetal blood flow dynamics. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, 936–944. [Google Scholar] [CrossRef]
- Brosens, J.J.; Pijnenborg, R.; Brosens, I.A. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: A review of the literature. Am. J. Obstet. Gynecol. 2002, 187, 1416–1423. [Google Scholar] [CrossRef]
- Hauzman, E.E.; Garcia-Velasco, J.A.; Pellicer, A. Oocyte donation and endometriosis: What are the lessons? Semin. Reprod. Med. 2013, 31, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Šalamun, V.; Verdenik, I.; Laganà, A.S.; Vrtačnik-Bokal, E. Should we consider integrated approach for endometriosis-associated infertility as gold standard management? Rationale and results from a large cohort analysis. Arch. Gynecol. Obstet. 2018, 297, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Düsterberg, B.; Nishino, Y. Pharmacokinetic and pharmacological features of estradiol valerate. Maturitas 1982, 4, 315–324. [Google Scholar] [CrossRef]
- Wellington, K.; Perry, C.M. Estradiol valerate/dienogest. Drugs 2002, 62, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.; Parisi, F.; Duca, P.; Savasi, V.M. Maternal estradiol and progesterone concentrations among singleton spontaneous pregnancies during the first trimester. J. Endocrinol. Investig. 2018, 42, 633–638. [Google Scholar] [CrossRef] [PubMed]


| Spontaneous Pregnancies n = 122 | Autologous IVF Pregnancies n = 48 | Autologous Frozen IVF Pregnancies n = 10 | OD * Pregnancies n = 55 | ||
|---|---|---|---|---|---|
| Media (DS) | Media (DS) | Media (DS) | Media (DS) | p ** | |
| Age | 32.8 (5.02) | 36.6 (4.24) | 34.4 (4.87) | 43. 2 (4.74) | p < 0.05 a,b,c |
| BMI | 22.4 (3.78) | 23.0 (3.86) | 22.3 (3.76) | 22.4 (3,61) | ns |
| GA | 12.4 (0.53) | 12.3 (0.68) | 12.5 (0.53) | 12.2 (0.60) | ns |
| CRL | 61.3 (6.86) | 61.5 (9.17) | 62.4 (7.06) | 59.7 (7.97) | Ns |
| Spontaneous Pregnancies | Autologous IVF Pregnancies | Autologous Frozen IVF Pregnancies | OD Pregnancies * | ||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | p ** | |
| Uta PI 11–13 + 6 weeks | 1.679 (0.456) | 1.706 (0.481) | 1.692 (0.466) | 1.415 (0.486) | p < 0.05 a,b,c |
| 17 β-Estradiol pg/ml | 2827.22 (1495.9) | 2532.4.50 (1564.38) | 2285.36 (592.78) | 1674.23 (372.1) | p < 0.05 a,b,c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandia, L.; Cavoretto, P.; Duca, P.; Candiani, M.; Cetin, I.; Savasi, V. Evaluation of Uterine Artery Doppler and Estrogen Milieu in Oocyte Donation Pregnancies—A Pilot Study. Diagnostics 2020, 10, 254. https://doi.org/10.3390/diagnostics10050254
Mandia L, Cavoretto P, Duca P, Candiani M, Cetin I, Savasi V. Evaluation of Uterine Artery Doppler and Estrogen Milieu in Oocyte Donation Pregnancies—A Pilot Study. Diagnostics. 2020; 10(5):254. https://doi.org/10.3390/diagnostics10050254
Chicago/Turabian StyleMandia, Luca, Paolo Cavoretto, Piergiorgio Duca, Massimo Candiani, Irene Cetin, and Valeria Savasi. 2020. "Evaluation of Uterine Artery Doppler and Estrogen Milieu in Oocyte Donation Pregnancies—A Pilot Study" Diagnostics 10, no. 5: 254. https://doi.org/10.3390/diagnostics10050254
APA StyleMandia, L., Cavoretto, P., Duca, P., Candiani, M., Cetin, I., & Savasi, V. (2020). Evaluation of Uterine Artery Doppler and Estrogen Milieu in Oocyte Donation Pregnancies—A Pilot Study. Diagnostics, 10(5), 254. https://doi.org/10.3390/diagnostics10050254






