MicroRNA Expression Profile Changes after Cardiopulmonary Bypass and Ischemia/Reperfusion-Injury in a Porcine Model of Cardioplegic Arrest
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
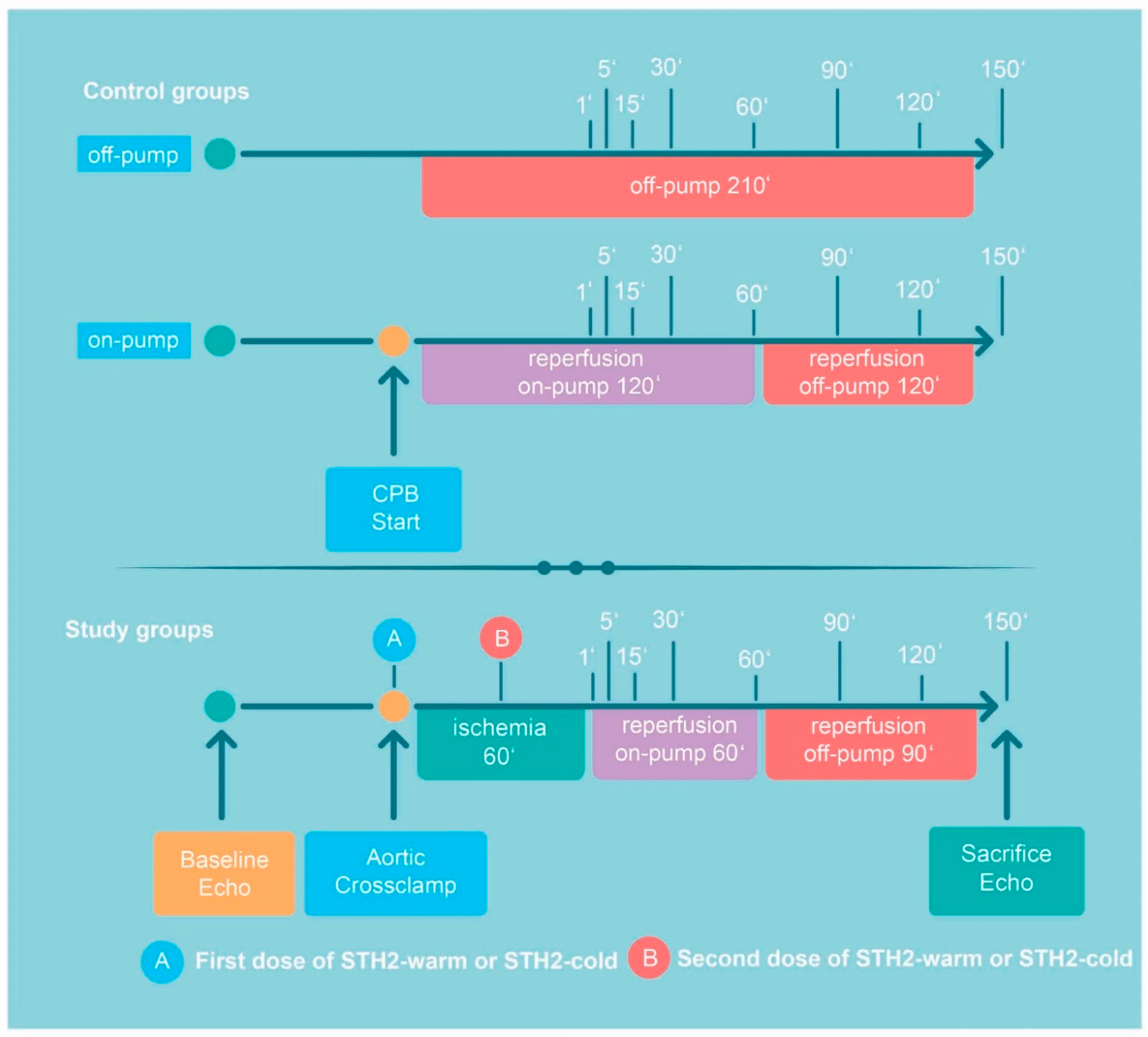
2.2. Experimental Protocol
2.3. Cardioplegic Solution
2.4. Hemodynamic Evaluation
2.5. Biochemical Analyses
2.6. Total RNA Extraction
2.7. Small RNA Sequencing
2.8. Reverse-Transcription Quantitative PCR (RT-qPCR) Messenger RNA Analysis
2.9. Statistical Analysis
3. Results
3.1. Animal Characteristics
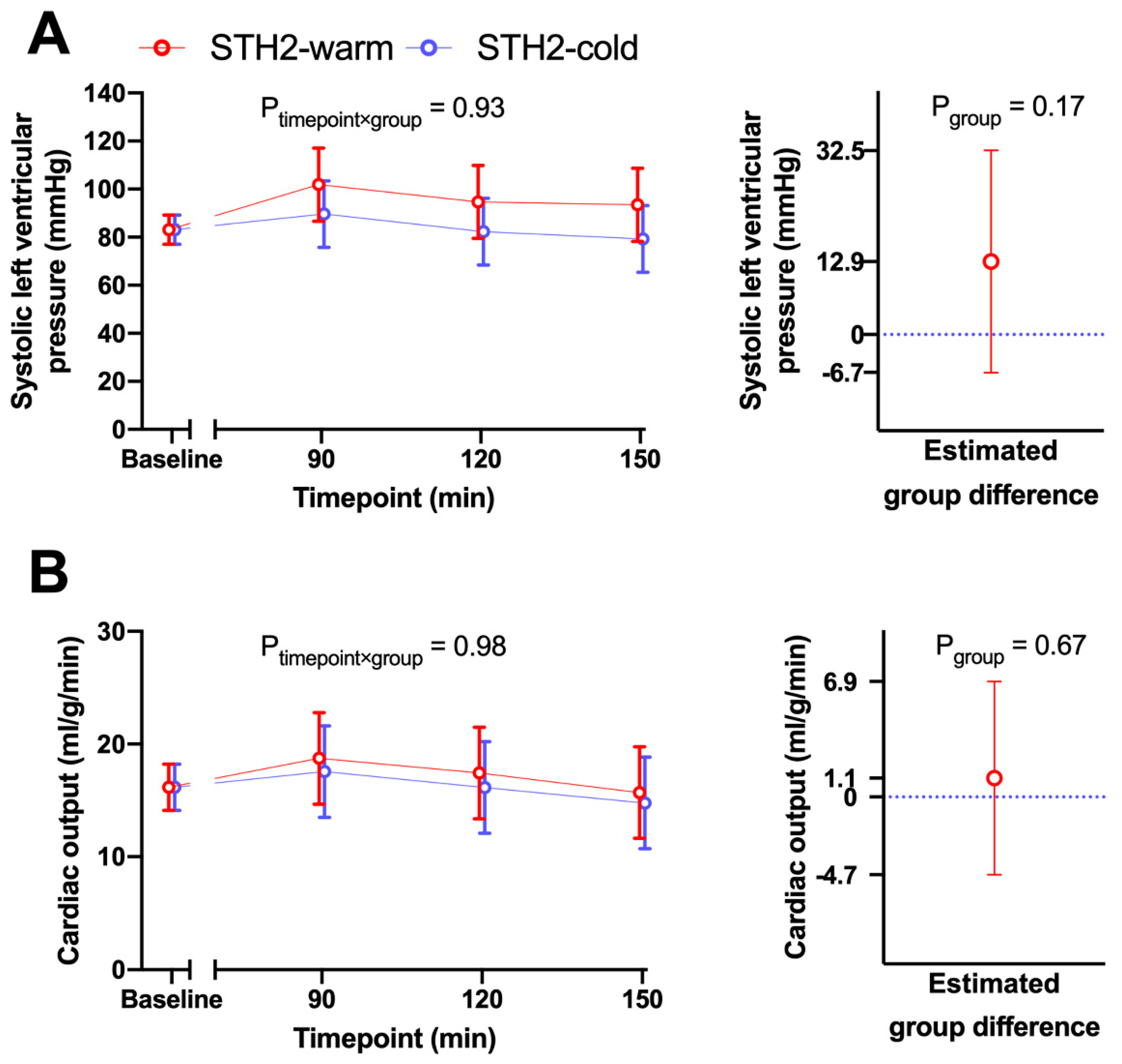
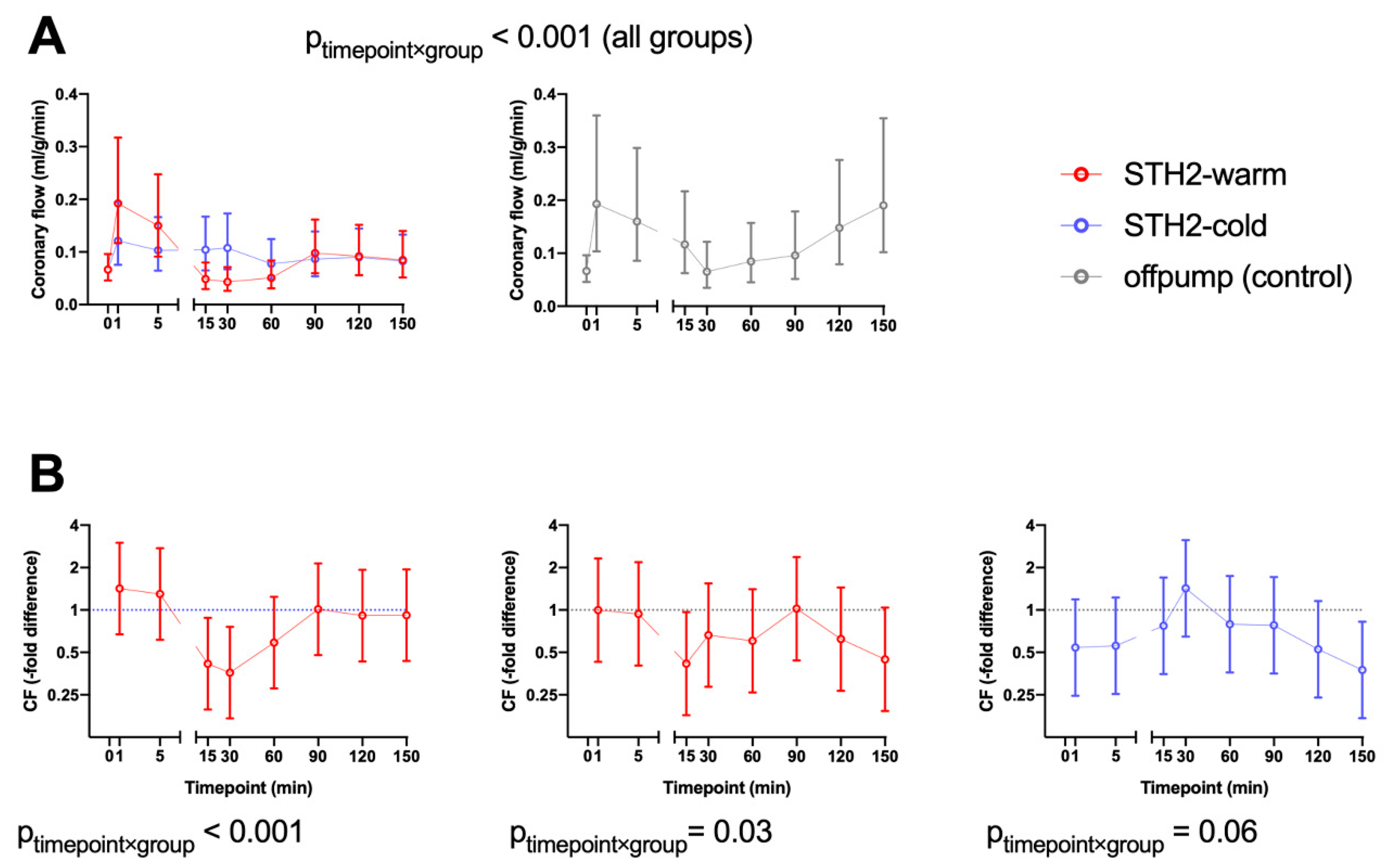
3.2. Hemodynamic Data
3.3. Biochemical Data
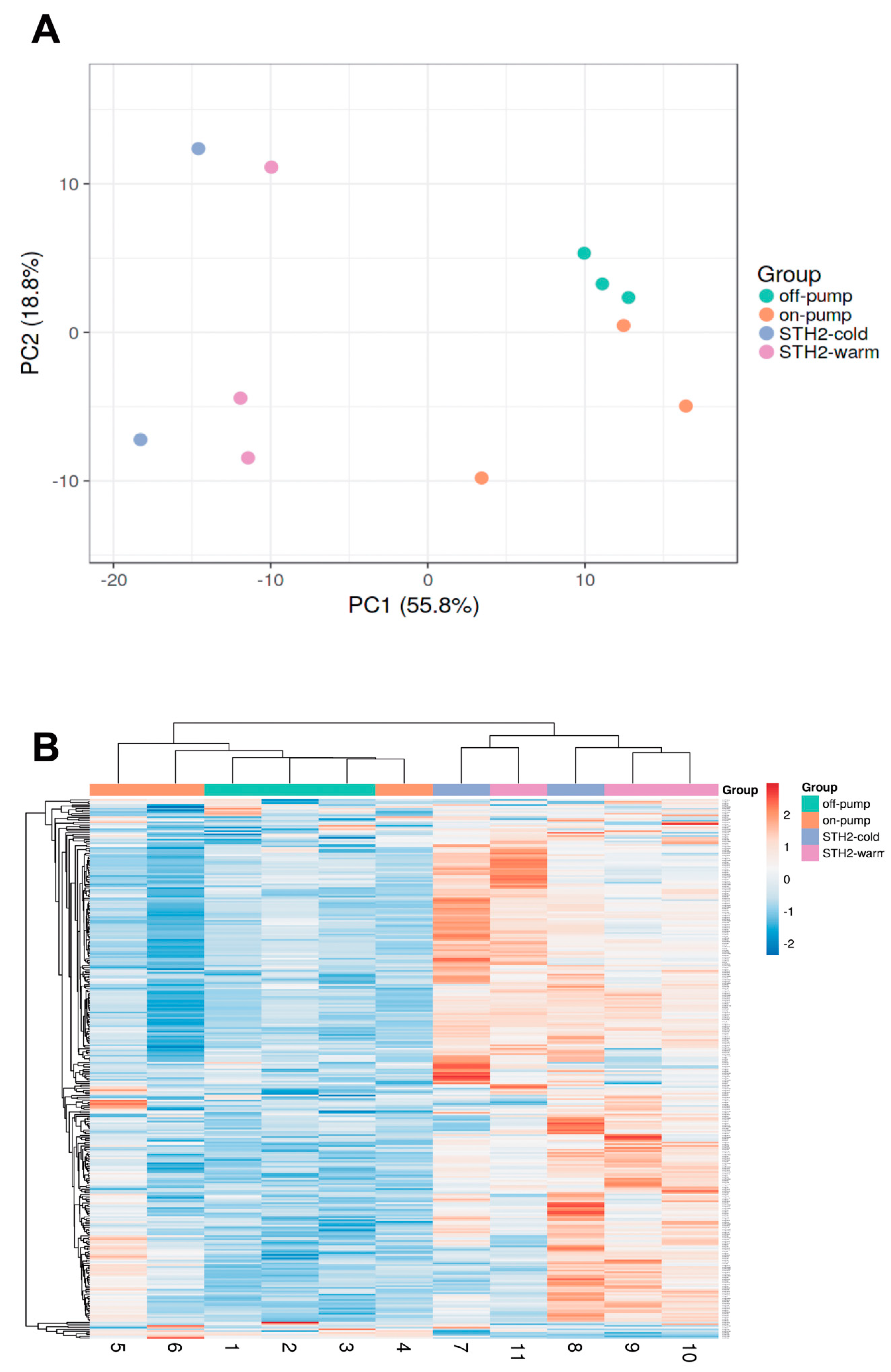
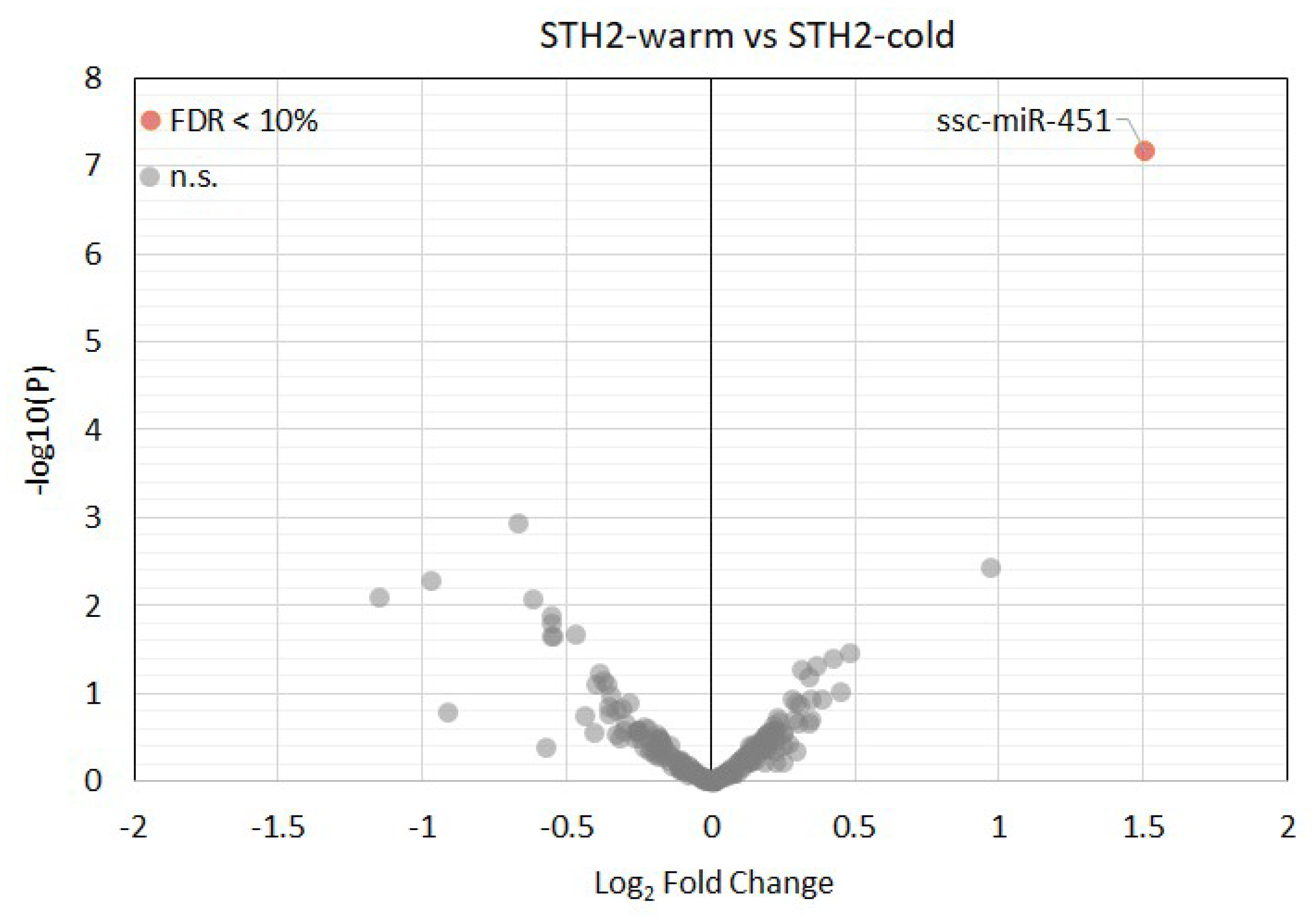
3.4. Expression of MicroRNAs in Left Ventricular Tissue Samples
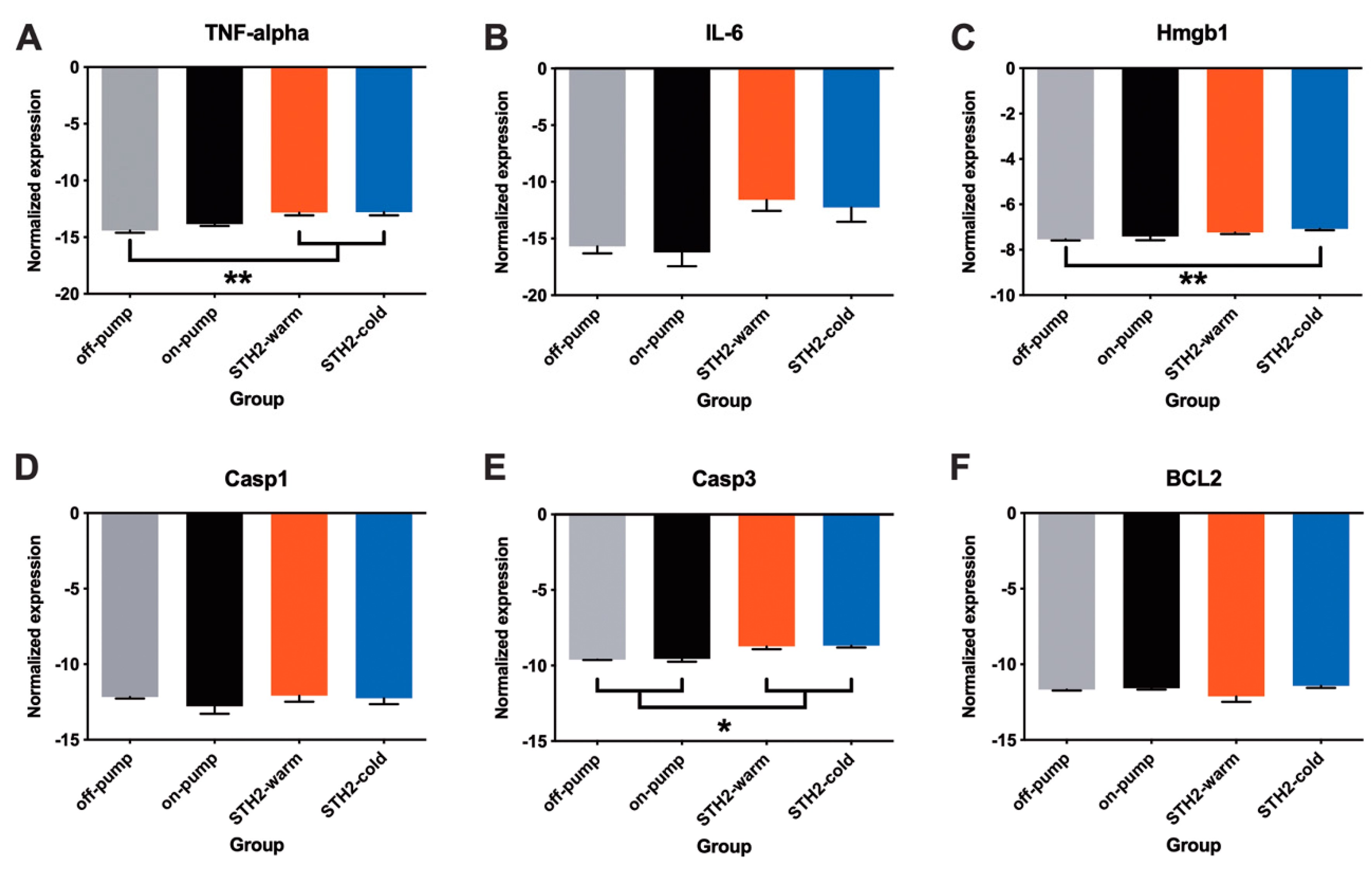
3.5. Expression of Pro-Inflammatory Cytokines and Apoptosis Markers
4. Discussion
5. Clinical Perspectives
- (1)
- microRNAs play a major role in myocardial ischemia and reperfusion injury and targeting microRNAs may be a potential therapeutic approach to achieve cardioprotecion in patients with acute myocardial infarction or elective cardiac surgery.
- (2)
- To the best of our knowledge, for the first time, we found evidence that CPB and cardioplegic arrest followed by IR leads to a specific microRNA expression profile change within the left ventricle in a translational large animal model. Notably, CPB-affected miRNAs’ are known to initiate cardioprotection.
- (3)
- Targeting specific miRNAs may be a potential therapeutic target for limiting IR injury in patients undergoing cardiac surgery.
6. Study Limitations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | arterial pressure |
| BW | body weight |
| CABG | coronary artery bypass surgery |
| CO | cardiac output |
| CF | coronary flow |
| CK-MB | creatine kinase- myocardial band |
| CPB | cardiopulmonary bypass |
| EF | ejection fraction |
| EHW | external heart work |
| FDR | false discovery rate |
| HR | heart rate |
| HW | heart weight |
| IR | ischemia-reperfusion |
| LAD | left anterior descending coronary artery |
| LV | left ventricle/left ventricular |
| LVP | left ventricular pressure |
| MAP | mean arterial pressure |
| mRNA | messenger RNA |
| miRNA | microRNA |
| PM | number of animals that demanded a pacemaker |
| RAP | right atrial pressure |
| RIC | remote ischemic conditioning |
| SEM | standard error of the mean |
| STH2 | St Thomas’ Hospital blood cardioplegia No. 2 |
| SV | stroke volume |
| SW | stroke work |
| TPM | tags per million |
References
- Sen, B.; Niemann, B.; Roth, P.; Aser, R.; Schönburg, M.; Böning, A. Short- and long-term outcomes in octogenarians after coronary artery bypass surgery. Eur. J. Cardio-Thorac. Surg. 2012, 42, 102–107. [Google Scholar] [CrossRef]
- Podesser, B.K. The ageing population—A challenge for cardiovascular surgery. Eur. Surg. Acta Chir. Austriaca 2011, 43, 63–68. [Google Scholar] [CrossRef]
- Karthik, S.; Grayson, A.D.; Oo, A.Y.; Fabri, B.M. A survey of current myocardial protection practices during coronary artery bypass grafting. Ann. R. Coll. Surg. Engl. 2004, 86, 413–415. [Google Scholar] [CrossRef]
- Barner, H.B. Blood cardioplegia: A review and comparison with crystalloid cardioplegia. Ann. Thorac. Surg. 1991, 52, 1354–1367. [Google Scholar] [CrossRef]
- Fremes, S.E.; Tamariz, M.G.; Abramov, D.; Christakis, G.T.; Sever, J.Y.; Sykora, K.; Goldman, B.S.; Feindel, C.M.; Lichtenstein, S.V. Late Results of the Warm Heart Trial: The Influence of Nonfatal Cardiac Events on Late Survival. Circulation 2000, 102 (Suppl. 3), III339–III345. [Google Scholar] [CrossRef]
- Zeriouh, M.; Heider, A.; Rahmanian, P.B.; Choi, Y.H.; Sabashnikov, A.; Scherner, M.; Popov, A.F.; Weymann, A.; Ghodsizad, A.; Deppe, A.C.; et al. Six-years survival and predictors of mortality after CABG using cold vs. warm blood cardioplegia in elective and emergent settings. J. Cardiothorac. Surg. 2015, 10, 1–13. [Google Scholar] [CrossRef]
- Trescher, K.; Gleiss, A.; Boxleitner, M.; Dietl, W.; Kassal, H.; Holzinger, C.; Podesser, B.K. Short-term clinical outcomes for intermittent cold versus intermittent warm blood cardioplegia in 2200 adult cardiac surgery patients. J. Cardiovasc. Surg. (Torino) 2017, 58, 105–112. [Google Scholar] [CrossRef]
- Boon, R.A.; Dimmeler, S. MicroRNAs in myocardial infarction. Nat. Rev. Cardiol. 2015, 12, 135–142. [Google Scholar] [CrossRef]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef]
- Wong, L.L.; Wang, J.; Liew, O.W.; Richards, A.M.; Chen, Y.T. MicroRNA and heart failure. Int. J. Mol. Sci. 2016, 17, 502. [Google Scholar] [CrossRef] [PubMed]
- Santer, D.; Kramer, A.; Kiss, A.; Aumayr, K.; Hackl, M.; Heber, S.; Chambers, D.J.; Hallström, S.; Podesser, B.K. St Thomas’ Hospital Polarizing blood cardioplegia improves hemodynamic recovery in a porcine model of cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 2019, 158, 1543–1554.e8. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2019, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.; Dihmis, W.; Bryan, A.J.; Suleiman, M.S.; Angelini, G.D. Warm Blood Hyperkalaemic Reperfusion (‘Hot Shot’) Prevents Myocardial Substrate Derangement in Patients Undergoing Coronary Artery Bypass Surgery. Eur. J. Cardio-Thorac. Surg. 1998, 13, 559–564. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, A.M.; Xiao, Y.B.; Weng, Y.G.; Hetzer, R. Warm versus cold cardioplegia for heart surgery: A meta-analysis. Eur. J. Cardio-Thorac. Surg. 2010, 37, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Christakis, G.T.; Liechtenstein, S.V.; Buth, K.J.; Fremes, S.E.; Weisel, R.D.; Naylor, C.D. The influence of risk on the results of warm heart surgery: A substudy of a randomized trial. Eur. J. Cardio-Thorac. Surg. 1997, 11, 515–520. [Google Scholar] [CrossRef]
- Pinchi, E.; Frati, P.; Aromatario, M.; Cipolloni, L.; Fabbri, M.; La Russa, R.; Maiese, A.; Neri, M.; Santurro, A.; Scopetti, M.; et al. miR-1, miR-499 and miR-208 are sensitive markers to diagnose sudden death due to early acute myocardial infarction. J. Cell. Mol. Med. 2019, 23, 6005–6016. [Google Scholar] [CrossRef]
- Schulte, C.; Barwari, T.; Joshi, A.; Theofilatos, K.; Zampetaki, A.; Barallobre-Barreiro, J.; Singh, B.; Sörensen, N.A.; Neumann, J.T.; Zeller, T.; et al. Comparative Analysis of Circulating Noncoding RNAs Versus Protein Biomarkers in the Detection of Myocardial Injury. Circ. Res. 2019, 125, 328–340. [Google Scholar] [CrossRef]
- Zhou, X.; Mao, A.; Wang, X.; Duan, X.; Yao, Y.; Zhang, C. Urine and Serum MicroRNA-1 as Novel Biomarkers for Myocardial Injury in Open-Heart Surgeries with Cardiopulmonary Bypass. PLoS ONE 2013, 8, e62245. [Google Scholar] [CrossRef]
- Engler, A.; Dreja, F.; Köberle, S.; Thielmann, M.; Peters, J.; Frey, U.H. Establishment of an easy and straight forward heparinase protocol to analyse circulating and myocardial tissue micro-RNA during coronary artery-bypass- graft surgery. Sci. Rep. 2018, 8, 1361. [Google Scholar] [CrossRef]
- Qin, H.; Chen, G.X.; Liang, M.Y.; Rong, J.; Yao, J.P.; Liu, H.; Wu, Z.K. The altered expression profile of microRNAs in cardiopulmonary bypass canine models and the effects of mir-499 on myocardial ischemic reperfusion injury. J. Transl. Med. 2013, 11, 154. [Google Scholar] [CrossRef]
- Huang, X.; Huang, F.; Yang, D.; Dong, F.; Shi, X.; Wang, H.; Zhou, X.; Wang, S.; Dai, S. Expression of microRNA-122 contributes to apoptosis in H9C2 myocytes. J. Cell. Mol. Med. 2012, 16, 2637–2646. [Google Scholar] [CrossRef]
- Liang, W.; Guo, J.; Li, J.; Bai, C.; Dong, Y. Downregulation of miR-122 attenuates hypoxia/reoxygenation (H/R)-induced myocardial cell apoptosis by upregulating GATA-4. Biochem. Biophys. Res. Commun. 2016, 478, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Vizi, D.; Khammy, O.; Mariani, J.A.; Kaye, D.M. The transcardiac gradient of cardio-microRNAs in the failing heart. Eur. J. Heart Fail. 2016, 18, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Skroblin, P.; Moschen, A.R.; Yin, X.; Kaudewitz, D.; Zampetaki, A.; Barwari, T.; Whitehead, M.; Ramírez, C.M.; Goedeke, L.; et al. Circulating MicroRNA-122 is associated with the risk of new-onset metabolic syndrome and type 2 diabetes. Diabetes 2017, 66, 347–357. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Bao, X.; Wang, X.; Wu, J.; Li, X.; Hong, W. MiR-499-5p protects cardiomyocytes against ischaemic injury via anti-apoptosis by targeting PDCD4. Oncotarget 2016, 7, 35607–35617. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Lv, X.; Ye, Z.; Sun, Y.; Kong, B.; Qin, Z.; Li, L. The mechanism of miR-142-3p in coronary microembolization-induced myocardiac injury via regulating target gene IRAK-1. Cell Death Dis. 2019, 10, 61. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, L.; You, F.; Zhou, J.; Ma, Y.; Yang, F.; Tao, L. MiR-145-5p regulates hypoxia-induced inflammatory response and apoptosis in cardiomyocytes by targeting CD40. Mol. Cell. Biochem. 2017, 431, 123–131. [Google Scholar] [CrossRef]
- Su, Q.; Liu, Y.; Lv, X.W.; Ye, Z.L.; Sun, Y.H.; Kong, B.H.; Qin, Z.B. Inhibition of lncRNA TUG1 upregulates miR-142-3p to ameliorate myocardial injury during ischemia and reperfusion via targeting HMGB1- and Rac1-induced autophagy. J. Mol. Cell. Cardiol. 2019, 133, 12–25. [Google Scholar] [CrossRef]
- Barsanti, C.; Trivella, M.G.; D’Aurizio, R.; El Baroudi, M.; Baumgart, M.; Groth, M.; Caruso, R.; Verde, A.; Botta, L.; Cozzi, L.; et al. Differential regulation of MicroRNAs in end-stage failing hearts is associated with left ventricular assist device unloading. BioMed Res. Int. 2015, 592512. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Zhang, X.; Liu, Y.; Chen, J.; Medvedovic, M.; Li, H.; Weiss, M.J.; Ren, X.; Fan, G.C. Loss of the miR-144/451 cluster impairs ischaemic preconditioning-mediated cardioprotection by targeting Rac-1. Cardiovasc. Res. 2012, 94, 379–390. [Google Scholar] [CrossRef]
- Meybohm, P.; Bein, B.; Brosteanu, O.; Cremer, J.; Gruenewald, M.; Stoppe, C.; Coburn, M.; Schaelte, G.; Böning, A.; Niemann, B.; et al. A multicenter trial of remote ischemic preconditioning for heart surgery. N. Engl. J. Med. 2015, 373, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Candilio, L.; Evans, R.; Ariti, C.; Jenkins, D.P.; Kolvekar, S.; Knight, R.; Kunst, G.; Laing, C.; Nicholas, J.; et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N. Engl. J. Med. 2015, 373, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, A.; Abbate, A.; Girola, F.; Nasso, G.; Biondi-Zoccai, G.G.; Possati, G.; Gaudino, M. Myocardial ischemia, stunning, inflammation, and apoptosis during cardiac surgery: A review of evidence. Eur. J. Cardio-Thorac. Surg. 2004, 25, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Paparella, D.; Yau, T.M.; Young, E. Cardiopulmonary bypass induced inflammation: Pathophysiology and treatment. An update. Eur. J. Cardio-Thorac. Surg. 2002, 21, 232–244. [Google Scholar] [CrossRef]
- Sangalli, F.; Guazzi, M.; Senni, S.; Sala, W.; Caruso, R.; Costa, M.C.; Formica, F.; Avalli, L.; Fumagalli, R. Assessing Endothelial Responsiveness after Cardiopulmonary Bypass: Insights on Different Perfusion Modalities. J. Cardiothorac. Vasc. Anesth. 2015, 29, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Toldo, S.; Mauro, A.G.; Cutter, Z.; Abbate, A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1553–H1568. [Google Scholar] [CrossRef]
- Haque, A.; Kunimoto, F.; Narahara, H.; Okawa, M.; Hinohara, H.; Kurabayashi, M.; Saito, S. High mobility group box 1 levels in on and off-pump cardiac surgery patients. Int. Heart J. 2011, 52, 170–174. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, A.; Heber, S.; Kramer, A.-M.; Hackl, M.; Skalicky, S.; Hallström, S.; Podesser, B.K.; Santer, D. MicroRNA Expression Profile Changes after Cardiopulmonary Bypass and Ischemia/Reperfusion-Injury in a Porcine Model of Cardioplegic Arrest. Diagnostics 2020, 10, 240. https://doi.org/10.3390/diagnostics10040240
Kiss A, Heber S, Kramer A-M, Hackl M, Skalicky S, Hallström S, Podesser BK, Santer D. MicroRNA Expression Profile Changes after Cardiopulmonary Bypass and Ischemia/Reperfusion-Injury in a Porcine Model of Cardioplegic Arrest. Diagnostics. 2020; 10(4):240. https://doi.org/10.3390/diagnostics10040240
Chicago/Turabian StyleKiss, Attila, Stefan Heber, Anne-Margarethe Kramer, Matthias Hackl, Susanna Skalicky, Seth Hallström, Bruno K. Podesser, and David Santer. 2020. "MicroRNA Expression Profile Changes after Cardiopulmonary Bypass and Ischemia/Reperfusion-Injury in a Porcine Model of Cardioplegic Arrest" Diagnostics 10, no. 4: 240. https://doi.org/10.3390/diagnostics10040240
APA StyleKiss, A., Heber, S., Kramer, A.-M., Hackl, M., Skalicky, S., Hallström, S., Podesser, B. K., & Santer, D. (2020). MicroRNA Expression Profile Changes after Cardiopulmonary Bypass and Ischemia/Reperfusion-Injury in a Porcine Model of Cardioplegic Arrest. Diagnostics, 10(4), 240. https://doi.org/10.3390/diagnostics10040240







