Coronary Artery Disease is More Severe in Patients with Non-Alcoholic Steatohepatitis than Fatty Liver
Abstract
1. Introduction
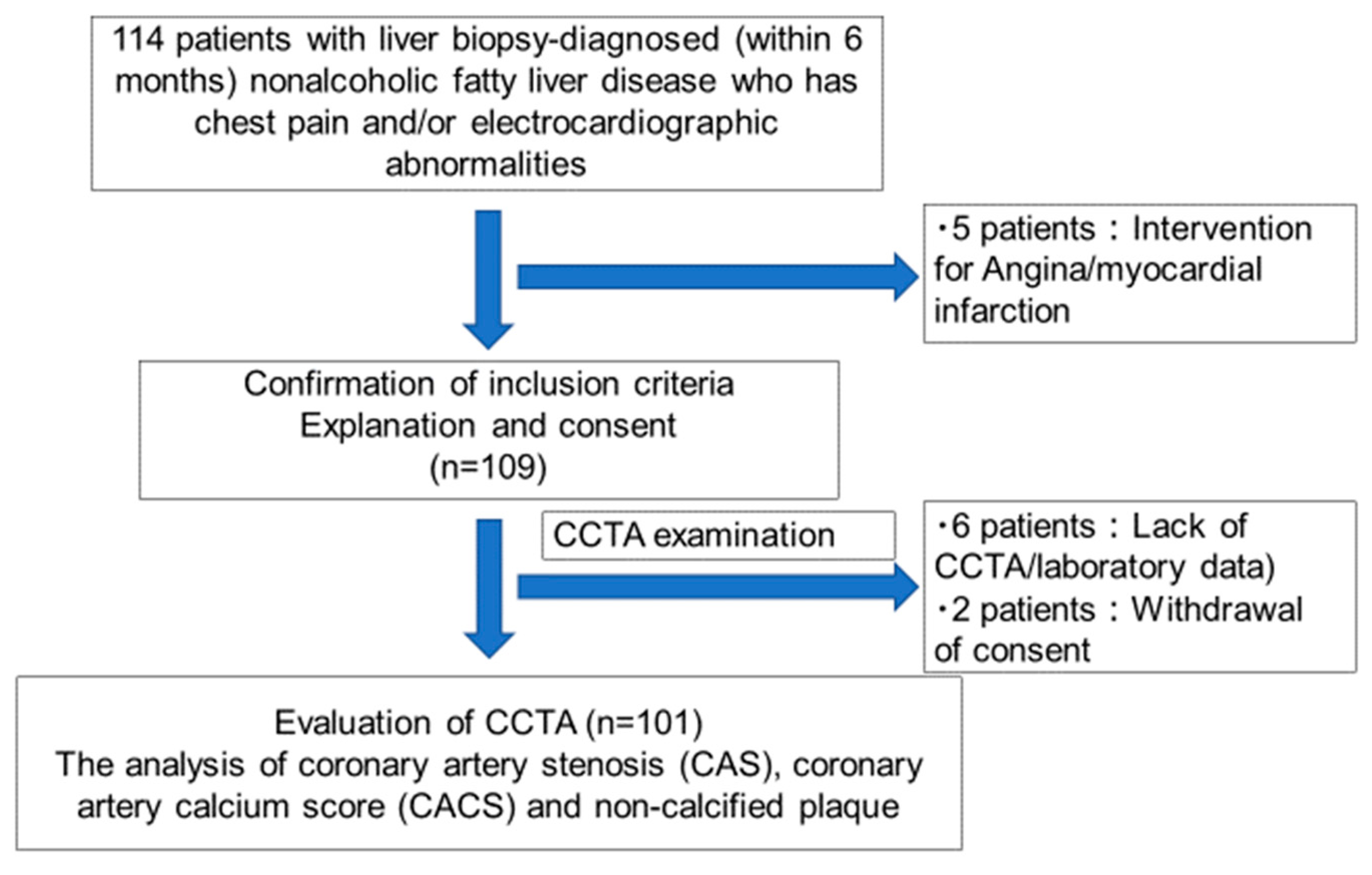
2. Patients and Methods
2.1. Ethical Approval of the Study Protocol
2.2. Study Population
2.3. Clinical and Laboratory Measurements
2.4. Histopathologic and Immunohistochemical Evaluations
2.5. CCTA
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
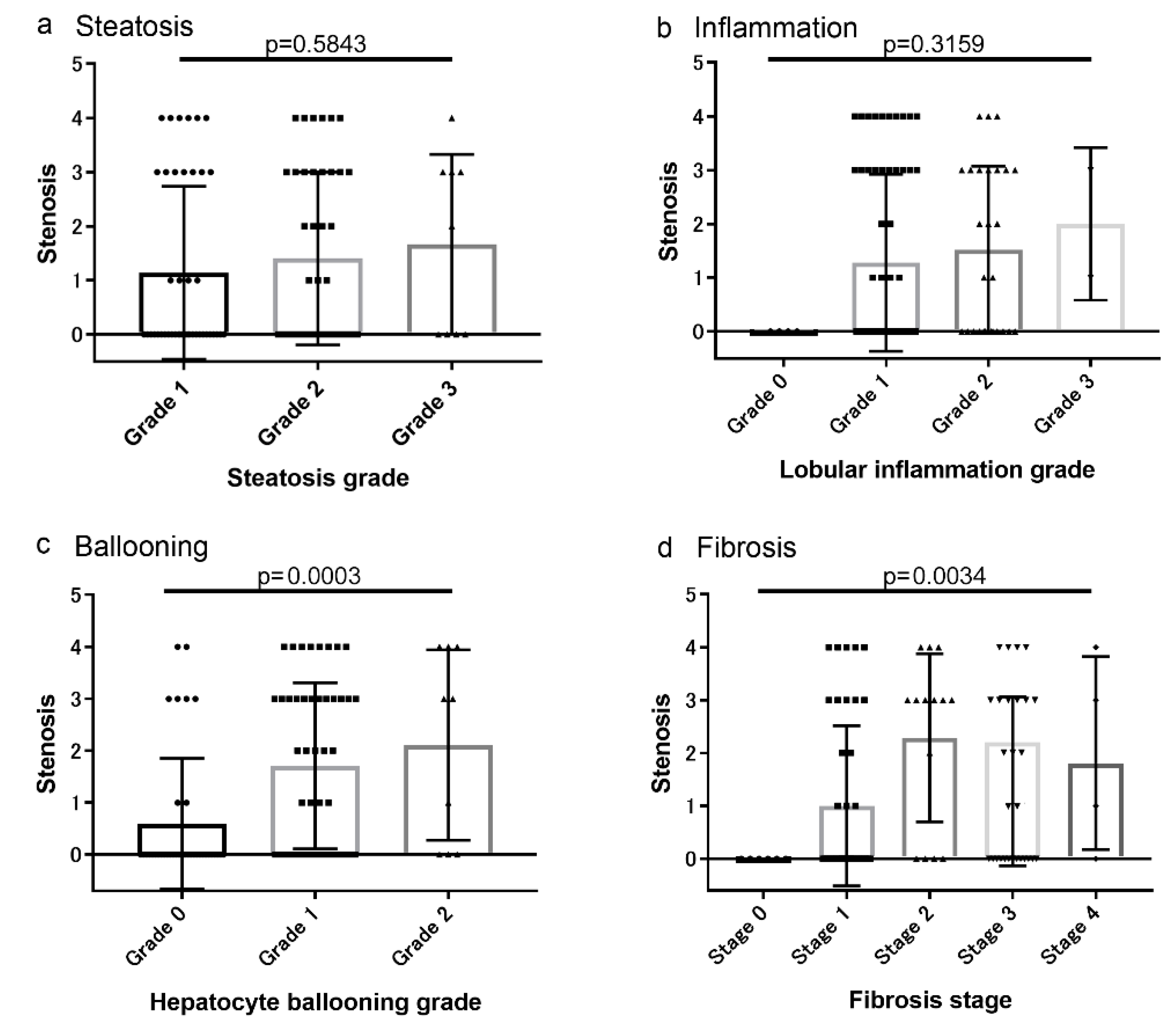
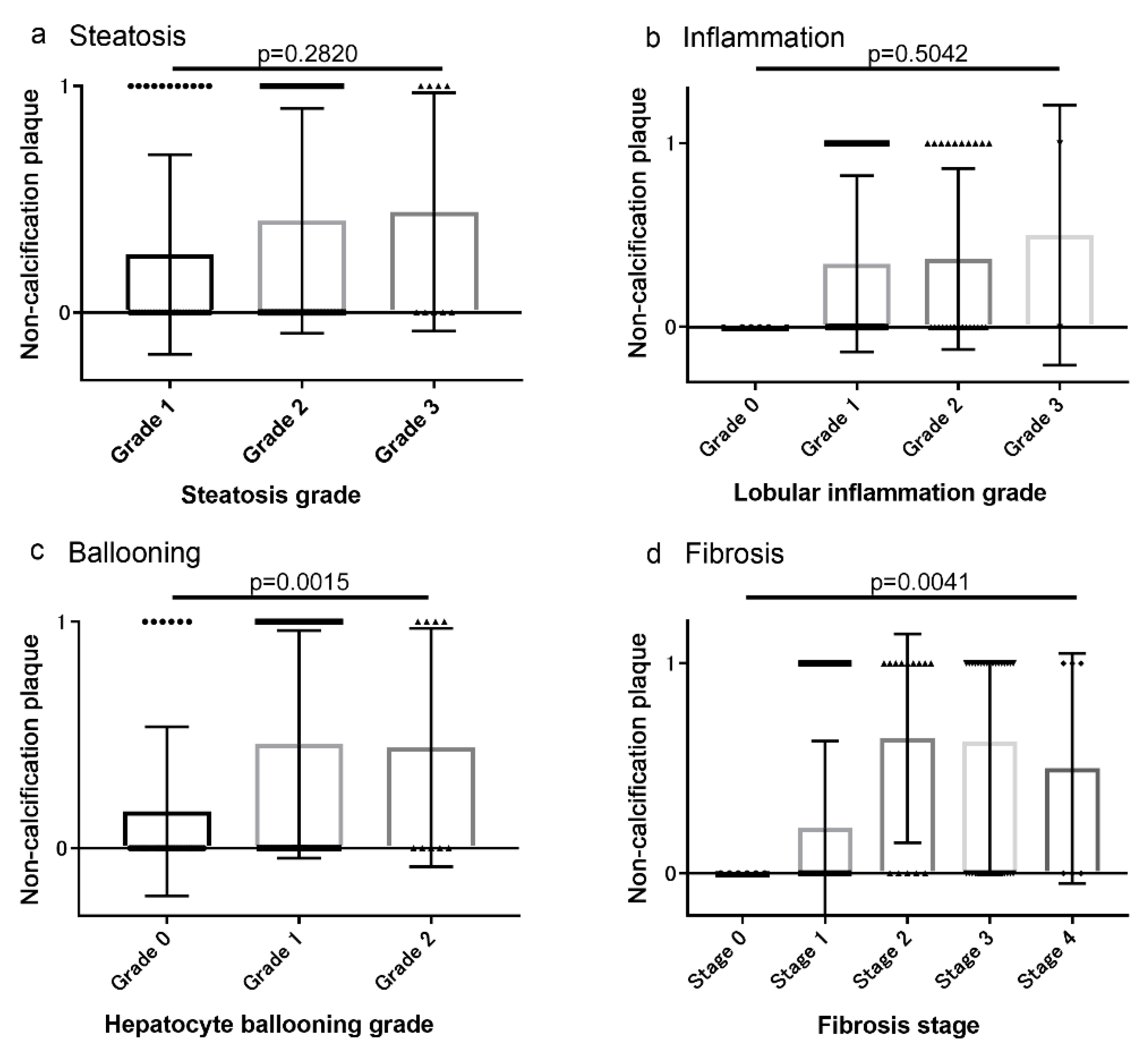
3.2. Associations between Coronary Artery Lesions and Histologic Findings
3.3. Multiple Regression Analysis of Potential Risk Factors for CAS, CACS, and non-Calcified Plaque
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| CACS | Coronary artery calcium score |
| CAD | Coronary artery disease |
| CAS | Coronary artery stenosis |
| CCTA | Coronary computed tomography angiography |
| CVD | Cardiovascular disease |
| DLP | Dyslipidemia |
| DM | Diabetes mellitus |
| FLIP | Fatty Liver Inhibition of Progression |
| GGT | Gamma-glutamyl transpeptidase |
| CRP | C-reactive protein |
| HbA1c | Hemoglobin A1c |
| HDL-C | High-density lipoprotein-cholesterol |
| HT | Hypertension |
| HU | Hounsfield units |
| LDL-MI | Low-density lipoprotein-migration index |
| MSCT | Multi-slice computer tomography |
| NAFL | Non-alcoholic fatty liver |
| NAFLD | Non-alcoholic fatty liver disease |
| NAS | Non-alcoholic fatty liver disease activity score |
| NASH | Non-alcoholic steatohepatitis |
| SCCT | Society of Cardiovascular Computed Tomography |
| SdLDL | Small dense low-density lipoprotein |
| VLDL | Very low-density lipoprotein |
References
- Brea, A.; Mosquera, D.; Martin, E.; Arizti, A.; Cordero, J.L.; Ros, E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: case-control study. Arter. Thromb Vasc Biol 2005, 25, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Arcaro, G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis 2007, 191, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mingfeng, X.; Ma, H.; Hofman, A.; Hu, Y.; Yan, H.; He, W.; Lin, H.; Jeekel, J.; Zhao, N.; et al. Liver fat content is associated with increased carotid atherosclerosis in Chinese middle-agedand elderly population: Shanghai Changfeng study. Atherosclerosis 2012, 224, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Padovani, R.; Rodella, S.; Zoppini, G.; Zenari, L.; Cigolini, M.; Falezza, G.; Arcaro, G. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care 2006, 29, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Schindhelm, R.K.; Diamant, M.; Bakker, S.J.; van Dijk, R.A.; Scheffer, P.G.; Teerlink, T.; Kostense, P.J.; Heine, R.J. Liver alanine aminotransferase, insulin resistance and endothelial dysfunction in normotriglyceridaemic subjects with type 2 diabetes mellitus. Eur. J. Clin Invest. 2005, 35, 369–374. [Google Scholar] [CrossRef]
- Chen, C.H.; Nien, C.K.; Yang, C.C.; Yeh, Y.H. Association between nonalcoholic fatty liver disease and coronary artery calcification. Dig. Dis Sci 2010, 55, 1752–1760. [Google Scholar] [CrossRef]
- Akabame, S.; Hamaguchi, M.; Tomiyasu, K.; Tanaka, M.; Kobayashi-Takenaka, Y.; Nakano, K.; Oda, Y.; Yoshikawa, T. Evaluation of vulnerable coronary plaques and non-alcoholic fatty liver disease (NAFLD) by 64-detector multislice computed tomography (MSCT). Circ. J. 2008, 72, 618–625. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, N.H.; Kim, B.S.; Kang, J.H. The association between nonalcoholic fatty liver disease, metabolic syndrome and arterial stiffness in nondiabetic, nonhypertensive individuals. Cardiology 2012, 123, 54–61. [Google Scholar] [CrossRef]
- Park, G.M.; Yun, S.C.; Cho, Y.R.; Gil, E.H.; Her, S.H.; Kim, S.H.; Jo, M.W.; Lee, M.S.; Lee, S.W.; Kim, Y.H. Prevalence of coronary atherosclerosis in an Asian population: findings from coronary computed tomographic angiography. Int J. Cardiovasc Imaging 2015, 31, 659–668. [Google Scholar] [CrossRef]
- Tesche, C.; Plank, F.; De Cecco, C.N.; Duguay, T.M.; Albrecht, M.H.; Varga-Szemes, A.; Bayer, R.R., 2nd; Yang, J.; Jacks, I.L.; Gramer, B.M.; et al. Prognostic implications of coronary CT angiography-derived quantitative markers for the prediction of major adverse cardiac events. J. Cardiovasc Comput Tomogr. 2016, 6, 8–465. [Google Scholar] [CrossRef]
- Oudkerk, M.; Stillman, A.E.; Halliburton, S.S.; Kalender, W.A.; Möhlenkamp, S.; McCollough, C.H.; Vliegenthart, R.; Shaw, L.J.; Stanford, W.; Taylor, A.J.; et al. Coronary artery calcium screening: current status and recommendations from the European Society of Cardiac Radiology and North American Society for Cardiovascular Imaging. Int J. Cardiovasc Imaging 2008, 24, 645–671. [Google Scholar] [CrossRef] [PubMed]
- Sinn, D.H.; Kang, D.; Chang, Y.; Ryu, S.; Gu, S.; Kim, H.; Seong, D.; Cho, S.J.; Yi, B.K.; Park, H.D. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut 2017, 66, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Nozaki, Y.; Wada, K.; Yoneda, M.; Fujimoto, Y.; Fujitake, M.; Endo, H.; Takahashi, H.; Inamori, M.; Kobayashi, N.; et al. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology 2009, 50, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Imajo, K.; Hyogo, H.; Yoneda, M.; Honda, Y.; Kessoku, T.; Tomeno, W.; Ogawa, Y.; Taguri, M.; Mawatari, H.; Nozaki, Y.; et al. LDL-migration index (LDL-MI), an indicator of small dense low-density lipoprotein (sdLDL), is higher in non-alcoholic steatohepatitis than in non-alcoholic fatty liver: a multicenter cross-sectional study. Plos One 2014, 9, e115403. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Kechagias, S.; Stål, P.; Bedossa, P.; Hultcrantz, R. SAF score and mortality in NAFLD after up to 41 years of follow-up. Scand J. Gastroenterol 2017, 52, 87–91. [Google Scholar] [CrossRef]
- Brunt, E.M. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis 2001, 21, 3–16. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zus- mer, N.R.; Viamonte, M., Jr.; Detrano, R. Quan- tification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll Cardiol 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Kumamaru, K.K.; Kondo, T.; Kumamaru, H.; Amanuma, M.; George, E.; Rybicki, F.J. Repeat coronary computed tomographic angiography in patients with a prior scan excluding significant stenosis. Circ Cardiovasc Imaging 2014, 7, 788–795. [Google Scholar] [CrossRef][Green Version]
- Lee, J.I.; Kim, M.C.; Moon, B.S.; Song, Y.S.; Han, E.N.; Lee, H.S.; Son, Y.; Kim, J.; Han, E.J.; Park, H.J.; et al. The Relationship between 10-Year Cardiovascular Risk Calculated Using the Pooled Cohort Equation and the Severity of Non-Alcoholic Fatty Liver Disease. Endocrinol Metab 2016, 3, 86–92. [Google Scholar] [CrossRef]
- Lee, M.K.; Park, H.J.; Jeon, W.S.; Park, S.E.; Park, C.Y.; Lee, W.Y.; Oh., K.W.; Park, S.W.; Rhee, E.J. Higher association of coronary artery calcification with non-alcoholic fatty liver disease than with abdominal obesity in middle-aged Korean men: the Kangbuk Samsung Health Study. Cardiovasc. Diabetol. 2015, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Jaruvongvanich, V.; Wirunsawanya, K.; Sanguankeo, A.; Upala, S. Nonalcoholic fatty liver disease is associated with coronary artery calcification: A systematic review and meta-analysis. Dig. Liver Dis 2016, 48, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Choi, S.Y.; Park, E.H.; Lee, W.; Kang, J.H.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Jeong, S.H.; Lee, D.H.; et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology 2012, 56, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.E.; Chang, S.A.; Choi, S.I.; Chun, E.J.; Cho, Y.S.; Youn, T.J.; Chung, W.Y.; Chae, I.H.; Choi, D.J.; Chang, H.J. The absence of coronary artery calcification does not rule out the presence of significant coronary artery disease in Asian patients with acute chest pain. Int J. Cardiovasc Imaging 2012, 28, 389–398. [Google Scholar] [CrossRef]
- Puchner, S.B.; Lu, M.T.; Mayrhofer, T.; Liu, T.; Pursnani, A.; Ghoshhajra, B.B.; Truong, Q.A.; Wiviott, S.D.; Fleg, J.L.; Hoffmann, U.; et al. High-risk coronary plaque at coronary CT angiography is associated with nonalcoholic fatty liver disease, independent of coronary plaque and stenosis burden: results from the ROMICAT II trial. Radiology 2015, 274, 693–701. [Google Scholar] [CrossRef]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef]
- Després, J.P.; Lamarche, B.; Mauriège, P.; Cantin, B.; Dagenais, G.R.; Moorjani, S.; Lupien, P.J. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J. Med. 1996, 334, 952–957. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N Engl J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Imajo, K.; Fujita, K.; Yoneda, M.; Shinohara, Y.; Suzuki, K.; Mawatari, H.; Takahashi, J.; Nozaki, Y.; Sumida, Y.; Kirikoshi, H.; et al. Plasma free choline is a novel non- invasive biomarker for early-stage non-alcoholic steatohepatitis: A multi-center validation study. Hepatol Res. 2012, 42, 757–766. [Google Scholar] [CrossRef]
- Imajo, K.; Yoneda, M.; Fujita, K.; Kessoku, T.; Tomeno, W.; Ogawa, Y.; Shinohara, Y.; Sekino, Y.; Mawatari, H.; Nozaki, Y.; et al. Oral choline tolerance test as a novel noninvasive method for predicting nonalcoholic steatohepatitis. J. Gastroenterol 2013, 49, 295–304. [Google Scholar] [CrossRef]
- Packard, C.J. Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoprotein. Biochem Soc Trans. 2003, 31, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Krauss, R.M. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 2004, 27, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.T.; Superko, H.R.; Haskell, W.L.; Alderman, E.L.; Blanche, P.J.; Holl, L.G.; Krauss, R.M. Smallest LDL particles are most strongly related to coronary disease progression in men. Arter. Thromb Vasc Biol 2003, 23, 314–321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Wong, V.W.S.; Castellanos, M.; Aller-de la Fuente, R.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Alvarez-Quiñones Sanz, M.; Conde-Martin, A.F.; et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018, 155, 443–457. [Google Scholar] [CrossRef]

| NAFL | NASH | p value * | |
|---|---|---|---|
| Number (n) | 41 | 60 | |
| Age (years) | 52.7 ± 13.9 | 59.6 ± 11.2 | 0.0090 * |
| Gender (male; female) | 24; 17 | 26; 34 | 0.0471 * |
| Body mass index (kg/m2) | 28.5 ± 4.2 | 27.8 ± 3.9 | 0.4803 |
| AST (IU/l) | 50.9 ± 33.1 | 51.1 ± 21.3 | 0.9749 |
| ALT (IU/l) | 73.6 ± 45.5 | 62.7 ± 30.5 | 0.1694 |
| C-reactive protein (mg/l) | 0.22 ± 0.35 | 0.19 ± 0.20 | 0.6555 |
| Creatine (mg/dl) | 0.73 ± 0.17 | 0.67 ± 0.17 | 0.1095 |
| Fasting blood glucose (mg/dl) | 117.6 ± 25.0 | 118.8 ± 30.0 | 0.8435 |
| Fasting insulin (mU/l) | 13.4 ± 7.09 | 18.6 ± 17.4 | 0.0915 |
| HbA1c | 6.23 ± 0.97 | 6.45 ± 1.08 | 0.3405 |
| Diabetes mellitus | 21 (51.2) | 42 (68.3) | 0.4714 |
| Hypertension (%) | 16 (39.0) | 28 (46.7) | 0.5188 |
| Dyslipidemia (%) | 24 (58.5) | 46 (76.7) | 0.3008 |
| Steatosis grade | 0.5734 | ||
| 5–33% | 21 | 25 | |
| 33–66% | 17 | 29 | |
| >66% | 3 | 6 | |
| Lobular inflammation | <0.0001 * | ||
| None | 6 | 0 | |
| <2 foci per 200× field | 32 | 32 | |
| 2–4 foci per 200× field | 3 | 26 | |
| >4 foci per 200× field | 0 | 2 | |
| Hepatocyte ballooning | <0.0001 * | ||
| None | 38 | 0 | |
| Few balloon cells | 3 | 51 | |
| Many balloon cells | 0 | 9 | |
| NAFLD activity score (NAS) | 2.52 ± 0.86 | 4.22 ± 1.17 | <0.0001 * |
| Fibrosis stage | 0.0009 * | ||
| None | 7 | 0 | |
| Perisinusoidal or periportal | 21 | 24 | |
| Perisinusoidal and portal/periportal | 3 | 13 | |
| Bridging fibrosis | 9 | 19 | |
| Cirrhosis | 1 | 4 |
| Factors | Absence (n = 51) | Presence (n = 50) | Univariate p value * p < 0.05 | Multiple p value * p < 0.05 |
|---|---|---|---|---|
| Age (years) | 52.4 ± 12.6 | 62.5 ± 10.8 | 0.0001 * | 0.0049 * |
| Gender (male; female) | 22; 29 | 29; 21 | 0.2201 | |
| Body mass index (kg/m2) | 28.0 ± 4.27 | 28.3 ± 3.73 | 0.8314 | |
| Smoking score | 120.8 ± 354.3 | 358.2 ± 454.6 | 0.0042 * | 0.0147 * |
| Serum ALT levels | 72.9 ± 44.9 | 62.8 ± 26.0 | 0.1730 | |
| Serum GGT levels | 90.3 ± 74.5 | 66.4 ± 41.5 | 0.0647 | |
| Renal dysfunction (%) | 3.9 | 9.3 | 0.2863 | |
| Diabetes mellitus (%) | 50.9 | 74.0 | 0.0354 * | 0.6901 |
| Hypertension (%) | 33.3 | 51.1 | 0.0801 * | 0.8215 |
| Dyslipidemia (%) | 56.9 | 79.0 | 0.0208 * | 0.0373 * |
| Nonalcoholic steatohepatitis | 43.1 | 82.0 | 0.0085 * | 0.0102 * |
| Fibrosis stage | 1.52 ± 1.06 | 2.34 ± 1.00 | 0.0002 * | 0.0450 * |
| Factors | Absence (score = 0) (n = 50) | Presence (score > 0) (n = 51) | Univariate p value * p < 0.05 | Multiple p value * p < 0.05 |
|---|---|---|---|---|
| Age (years) | 51.6 ± 12.7 | 63.1 ± 9.6 | <0.0001 * | 0.0023 * |
| Gender (male; female) | 23; 27 | 28; 23 | 0.3707 | |
| Body mass index (kg/m2) | 28.2 ± 4.19 | 28.1 ± 3.70 | 0.9470 | |
| Smoking score | 165.4 ± 377.2 | 309.8 ± 454.4 | 0.0857 | |
| Serum ALT levels | 73.1 ± 42.7 | 62.8 ± 29.8 | 0.1648 | |
| Serum GGT levels | 90.8 ± 74.9 | 66.4 ± 41.7 | 0.0588 | |
| Renal dysfunction (%) | 3.9 | 9.3 | 0.2863 | |
| Diabetes mellitus (%) | 44.0 | 80.4 | 0.0001 * | 0.0232 * |
| Hypertension (%) | 28.0 | 58.9 | 0.0016 * | 0.2039 |
| Dyslipidemia (%) | 56.0 | 82.3 | 0.0037 * | 0.0118 * |
| Nonalcoholic steatohepatitis | 48.0 | 76.4 | 0.0029 * | 0.0987 |
| Fibrosis stage | 1.62 ± 1.06 | 2.23 ± 1.07 | 0.0047 * | 0.1892 |
| Factors | Absence (n = 63) | Presence (n = 38) | Univariate p value * p < 0.05 | Multiple p value * p < 0.05 |
|---|---|---|---|---|
| Age (years) | 54.3 ± 12.8 | 62.2 ± 10.8 | 0.0012 * | 0.0688 |
| Gender (male; female) | 28; 35 | 23; 15 | 0.1163 | |
| Body mass index (kg/m2) | 28.1 ± 3.91 | 28.3 ± 4.01 | 0.8136 | |
| Smoking score | 156.5 ± 384.2 | 373.9 ± 451.7 | 0.0114 * | 0.0684 |
| Serum ALT levels | 68.8 ± 42.0 | 66.4 ± 26.9 | 0.7494 | |
| Serum GGT levels | 86.0 ± 69.3 | 66.5 ± 44.9 | 0.1532 | |
| Renal dysfunction (%) | 4.8 | 9.4 | 0.4058 | |
| Diabetes mellitus (%) | 55.6 | 73.7 | 0.0255 * | 0.9736 |
| Hypertension (%) | 34.9 | 57.9 | 0.0240 * | 0.6271 |
| Dyslipidemia (%) | 58.7 | 86.8 | 0.0020 * | 0.0240 * |
| Nonalcoholic steatohepatitis | 49.2 | 84.2 | 0.0003 * | 0.0307 * |
| Fibrosis stage | 1.67 ± 1.12 | 2.37 ± 0.94 | 0.0017 * | 0.0947 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niikura, T.; Imajo, K.; Ozaki, A.; Kobayashi, T.; Iwaki, M.; Honda, Y.; Kessoku, T.; Ogawa, Y.; Yoneda, M.; Kirikoshi, H.; et al. Coronary Artery Disease is More Severe in Patients with Non-Alcoholic Steatohepatitis than Fatty Liver. Diagnostics 2020, 10, 129. https://doi.org/10.3390/diagnostics10030129
Niikura T, Imajo K, Ozaki A, Kobayashi T, Iwaki M, Honda Y, Kessoku T, Ogawa Y, Yoneda M, Kirikoshi H, et al. Coronary Artery Disease is More Severe in Patients with Non-Alcoholic Steatohepatitis than Fatty Liver. Diagnostics. 2020; 10(3):129. https://doi.org/10.3390/diagnostics10030129
Chicago/Turabian StyleNiikura, Toshihiro, Kento Imajo, Anna Ozaki, Takashi Kobayashi, Michihiro Iwaki, Yasushi Honda, Takaomi Kessoku, Yuji Ogawa, Masato Yoneda, Hiroyuki Kirikoshi, and et al. 2020. "Coronary Artery Disease is More Severe in Patients with Non-Alcoholic Steatohepatitis than Fatty Liver" Diagnostics 10, no. 3: 129. https://doi.org/10.3390/diagnostics10030129
APA StyleNiikura, T., Imajo, K., Ozaki, A., Kobayashi, T., Iwaki, M., Honda, Y., Kessoku, T., Ogawa, Y., Yoneda, M., Kirikoshi, H., Saito, S., & Nakajima, A. (2020). Coronary Artery Disease is More Severe in Patients with Non-Alcoholic Steatohepatitis than Fatty Liver. Diagnostics, 10(3), 129. https://doi.org/10.3390/diagnostics10030129







