Analytical Evaluation of the New Beckman Coulter Access Procalcitonin (PCT) Chemiluminescent Immunoassay
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Access PCT Immunoassay
2.2. Evaluation of the Analytical Performance of Access PCT
2.3. Imprecision Studies
2.4. Linearity
2.5. Method Comparison
2.6. Statistics and Ethics Committee Approval
3. Results
3.1. Analytical Performance
3.2. Imprecision Studies
3.3. Linearity Studies
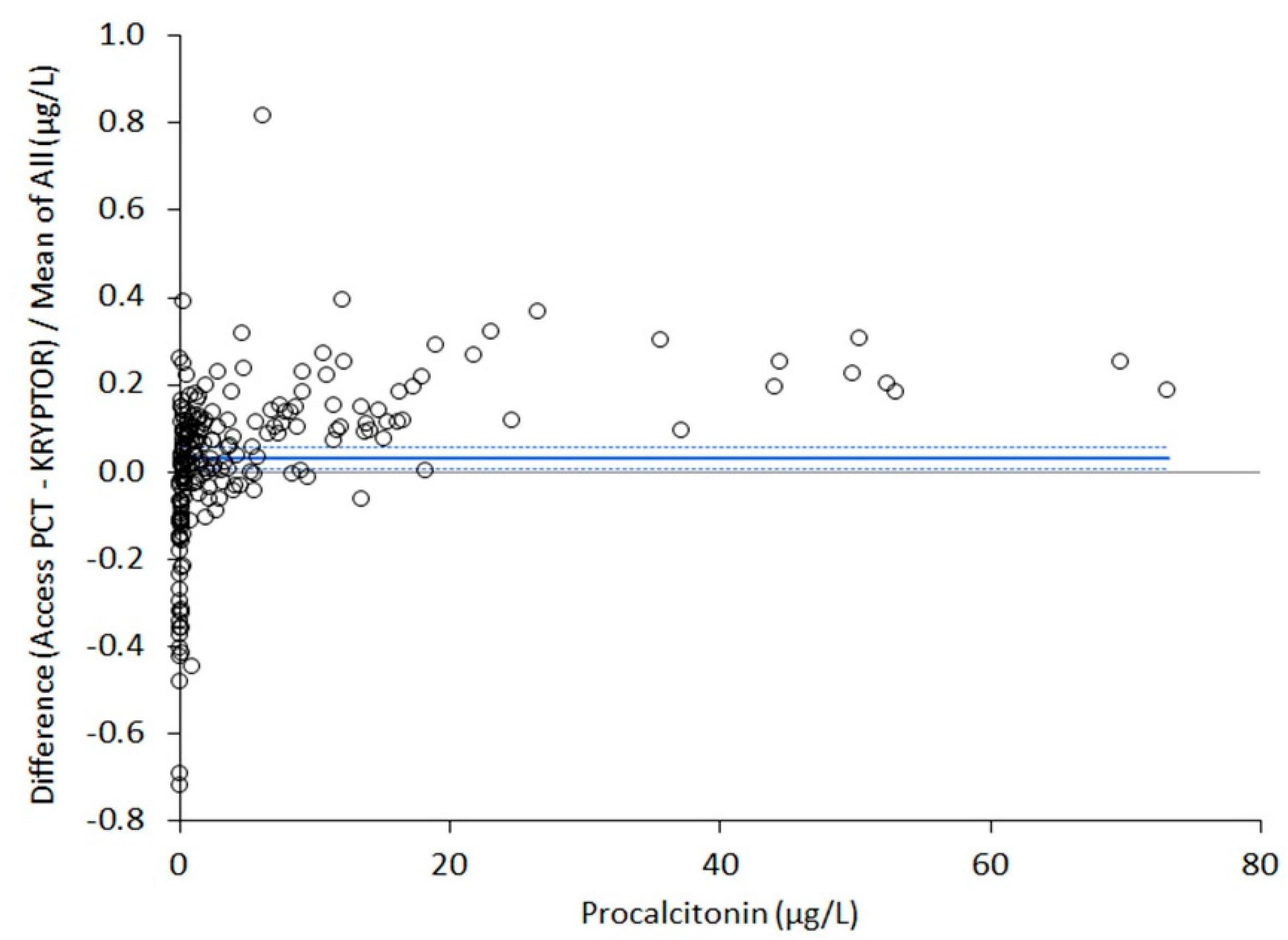
3.4. Method Comparison
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Meisner, M. Procalcitonin-Biochemistry and Clinical Diagnosis, 1st ed.; UNI-MED: Bremen, Germany, 2010; ISBN 978-3-8374-1241-3. [Google Scholar]
- Meisner, M. Update on procalcitonin measurements. Ann. Lab. Med. 2014, 34, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Meschi, T.; Cervellin, G. Inflammatory biomarkers for the diagnosis, monitoring and follow-up of community-acquired pneumonia: Clinical evidence and perspectives. Eur. J. Intern. Med. 2011, 22, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.A.; Batts, D.H.; Colville, J.M.; Lauter, C. Hypocalcaemia and toxic shock syndrome. Lancet 1981, 1, 1208. [Google Scholar] [CrossRef]
- Assicot, M.; Gendrel, D.; Carsin, H.; Guilbaud, J. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993, 341, 515–518. [Google Scholar] [CrossRef]
- Lippi, G.; Montagnana, M.; Balboni, F.; Bellone, A.; Casagranda, I.; Cavazza, M.; Da Rin, G.; Coen, D.; Giavarina, D.; Giostra, F.; et al. Academy of Emergency Medicine and Care-Society of Clinical Biochemistry and Clinical Molecular Biology consensus recommendations for clinical use of sepsis biomarkers in the emergency department. Emerg. Care J. 2017, 13, 6877. [Google Scholar] [CrossRef]
- Bartoletti, M.; Antonelli, M.; Bruno Blasi, F.A.; Casagranda, I.; Chieregato, A.; Fumagalli, R.; Girardis, M.; Pieralli, F.; Plebani, M.; Rossolini, G.M.; et al. Procalcitonin-guided antibiotic therapy: An expert consensus. Clin. Chem. Lab. Med. 2018, 56, 1223–1229. [Google Scholar] [CrossRef]
- Hey, J.; Thompson-Leduc, P.; Kirson, N.Y.; Zimmer, L.; Wilkins, D.; Rice, B.; Iankova, I.; Krause, A.; Schonfeld, S.A.; DeBrase, C.R.; et al. Procalcitonin guidance in patients with lower respiratory tract infections: A systematic review and meta-analysis. Clin. Chem. Lab. Med. 2018, 56, 1200–1209. [Google Scholar] [CrossRef]
- Schuetz, P.; Beishuizen, A.; Broyles, M.; Ferrer, R.; Gavazzi, G.; Gluck, E.H.; Del Castillo, J.G.; Jensen, J.U.; Kanizsai, P.L.; Kwa, A.L.; et al. Procalcitonin (PCT)-guided antibiotic stewardship: An international experts consensus on optimized clinical use. Clin. Chem. Lab. Med. 2019, 57, 1308–1318. [Google Scholar] [CrossRef]
- Shrivastava, S.R.; Shrivastava, P.S.; Ramasamy, J. Responding to the challenge of antibiotic resistance: World Health Organization. J. Res. Med. Sci. 2018, 23, 21. [Google Scholar] [CrossRef]
- Lippi, G. Sepsis biomarkers: Past, present and future. Clin. Chem. Lab. Med. 2019, 57, 1281–1283. [Google Scholar] [CrossRef]
- Dipalo, M.; Guido, L.; Micca, G.; Pittalis, S.; Locatelli, M.; Motta, A.; Bianchi, V.; Callegari, T.; Aloe, R.; Da Rin, G.; et al. Multicenter comparison of automated procalcitonin immunoassays. Pract. Lab. Med. 2015, 2, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, A. A new sensitive automated assay for procalcitonin detection: LIAISON® BRAHMS PCT® II GEN. Pract. Lab. Med. 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ruzzenente, O.; Salvagno, G.L.; Gelati, M.; Lippi, G. Analytical evaluation of the novel Lumipulse G BRAHMS procalcitonin immunoassay. Pract. Lab. Med. 2016, 6, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Kutz, A.; Hausfater, P.; Oppert, M.; Alan, M.; Grolimund, E.; Gast, C.; Alonso, C.; Wissmann, C.; Kuehn, C.; Bernard, M.; et al. Comparison between B·R·A·H·M·S PCT direct, a new sensitive point-of-care testing device for rapid quantification of procalcitonin in emergency department patients and established reference methods—A prospective multinational trial. Clin. Chem. Lab. Med. 2016, 54, 577–584. [Google Scholar] [PubMed]
- Ceriotti, F.; Marino, I.; Motta, A.; Carobene, A. Analytical evaluation of the performances of Diazyme and BRAHMS procalcitonin applied to Roche Cobas in comparison with BRAHMS PCT-sensitive Kryptor. Clin. Chem. Lab. Med. 2017, 56, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Soh, A.; Binder, L.; Clough, M.; Hernandez, M.H.; Lefèvre, G.; Mostert, K.; Nguyen, T.B.; Otte, K.M.; Portakal, O.; Sandri, M.S.; et al. Comparison of the novel ARCHITECT procalcitonin assay with established procalcitonin assay systems. Pract. Lab. Med. 2018, 12, e00110. [Google Scholar] [CrossRef] [PubMed]
- Eidizadeh, A.; Asif, A.R.; von Ahsen, N.; Binder, L.; Schnelle, M. Differences in procalcitonin measurements between three BRAHMS-partnered immunoassays (Liaison, Elecsys and Architect). Clin. Chem. Lab. Med. 2019, 57, e207–e210. [Google Scholar] [CrossRef]
- Dipalo, M.; Gnocchi, C.; Avanzini, P.; Musa, R.; Di Pietro, M.; Aloe, R. Comparison of Procalcitonin Assays on KRYPTOR and LIAISON® XL Analyzers. Diagnostics 2019, 9, 94. [Google Scholar] [CrossRef]
- Krouwer, J.S.; Rabinowitz, R. How to improve estimates of imprecision. Clin. Chem. 1984, 30, 290–292. [Google Scholar] [CrossRef]
- Farooq, A.; Colón-Franco, J.M. Procalcitonin and Its Limitations: Why a Biomarker’s Best Isn’t Good Enough. J. Appl. Lab. Med. 2019, 3, 716–719. [Google Scholar] [CrossRef]
- Samsudin, I.; Vasikaran, S.D. Clinical Utility and Measurement of Procalcitonin. Clin. Biochem. Rev. 2017, 38, 59–68. [Google Scholar] [PubMed]
- Sager, R.; Kutz, A.; Mueller, B.; Schuetz, P. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; harti Mittu, B.; Chauhan, P. Analytical Method Development and Validation: A Concise Review. J. Anal. Bioanal. Tech. 2015, 6, 233. [Google Scholar]
| Pools | Intra-Assay | Inter-Assay | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (µg/L) | SD (µg/L) | CV | n | Mean (µg/L) | SD (µg/L) | CV | CV | |
| Pool low | 20 | 0.29 | 0.01 | 1.8% | 10 | 0.30 | 0.01 | 3.6% | 4.0% |
| Pool medium | 20 | 2.83 | 0.05 | 1.9% | 10 | 2.85 | 0.07 | 2.4% | 3.1% |
| Pool high | 20 | 11.23 | 0.23 | 2.1% | 10 | 10.92 | 0.41 | 3.7% | 4.3% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lippi, G.; Salvagno, G.L.; Gelati, M.; Pucci, M.; Demonte, D.; Faggian, D.; Plebani, M. Analytical Evaluation of the New Beckman Coulter Access Procalcitonin (PCT) Chemiluminescent Immunoassay. Diagnostics 2020, 10, 128. https://doi.org/10.3390/diagnostics10030128
Lippi G, Salvagno GL, Gelati M, Pucci M, Demonte D, Faggian D, Plebani M. Analytical Evaluation of the New Beckman Coulter Access Procalcitonin (PCT) Chemiluminescent Immunoassay. Diagnostics. 2020; 10(3):128. https://doi.org/10.3390/diagnostics10030128
Chicago/Turabian StyleLippi, Giuseppe, Gian Luca Salvagno, Matteo Gelati, Mairi Pucci, Davide Demonte, Diego Faggian, and Mario Plebani. 2020. "Analytical Evaluation of the New Beckman Coulter Access Procalcitonin (PCT) Chemiluminescent Immunoassay" Diagnostics 10, no. 3: 128. https://doi.org/10.3390/diagnostics10030128
APA StyleLippi, G., Salvagno, G. L., Gelati, M., Pucci, M., Demonte, D., Faggian, D., & Plebani, M. (2020). Analytical Evaluation of the New Beckman Coulter Access Procalcitonin (PCT) Chemiluminescent Immunoassay. Diagnostics, 10(3), 128. https://doi.org/10.3390/diagnostics10030128









