Preliminary Assessment of Burn Depth by Paper-Based ELISA for the Detection of Angiogenin in Burn Blister Fluid—A Proof of Concept
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Samples
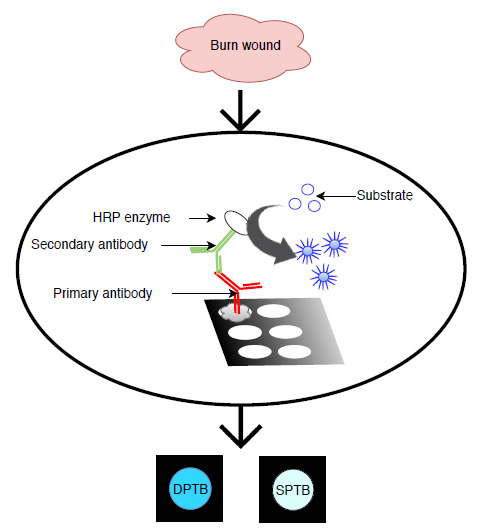
2.2. Preparation of Paper-Based 96-Well Plates Via Wax Printing
2.3. Procedure of Paper-Based ELISA and Plate ELISA
2.4. Data Analysis
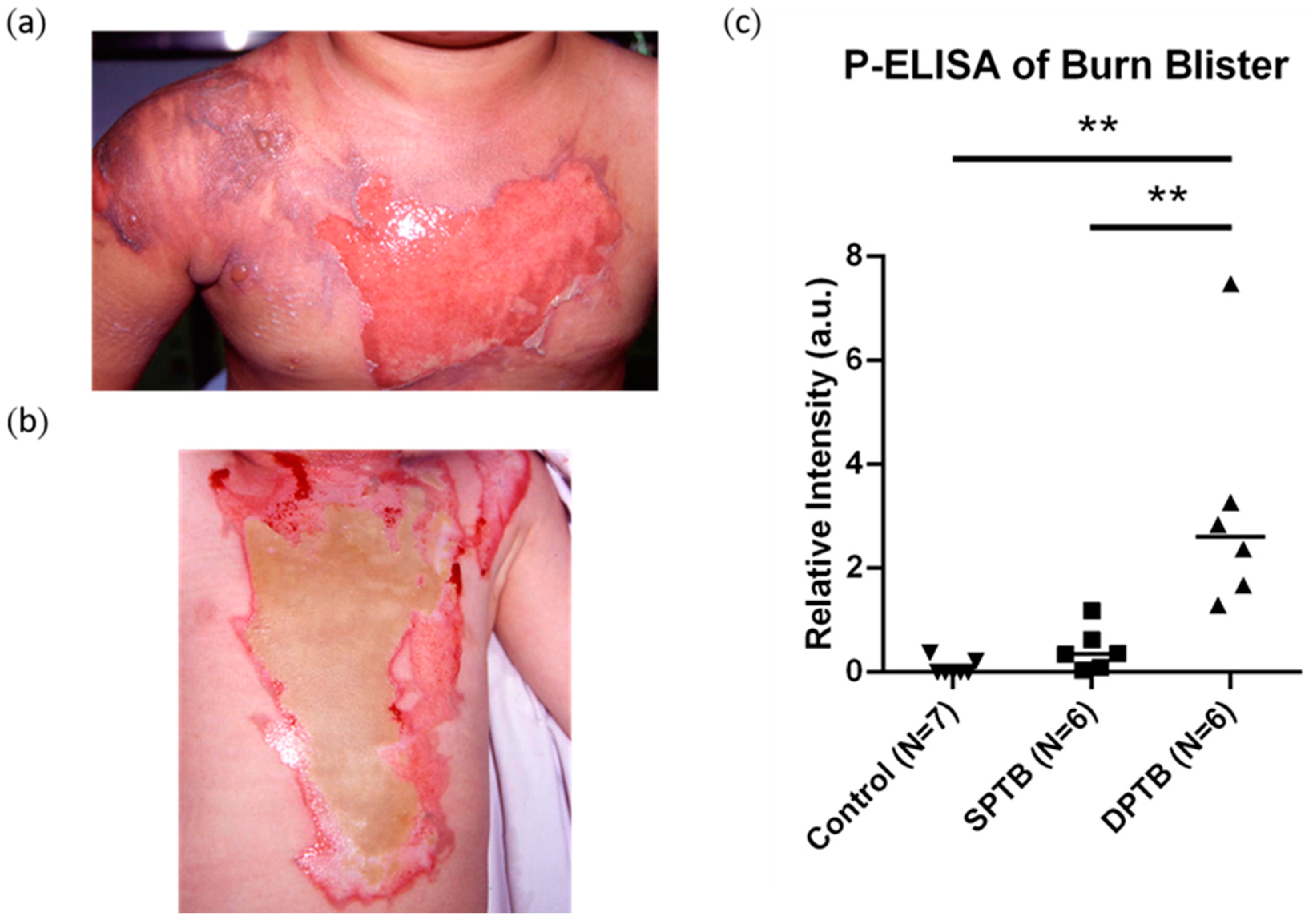
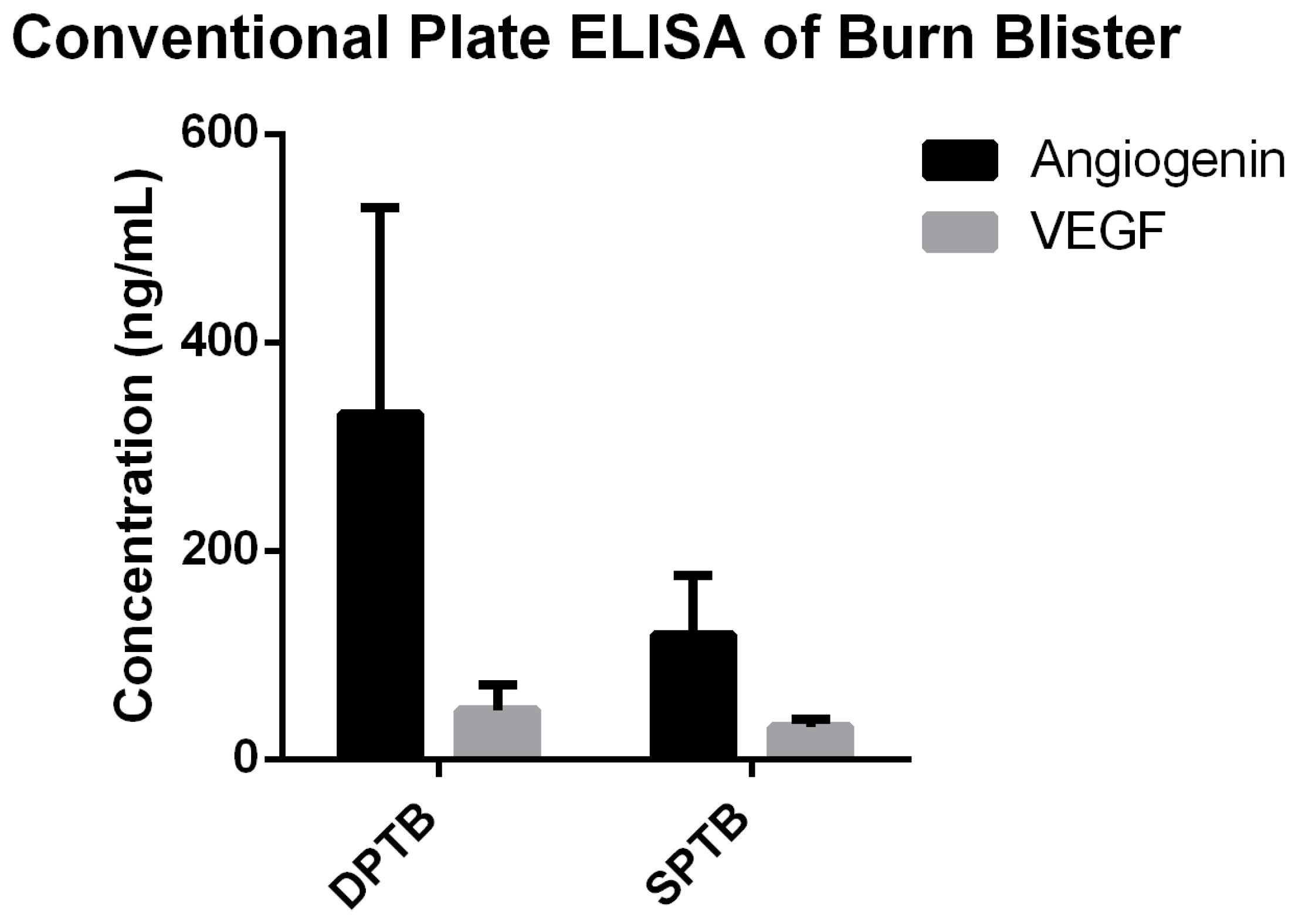
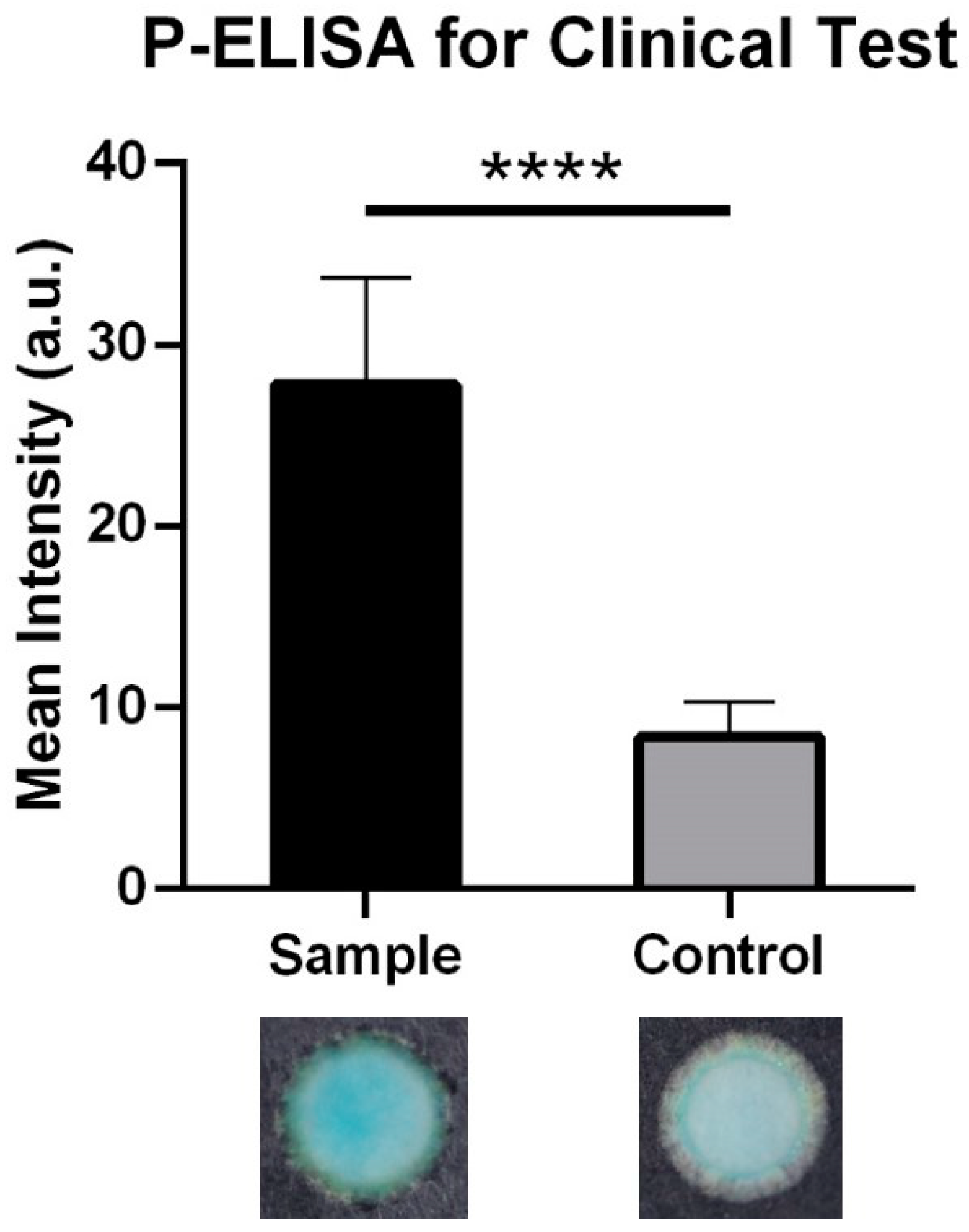
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Monstrey, S.; Hoeksema, H.; Verbelen, J.; Pirayesh, A.; Blondeel, P. Assessment of burn depth and burn wound healing potential. Burns 2008, 34, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, D.; Engrav, L.; Grube, B.; Marvin, J. Burn depth: A review. World J. Surg. 1992, 16, 10–15. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Gunn, S.W.; Hayek, S.N. State of the art in burn treatment. World J. Surg. 2005, 29, 131–148. [Google Scholar] [CrossRef]
- Pape, S.A.; Skouras, C.A.; Byrne, P.O. An audit of the use of laser Doppler imaging (LDI) in the assessment of burns of intermediate depth. Burns 2001, 27, 233–239. [Google Scholar] [CrossRef]
- Droog, E.J.; Steenbergen, W.; Sjoberg, F. Measurement of depth of burns by laser Doppler perfusion imaging. Burns 2001, 27, 561–568. [Google Scholar] [CrossRef]
- Holland, A.J.; Martin, H.C.; Cass, D.T. Laser Doppler imaging prediction of burn wound outcome in children. Burns 2002, 28, 11–17. [Google Scholar] [CrossRef]
- Lawrence, C. Treating minor burns. Nurs Times 1989, 85, 69–73. [Google Scholar]
- Gillitzer, R.; Goebeler, M. Chemokines in cutaneous wound healing. J. Leukoc Biol. 2001, 69, 513–521. [Google Scholar]
- Pan, S.C.; Wu, L.W.; Chen, C.L.; Shieh, S.J.; Chiu, H.Y. Angiogenin expression in burn blister fluid: Implications for its role in burn wound neovascularization. Wound Repair Regen. 2012, 20, 731–739. [Google Scholar] [CrossRef]
- Fett, J.W.; Strydom, D.J.; Lobb, R.R.; Alderman, E.M.; Bethune, J.L.; Riordan, J.F.; Vallee, B.L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry 1985, 24, 5480–5486. [Google Scholar] [CrossRef]
- Katona, T.M.; Neubauer, B.L.; Iversen, P.W.; Zhang, S.; Baldridge, L.A.; Cheng, L. Elevated expression of angiogenin in prostate cancer and its precursors. Clin. Cancer Res. 2005, 11, 8358–8363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pan, S.C.; Wu, L.W.; Chen, C.L.; Shieh, S.J.; Chiu, H.Y. Deep partial thickness burn blister fluid promotes neovascularization in the early stage of burn wound healing. Wound Repair Regen. 2010, 18, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, R.; Niday, E.; Gordon, J. A dot-immunobinding assay for monoclonal and other antibodies. Anal. Biochem. 1982, 119, 142–147. [Google Scholar] [CrossRef]
- Beyer, C.F. A ‘dot-immunobinding assay’ on nitrocellulose membrane for the determination of the immunoglobulin class of mouse monoclonal antibodies. J. Immunol. Methods 1984, 67, 79–87. [Google Scholar] [CrossRef]
- Hawkes, R. The dot immunobinding assay. Methods Enzymol. 1986, 121, 484–491. [Google Scholar]
- Cheng, C.M.; Martinez, A.W.; Gong, J.; Mace, C.R.; Phillips, S.T.; Carrilho, E.; Mirica, K.A.; Whitesides, G.M. Paper-based ELISA. Angew. Chem. Int. Ed. Engl. 2010, 49, 4771–4774. [Google Scholar] [CrossRef]
- Hsu, M.Y.; Yang, C.Y.; Hsu, W.H.; Lin, K.H.; Wang, C.Y.; Shen, Y.C.; Chen, Y.C.; Chau, S.F.; Tsai, H.Y.; Cheng, C.M. Monitoring the VEGF level in aqueous humor of patients with ophthalmologically relevant diseases via ultrahigh sensitive paper-based ELISA. Biomaterials 2014, 35, 3729–3735. [Google Scholar] [CrossRef]
- Wang, H.K.; Tsai, C.H.; Chen, K.H.; Tang, C.T.; Leou, J.S.; Li, P.C.; Tang, Y.L.; Hsieh, H.J.; Wu, H.C.; Cheng, C.M. Cellulose-based diagnostic devices for diagnosing serotype-2 dengue fever in human serum. Adv. Healthc Mater 2014, 3, 187–196. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Feng, M.J.; Wu, C.C.; Wang, J.; Chang, T.C.; Cheng, C.M. Development of a Sampling Collection Device with Diagnostic Procedures. Anal. Chem. 2016, 88, 7591–7596. [Google Scholar] [CrossRef]
- Hsu, C.K.; Huang, H.Y.; Chen, W.R.; Nishie, W.; Ujiie, H.; Natsuga, K.; Fan, S.T.; Wang, H.K.; Lee, J.Y.; Tsai, W.L.; et al. Paper-based ELISA for the detection of autoimmune antibodies in body fluid-the case of bullous pemphigoid. Anal. Chem. 2014, 86, 4605–4610. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Udugama, B.; Kadhiresan, P.; Kim, J.; Mubareka, S.; Weiss, P.S.; Parak, W.J. Patients, Here Comes More Nanotechnology. ACS Nano. 2016, 10, 8139–8142. [Google Scholar] [CrossRef] [PubMed]
- Marquez, S.; Morales-Narváez, E. Nanoplasmonics in Paper-Based Analytical Devices. Front. Bioeng. Biotechnol. 2019, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, K.; Liu, S.; Tsuji, T.; Olson, K.A.; Hu, G.F. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene 2005, 24, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Devgan, L.; Bhat, S.; Aylward, S.; Spence, R.J. Modalities for the assessment of burn wound depth. J. Burns Wounds. 2006, 5, e2. [Google Scholar]
- Park, D.H.; Hwang, J.W.; Jang, K.S.; Han, D.G.; Ahn, K.Y.; Baik, B.S. Use of laser Doppler flowmetry for estimation of the depth of burns. Plast Reconstr Surg. 1998, 101, 1516–1523. [Google Scholar] [CrossRef]
- O’Reilly, T.J.; Spence, R.J.; Taylor, R.M.; Scheulen, J.J. Laser Doppler flowmetry evaluation of burn wound depth. J. Burn Care Rehabil. 1989, 10, 1–6. [Google Scholar] [CrossRef]
- Anselmo, V.J.; Zawacki, B.E. Multispectral photographic analysis. A new quantitative tool to assist in the early diagnosis of thermal burn depth. Ann. Biomed. Eng. 1977, 5, 179–193. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Sharpe, J.R.; Booth, S.; Jubin, K.; Jordan, N.R.; Lawrence-Watt, D.J.; Dheansa, B.S. Progression of wound pH during the course of healing in burns. J. Burn Care Res. 2013, 34, e201–e208. [Google Scholar] [CrossRef]
- Murphy, G.R.; Dunstan, R.H.; Macdonald, M.M.; Gottfries, J.; Roberts, T.K. Alterations in amino acid metabolism during growth by Staphylococcus aureus following exposure to H2O2—A multifactorial approach. Heliyon 2018, 4, e00620. [Google Scholar] [CrossRef]


| Paper-Based ELISA | Conventional Plate ELISA | |||
|---|---|---|---|---|
| antigen | angiogenin | angiogenin | ||
| Primary antibody | Rabbit polyclonal anti-human angiogenin | Mouse monoclonal anti-human angiogenin | ||
| Secondary antibody | Goat anti-rabbit lgG (HRP) | Goat anti-mouse lgG (HRP) | ||
| Enzyme/substrate | HRP/TMB + H2O2 | HRP/TMB + H2O2 | ||
| Detection device | camera | plate reader | ||
| analysis | qualitative | quantitative | ||
| Time and reagents | Volume (μL) | Time (min) | Volume (μL) | Time (min) |
| (1) antigen immobilization | 3 | 10 | 50 | 120 |
| (2) blocking | 3 | 10 | 200 | 120 |
| (3) primary antibody | 3 | 10 | 100 | 120 |
| (4) secondary antibody | 3 | 10 | 100 | 120 |
| (5) signal amplification | 3 | 10 | 100 | 15 |
| total | 15 | 50 | 550 | 495 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, S.-C.; Tsai, Y.-H.; Chuang, C.-C.; Cheng, C.-M. Preliminary Assessment of Burn Depth by Paper-Based ELISA for the Detection of Angiogenin in Burn Blister Fluid—A Proof of Concept. Diagnostics 2020, 10, 127. https://doi.org/10.3390/diagnostics10030127
Pan S-C, Tsai Y-H, Chuang C-C, Cheng C-M. Preliminary Assessment of Burn Depth by Paper-Based ELISA for the Detection of Angiogenin in Burn Blister Fluid—A Proof of Concept. Diagnostics. 2020; 10(3):127. https://doi.org/10.3390/diagnostics10030127
Chicago/Turabian StylePan, Shin-Chen, Yao-Hung Tsai, Chin-Chuan Chuang, and Chao-Min Cheng. 2020. "Preliminary Assessment of Burn Depth by Paper-Based ELISA for the Detection of Angiogenin in Burn Blister Fluid—A Proof of Concept" Diagnostics 10, no. 3: 127. https://doi.org/10.3390/diagnostics10030127
APA StylePan, S.-C., Tsai, Y.-H., Chuang, C.-C., & Cheng, C.-M. (2020). Preliminary Assessment of Burn Depth by Paper-Based ELISA for the Detection of Angiogenin in Burn Blister Fluid—A Proof of Concept. Diagnostics, 10(3), 127. https://doi.org/10.3390/diagnostics10030127