Heart of the World’s Top Ultramarathon Runner—Not Necessarily Much Different from Normal
Abstract
1. Introduction
2. Materials and Methods
2.1. Sports Biography and Main Achievements
2.2. Methods
2.2.1. Study Protocol
2.2.2. Laboratory Examinations
2.2.3. ECG Tests
2.2.4. Transthoracic Echocardiography
2.2.5. MRI
2.2.6. P MRS
2.2.7. Ethical Approval
3. Results
3.1. Laboratory Examinations (Morphological, Biochemical, Coagulation)
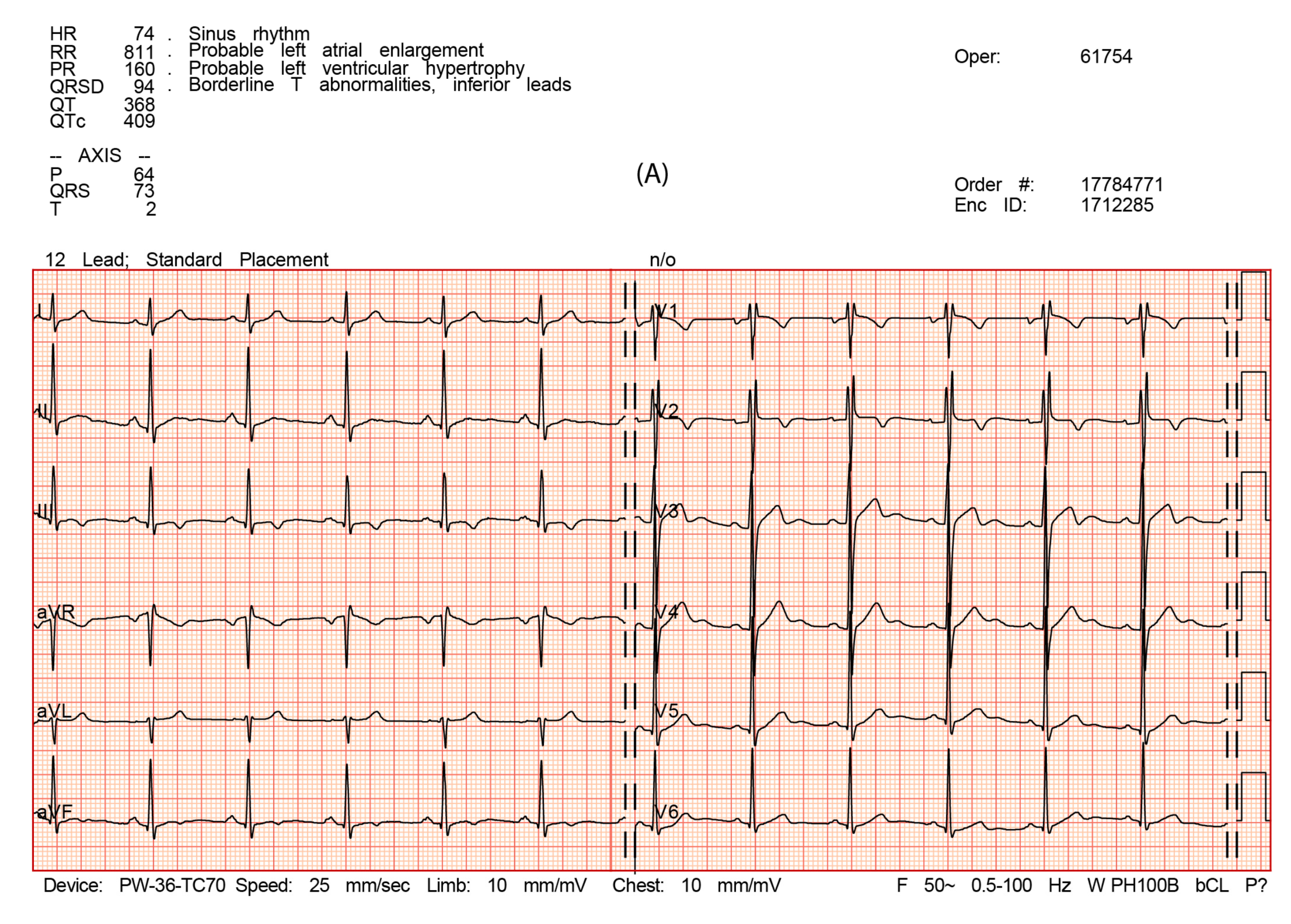
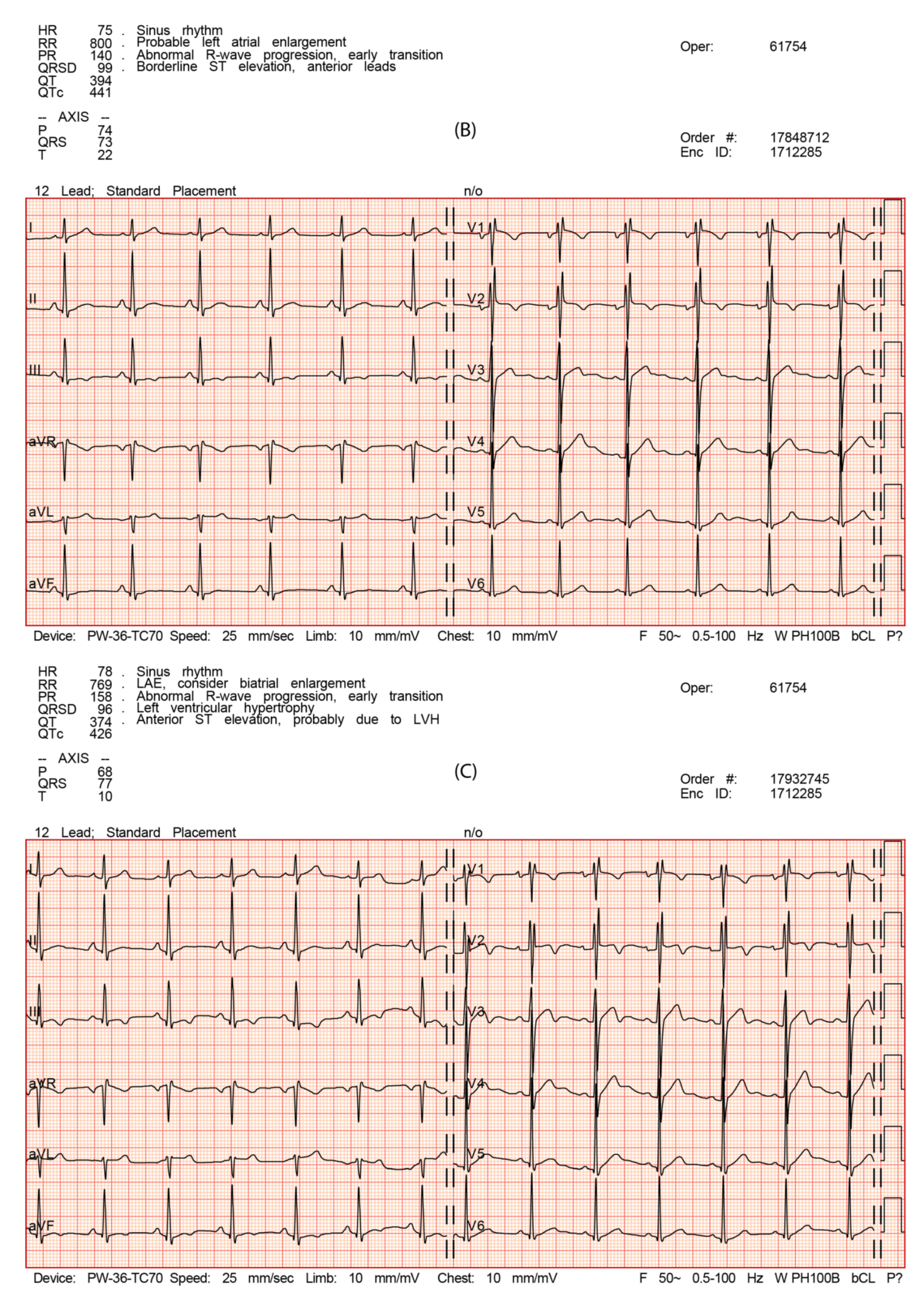
3.2. Electrocardiography
3.3. Echocardiography
3.4. MRI
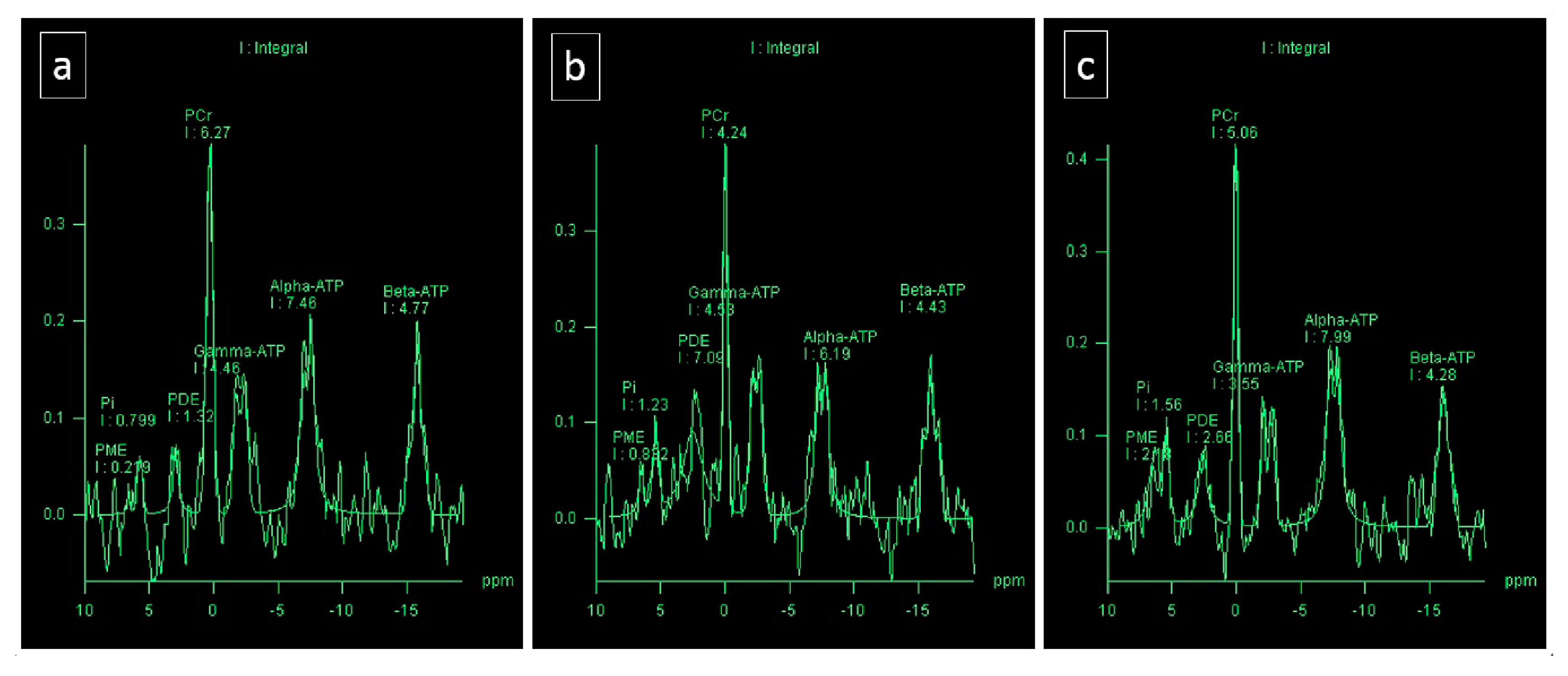
3.5. P MRS
4. Discussion
4.1. How Much Physical Activity Is Too Much or Too Little?
4.2. Lipids
4.3. Hemoglobin
4.4. Enzymes and Echocardiography
4.5. MRI
4.6. Cardiac 31P MRS
4.7. What Makes Him the Champion of Ultramarathon?
4.8. Strength, Limitations, and Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Finn, A. When 26.2 Miles Just Isn’t Enough—The Phenomenal Rise of the Ultramarathon. Available online: https://www.theguardian.com/lifeandstyle/2018/apr/02/ultrarunner-ultramarathon-racing-100-miles (accessed on 2 April 2018).
- Nikolaidis, P.T.; Knechtle, B. Age of peak performance in 50-km ultramarathoners - is it older than in marathoners? Open. Access. J. Sports. Med. 2018, 9, 37–45. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Lebus, D.K.; Ganong, A.C.; Casazza, G.A.; Loan, M.V. Body composition of 161-km ultramarathoners. Int. J. Sports Med. 2010, 31, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Jastrzebski, Z.; Rosemann, T.; Nikolaidis, P.T. Pacing during and physiological response after a 12-hour ultra-marathon in a 95-year-old male runner. Front. Physiol. 2019, 9, 1875. [Google Scholar] [CrossRef] [PubMed]
- Renfree, A.; Crivoi do Carmo, E.; Martin, L. The influence of performance level, age and gender on pacing strategy during a 100-km ultramarathon. Eur. J. Sport. Sci. 2016, 16, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Timms, M. Ultra Pain, Ultra Gain: The Rise of the Ultramarathon. Available online: https://www.businessdestinations.com/relax/health-and-fitness/rise-of-the-ultramarathon-best-events/ (accessed on 19 November 2014).
- Zaryski, C.; Smith, D.J. Training principles and issues for ultra-endurance athletes. Curr. Sports. Med. Rep. 2005, 4, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Kastner, C.B. Bunion Derby: The 1928 Footrace across America; University of New Mexico Press: Albuquerque, NM, USA, 2007. [Google Scholar]
- Schütz, U.H.; Schmidt-Trucksäss, A.; Knechtle, B.; Machann, J.; Wiedelbach, H.; Ehrhardt, M.; Freund, W.; Gröninger, S.; Brunner, H.; Schulze, I.; et al. The TransEurope FootRace Project: Longitudinal data acquisition in a cluster randomized mobile MRI observational cohort study on 44 endurance runners at a 64-stage 4,486 km transcontinental ultramarathon. BMC. Med. 2012, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Badenhausen, K. Ultramarathoner Dean Karnazes on Endurance, Training and Running 350 Miles Straight. Available online: https://www.forbes.com/sites/kurtbadenhausen/2012/05/09/ultramarathoner-dean-karnazes-on-endurance-training-and-running-350-miles-straight/#5400889f43ac (accessed on 9 May 2012).
- McDougall, C. Born to Run: A Hidden Tribe, Superathletes, and the Greatest Race the World Has Never Seen. Available online: https://en.wikipedia.org/wiki/Born_to_Run:_A_Hidden_Tribe,_Superathletes,_and_the_Greatest_Race_the_World_Has_Never_Seen (accessed on 7 September 2019).
- Pluim, B.M.; Zwinderman, A.H.; van der Laarse, A.; van der Wall, E.E. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation. 2000, 101, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Riding, N.R.; Salah, O.; Sharma, S.; Carré, F.; O’Hanlon, R.; George, K.P.; Hamilton, B.; Chalabi, H.; Whyte, G.P.; Wilson, M.G. Do big athletes have big hearts? Impact of extreme anthropometry upon cardiac hypertrophy in professional male athletes. Br. J. Sports. Med. 2012, 46, i90–i97. [Google Scholar] [CrossRef]
- Frassl, W.; Kowoll, R.; Katz, N.; Speth, M.; Stangl, A.; Brechtel, L.; Joscht, B.; Boldt, L.H.; Meier-Buttermilch, R.; Schlemmer, M.; et al. Cardiac markers (BNP, NT-pro-BNP, Troponin I, Troponin T, in female amateur runners before and up until three days after a marathon. Clin. Lab. 2008, 54, 81–87. [Google Scholar]
- Kim, Y.J.; Shin, Y.O.; Lee, J.B.; Lee, Y.H.; Shin, K.A.; Kim, A.C.; Goh, C.W.; Kim, C.; Oh, J.K.; Min, Y.K.; et al. The effects of running a 308 km ultra-marathon on cardiac markers. Eur. J. Sport Sci. 2014, 14, S92–S97. [Google Scholar] [CrossRef]
- Biernacka, K. Atrial fibrillation in sportsmen. Kardiologia po Dyplomie 2016, 3, 32–37. [Google Scholar]
- Bosomworth, N.J. Atrial fibrillation and physical activity: Should we exercise caution? Can. Fam. Physician 2015, 61, 1061–1070. [Google Scholar] [PubMed]
- Guasch, E.; Mont, L. Diagnosis, pathophysiology, and management of exercise-induced arrhythmias. Nat. Rev. Cardiol. 2016, 14, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Matelot, D.; Schnell, F.; Khodor, N.; Endjah, N.; Kervio, G.; Carrault, G.; Thillaye du Boullay, N.; Carre, F. Does deep bradycardia increase the risk of arrhythmias and syncope in endurance athletes? Int. J. Sports Med. 2016, 37, 792–798. [Google Scholar] [CrossRef]
- Krysztofiak, H.; Dimitrow, P. Differentiating physiology from pathology in elite athletes. Left ventricular hypertrophy versus hypertrophic cardiomyopathy. Kardiologia Polska 2016, 74, 705–716. [Google Scholar] [CrossRef]
- Baggish, A.L.; Wood, M.J. Athlete’s heart and cardiovascular care of the athlete: Scientific and clinical update. Circulation 2011, 123, 2723–2735. [Google Scholar] [CrossRef]
- Biffi, A.; Maron, B.J.; Di Giacinto, B.; Porcacchia, P.; Verdile, L.; Fernando, F.; Spataro, A.; Culasso, F.; Casasco, M.; Pelliccia, A. Relation between training-induced left ventricular hypertrophy and risk for ventricular tachyarrhythmias in elite athletes. Am. J. Cardiol. 2008, 101, 1792–1795. [Google Scholar] [CrossRef]
- Inama, G.; Pedrinazzi, C.; Durin, O.; Nanetti, M.; Donato, G.; Pizzi, R. Ventricular arrhythmias in competitive athletes: Risk stratification with T-wave alternans. Heart. Int. 2007, 3, 58. [Google Scholar] [CrossRef]
- Biffi, A.; Maron, B.J.; Verdile, L.; Fernando, F.; Spataro, A.; Marcello, G.; Ciardo, R.; Ammirati, F.; Colivicchi, F.; Pelliccia, A. Impact of physical deconditioning on ventricular tachyarrhythmias in trained athletes. J. Am. Coll. Cardiol. 2004, 44, 1053–1058. [Google Scholar] [CrossRef]
- Sharma, S.; Zaidi, A. Exercise-induced arrhythmogenic right ventricular cardiomyopathy: Fact or fallacy? Eur. Heart J. 2012, 33, 938–940. [Google Scholar] [CrossRef]
- Trivax, J.E.; McCullough, P.A. Phidippides cardiomyopathy: A review and case illustration. Clin. Cardiol. 2012, 35, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Mont, L.; Sambola, A.; Brugada, J.; Vacca, M.; Marrugat, J.; Elosua, R.; Paré, C.; Azqueta, M.; Sanz, G. Long-lasting sport practice and lone atrial fibrillation. Eur. Heart J. 2002, 23, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Sharma, S. Arrhythmogenic right ventricular remodelling in endurance athletes: Pandora’s box or Achilles’ heel? Eur. Heart J. 2015, 36, 1955–1957. [Google Scholar] [CrossRef] [PubMed]
- Belli, T.; Macedo, D.V.; de Araújo, G.G.; Nunes, L.A.S.; Brenzikofer, R.; Gobatto, C.A. Mountain Ultramarathon Induces Early Increases of Muscle Damage, Inflammation, and Risk for Acute Renal Injury. Front. Physiol. 2018, 9, 1368. [Google Scholar] [CrossRef]
- Barr, S.I.; Costill, D.L. Water: Can the endurance athlete get too much of a good thing? J. Am. Diet. Assoc. 1989, 89, 1629–1632, 1635. [Google Scholar]
- Sharma, S.; Drezner, J.A.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International recommendations for electrocardiographic interpretation in athletes. Eur. Heart J. 2018, 39, 1466–1480. [Google Scholar] [CrossRef]
- Tokudome, S.; Kuriki, K.; Yamada, N.; Ichikawa, H.; Miyata, M.; Shibata, K.; Hoshino, H.; Tsuge, S.; Tokudome, M.; Goto, C.; et al. Anthropometric, lifestyle and biomarker assessment of Japanese non-professional ultra-marathon runners. J. Epidemiol. 2004, 14, 161–167. [Google Scholar] [CrossRef]
- Zachariah, G.; Alex, A.G. Exercise for prevention of cardiovascular disease: Evidence-based recommendations. J. Clin. Prev. Cardiol. 2017, 6, 109–114. [Google Scholar]
- Lee, D.C.; Pate, R.R.; Lavie, C.J.; Sui, X.; Church, T.S.; Blair, S.N. Leisure-time running reduces all-cause and cardiovascular mortality risk. J. Am. Coll. Cardiol. 2014, 64, 472–481. [Google Scholar] [CrossRef]
- Merghani, A.; Malhotra, A.; Sharma, S. The U-shaped relationship between exercise and cardiac morbidity. Trends Cardiovasc. Med. 2015, 26, 232–240. [Google Scholar] [CrossRef]
- Schnohr, P.; O’Keefe, J.H.; Marott, J.L.; Lange, P.; Jensen, G.B. Dose of jogging and long-term mortality: The Copenhagen City Heart Study. J. Am. Coll. Cardiol. 2015, 65, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, D.; Duncan, E. Atrial fibrillation (AF) in endurance athletes: A complicated affair. Curr. Treat. Opt. Cardiovasc. Med. 2018, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Gajda, R.; Biernacka, E.K.; Drygas, W. Are heart rate monitors valuable tools for diagnosing arrhythmias in endurance athletes? Scand. J. Med. Sci. Sports 2018, 28, 496–516. [Google Scholar] [CrossRef] [PubMed]
- Gajda, R.; Biernacka, E.K.; Drygas, W. The Problem of Arrhythmias in Endurance Athletes: Are Heart Rate Monitors Valuable Tools for Diagnosing Arrhythmias? Horizons in World Cardiovascular Research; Nova Science Publishers, Inc.: New York, NY, USA, 2018; pp. 1–64. [Google Scholar]
- Gajda, R.; Kowalik, E.; Rybka, S.; Rębowska, E.; Śmigielski, W.; Nowak, M.; Kwaśniewska, M.; Hoffman, P.; Drygas, W. Evaluation of the heart function of swimmers subjected to exhaustive repetitive endurance efforts during a 500-km relay. Front. Physiol. 2019, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Creighton, B.C.; Hyde, P.N.; Maresh, C.M.; Kraemer, W.J.; Phinney, S.D.; Volek, J.S. Paradox of hypercholesterolaemia in highly trained, keto-adapted athletes. BMJ Open Sport Exerc. Med. 2018, 4, e000429. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Chen, K.T.; Shee, B.W.; Chang, H.C.; Huang, Y.J.; Yang, R.S. Effects of 24 h ultra-marathon on biochemical and hematological parameters. World J. Gastroenterol. 2004, 10, 2711–2714. [Google Scholar] [CrossRef] [PubMed]
- Mairbaurl, H. Red blood cells in sports: Effects of exercise and training on oxygen supply by red blood cells. Front. Physiol. 2013, 4, 332. [Google Scholar] [CrossRef]
- Wirnitzer, K.C.; Faulhaber, M. Hemoglobin and Hematocrit During an 8 Day Mountainbike Race: A Field Study. J. Sports Sci. Med. 2007, 6, 265–266. [Google Scholar]
- Noakes, T.D. Effect of exercise on serum enzyme activities in humans. Sports Med. 1987, 4, 245–267. [Google Scholar] [CrossRef]
- Passaglia, D.G.; Emed, L.G.; Barberato, S.H.; Guerios, S.T.; Moser, A.I.; Silva, M.M.; Ishie, E.; Guarita-Souza, L.C.; Costantini, C.R.; Faria-Neto, J.R. Acute effects of prolonged physical exercise: Evaluation after a twenty-four-hour ultramarathon. Arq. Bras. Cardiol. 2013, 100, 21–28. [Google Scholar] [CrossRef]
- Urhausen, A.; Scharhag, J.; Herrmann, M.; Kindermann, W. Clinical significance of increased cardiac troponins T and I in participants of ultra-endurance events. Am. J. Cardiol. 2004, 94, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Scharhag, J.; Herrmann, M.; Urhausen, A.; Haschke, M.; Herrmann, W.; Kindermann, W. Independent elevations of N-terminal pro-brain natriuretic peptide and cardiac troponins in endurance athletes after prolonged strenuous exercise. Am. Heart J. 2005, 150, 1128–1134. [Google Scholar] [CrossRef]
- Scharhag, J.; George, K.; Shave, R.; Urhausen, A.; Kindermann, W. Exercise-associated increases in cardiac biomarkers. Med. Sci. Sports Exerc. 2008, 40, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Kosowski, M.; Młynarska, K.; Chmura, J.; Kustrzycka-Kratochwil, D.; Sukiennik-Kujawa, M.; Todd, J.A.; Jankowska, E.A.; Banasiak, W.; Reczuch, K.; Ponikowski, P. Cardiovascular stress biomarker assessment of middle-aged non-athlete marathon runners. Eur. J. Prev. Cardiol. 2019, 26, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Januzzi, J.L.; Lee-Lewandrowski, E.; Ton-Nu, T.T.; Yoerger, D.M.; Jassal, D.S.; Lewandrowski, K.B.; Siegel, A.J.; Marshall, J.E.; Douglas, P.S.; et al. Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the Boston marathon. Circulation 2006, 114, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Oxborough, D.; Shave, R.; Warburton, D.; Williams, K.; Oxborough, A.; Charlesworth, S.; Foulds, H.; Hoffman, M.D.; Birch, K.; George, K. Dilatation and dysfunction of the right ventricle immediately after ultraendurance exercise: Exploratory insights from conventional two-dimensional and speckle tracking echocardiography. Circ. Cardiovasc. Imaging 2011, 4, 253–263. [Google Scholar] [CrossRef]
- Scott, J.M.; Esch, B.T.; Shave, R.; Warburton, D.E.; Gaze, D.; George, K. Cardiovascular consequences of completing a 160-km ultramarathon. Med. Sci. Sports Exerc. 2004, 41, 26–34. [Google Scholar] [CrossRef]
- Dávila-Román, V.G.; Guest, T.M.; Tuteur, P.G.; Rowe, W.J.; Ladenson, J.H.; Jaffe, A.S. Transient right but not left ventricular dysfunction after strenuous exercise at high altitude. J. Am. Coll. Cardiol. 1997, 30, 468–473. [Google Scholar] [CrossRef]
- Klenk, C.; Brunner, H.; Nickel, T.; Sagmeister, F.; Infanger, D.; Billich, C.; Beer, M.; Schuetz, U.; Schmidt-Trucksaess, A. P649 Harmonic cardiac adaptation of myocardial structure and mass in the course of a multistage marathon over 4.486 km. Eur. Heart J. 2018, 39. [Google Scholar] [CrossRef]
- Conway, M.A.; Bristow, J.D.; Blackledge, M.J.; Rajagopalan, B.; Radda, G.K. Cardiac metabolism during exercise in healthy volunteers measured by 31P magnetic resonance spectroscopy. Br. Heart J. 1991, 65, 25–30. [Google Scholar] [CrossRef]
- Jung, W.I.; Dietze, G.J. 31P nuclear magnetic resonance spectroscopy: A noninvasive tool to monitor metabolic abnormalities in left ventricular hypertrophy in human. Am. J. Cardiol. 1999, 83, 19H–24H. [Google Scholar] [CrossRef]
- Neubauer, S.; Horn, M.; Hahn, D.; Kochsiek, K. Clinical cardiac magnetic resonance spectroscopy--present state and future directions. Mol. Cell. Biochem. 1998, 184, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Bakermans, A.J.; Bazil, J.N.; Nederveen, A.J.; Strijkers, G.J.; Boekholdt, S.M.; Beard, D.A.; Jeneson, J.A.L. Human cardiac 31P-MR spectroscopy at 3 Tesla cannot detect failing myocardial energy homeostasis during exercise. Front. Physiol. 2017, 8, 939. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, E.J.; Evanochko, W.T.; Pohost, G.M. The 31P-NMR stress test: An approach for detecting myocardial ischemia. Ann. Biomed. Eng. 2000, 28, 930–933. [Google Scholar] [CrossRef]
- Secchi, F.; Di Leo, G.; Petrini, M.; Spairani, R.; Alì, M.; Guazzi, M.; Sardanelli, F. 1H- and 31P-myocardial magnetic resonance spectroscopy in non-obstructive hypertrophic cardiomyopathy patients and competitive athletes. Radiol. Med. 2017, 122, 265–272. [Google Scholar] [CrossRef]
- Bottomley, P.A. NMR Spectroscopy of the human heart. encyclopedia of magnetic resonance. eMagRes 2009. [Google Scholar] [CrossRef]
- Neubauer, S.; Horn, M.; Cramer, M.; Harre, K.; Newell, J.B.; Peters, W.; Pabst, T.; Ertl, G.; Hahn, D.; Ingwall, J.S.; et al. Myocardial Phosphocreatine-to-ATP Ratio Is a Predictor of Mortality in Patients with Dilated Cardiomyopathy. Circulation 1997, 96, 2190–2196. [Google Scholar] [CrossRef]
- Mitsunami, K.; Yabe, T.; Kinoshita, M. Diagnosis of myocardial ischemia and viability by 31P nuclear magnetic resonance spectroscopy. Rinsho Byori 1998, 46, 348–353. [Google Scholar]
- Kuno, S.; Ogawa, T.; Katsuta, S.; Itai, Y. In vivo human myocardial metabolism during aerobic exercise by phosphorus-31 nuclear magnetic resonance spectroscopy. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 488–491. [Google Scholar] [CrossRef]
- George, K.; Whyte, G.P.; Green, D.J.; Oxborough, D.; Shave, R.E.; Gaze, D.; Somauroo, J. The endurance athletes heart: Acute stress and chronic adaptation. Br. J. Sports Med. 2012, 46, i29–i36. [Google Scholar] [CrossRef]
| Days before or after the Run | Before Run | 1 Day after the Run | 2 Days after the Run | 10 Days after the Run | 11 Days after the Run |
|---|---|---|---|---|---|
| ECG | x | x | x | ||
| TTE | x | x | x | ||
| Blood tests | x | x | x | ||
| MRI | x | x | x | ||
| Cardiac 31P MRS | x | x | x |
| Parameters | Units | Before Run | 1 Day after the Run | 10 Days after the Run | Reference Values |
|---|---|---|---|---|---|
| Morphology | |||||
| White blood cells | 109/L | 4 | ↑ 10.87 | 5.36 | 4.0–10.0 |
| Neutrophils | 109/L | ↓1.81 | ↑ 8.46 | 2.67 | 2.5–5.0 |
| Neutrophils (%) | % | 45.2 | ↑ 77.8 | 49.8 | 45.0–70.0 |
| Lymphocytes | 109/L | ↓ 1.22 | ↓ 1.18 | 1.74 | 1.5–3.5 |
| Lymphocytes (%) | % | 30.5 | ↓ 10.9 | 32.5 | 20.0–45.0 |
| Monocytes | 109/L | 0.44 | 0.72 | 0.54 | 0.2–0.8 |
| Monocytes (%) | % | ↑ 11.0 | 6.6 | ↑10.1 | 3.0–8.0 |
| Eosinophils | 109/L | ↑ 0.46 | ↑0.49 | 0.36 | 0.04–0.40 |
| Eosinophils (%) | % | ↑ 11.5 | 4.5 | ↑6.7 | 1.0–5.0 |
| Basophils | 109/L | 0.07 | 0.02 | 0.05 | 0.020–0.100 |
| Basophils (%) | % | ↑ 1.8 | 0.2 | 0.9 | 0.0–1.0 |
| Red blood cells | 1012/L | 4.96 | 4.39 | 5.02 | 4.1–6.2 |
| Hemoglobin | g/dL | 15 | ↓ 13.4 | 15.2 | 14.0–18.0 |
| Hematocrit | % | 42.8 | ↓ 38.0 | 44.2 | 40.0–54.0 |
| Mean corpuscular volume | fL | 86.3 | 86.6 | 88 | 77.0–95.0 |
| Mean corpuscular hemoglobin concentration | g/dL | 35 | 35.3 | 34.4 | 32.0–36.0 |
| Biochemistry | |||||
| Na | mmol/L | 139.28 | ↓ 135.8 | 140.02 | 136.0–145.0 |
| K | mmol/L | 4.75 | 4.13 | 5.12 | 3.8–5.2 |
| Cl | mmol/L | 99.29 | 98.89 | 101.36 | 98.0–110.0 |
| Protein | g/dL | 7 | / | 6.9 | 6.0–8.0 |
| Glucose | mg/dL | ↑ 105 | ↑109.0 | 95 | 70–99 |
| Creatinine | mg/dL | 0.76 | 0.74 | 0.64 | 0.30–1.20 |
| Estimated glomerular filtration rate | mL/min/1.73 m2 | ≥60 | ≥60 | ≥60 | / |
| Urea | mg/dL | 25 | 35 | 28 | <50 |
| Uric acid | mg/dL | 4.2 | 4.2 | 4.7 | 2.7–7.0 |
| Alanine aminotransferase | U/L | 12.74 | ↑73.65 | 32.96 | <40.0 |
| Aspartate amino transferase | U/L | 18.42 | ↑249.18 | 24.93 | <37.0 |
| Gamma-G glutamyl transpeptidase | U/L | 25.42 | 20.64 | 37 | 6.00–71.00 |
| Amylase | U/L | 51.54 | 47.21 | 56.02 | 10.0–100.0 |
| Creatine kinase | U/L | 102.9 | ↑ 5079.6 | 91.1 | 26–174 |
| Cholesterol | mg/dL | ↑ 255 | 177 | ↑ 240 | 115–190 |
| HDL-C | mg/dL | 67 | 80 | 72 | >40 |
| Triglycerides | mg/dL | 55.04 | 34.9 | 82.81 | <150.0 |
| Low-density lipoprotein direct measured | mg/dL | 185 | 106 | 163 | <115 |
| Non-HDL-C | mg/dL | 188 | 97 | 168 | 40–160 |
| Fe | µg/dL | 120 | 70 | 134 | 70–181 |
| Unsaturated iron binding capacity | ug/dL | 193.9 | 179 | 197.3 | 150.0–349.0 |
| Transferrin saturation | % | 39.75 | 29.27 | 40.69 | 20.00–45.00 |
| Total iron binding capacity | µg/dL | 321.85 | 253.07 | 332.68 | 200–400 |
| C-reactive protein | mg/L | 0.09 | ↑144.12 | 1.52 | 0.0–5.0 |
| D-Dimers | ng/mL | 352.84 | 398.09 | 277.3 | <113.0 |
| N-terminal-pro hormone BNP | pg/mL | 9.9 | 213.6 | 16.1 | <125.00 |
| Troponin T | pg/mL | 3.67 | 8.11 | 6.32 | <14 |
| BNP | pg/mL | / | 29 | <2 | <35 |
| Coagulology | |||||
| Activated partial thromboplastin time | sek. | 23.9 | 33 | 28.7 | / |
| Activated partial thromboplastin time ratio | / | ↓0.70 | 0.97 | 0.84 | 0.80–1.20 |
| Prothrombin time | sek. | 10.1 | 10.4 | 9.5 | / |
| Prothrombin ratio | % | 101 | 98 | 107 | 80.0–120.0 |
| International normalized ratio | / | 0.99 | 1.02 | 0.93 | 0.80–1.20 |
| Fibrinogen | g/L | 3.7 | ↑ 8.24 | ↑ 4.26 | 1.80–4.00 |
| Parameters | Units | Before Run | 1 Day after the Run | 10 Days after the Run |
|---|---|---|---|---|
| Rhythm | / | Sinus, 74’ | Sinus, 75’ | Sinus, 78’ |
| PQ duration | ms | 160 | 140 | 160 |
| The length of PII | ms | 120 | 100 | 120 |
| P V1 negative deflection amplitude | mm | 1 | 1.5 | 1 |
| QRS duration | ms | 100 | 100 | 96 |
| rSr’ (leads where present) | / | V1-V2 | V1-V2 | V1-V2 |
| QRS morphology | / | rSr’ V1-V2 | rSr’ V1-V2 | rSr’ V1-V2 |
| QRS voltage criteria for left ventricular hypertrophy SV1 + RV5 or RV6 >3.5 mV (35 mm) | mm | 32/16? | 32/16 | 32/16 |
| QRS voltage criteria for right ventricular hypertrophy RV1 + SV5 or SV6 >1.1 mV (11 mm) | mm | 5.0/1? | 4.5/1 | 4.5/1 |
| QTc duration | ms | 410 | 440 | 420 |
| T negative (leads when present) | / | III, aVF, V1, V2 | III, V1, V2 | III, aVF, V1, V2 |
| Parameters | Units (Normal Values) | Before the Run | 1 Day after the Run | 10 Days after the Run |
|---|---|---|---|---|
| Left ventricle end-diastolic diameter volume | mL (106 ± 22) | 109 | 126 | 113 |
| Left ventricle end-systolic diameter volume | mL (41 ± 10) | 33 | 35 | 33 |
| Ejection fraction 2D (%) bi-plane | % (62 ± 5) | 70 | 72 | 71 |
| Global longitudinal strain | % (−20) | 20.3 | 21.9 | 20.3 |
| Interventricular septum diameter | mm (6–10) | 9 | 10 | 10 |
| Posterior wall diastolic diameter | mm (6–10) | 9 | 9 | 9 |
| Right ventricular end-diastolic diameter | mm (20–30) | 31 | 34 | 29 |
| S’ right ventricle | cm/s (14.1 ± 2.3) | 16 | 14 | 17 |
| Left atrium | mm (30–40) | 33 | 36 | 34 |
| Left atrial volume index | mL/m2 (16–34) | 31.8 | 32.3 | 33.5 |
| Right atrial area | cm2 (16 ± 5) | 17.4 | 20.7 | 18.7 |
| Mitral valve E-wave | cm/s (73 ± 19) | 87 | 75 | 75 |
| Mitral valve A-wave | cm/s (69 ± 17) | 50 | 57 | 46 |
| E’ lateral | cm/s (>10) | 21 | 21 | 20 |
| E’ septal | cm/s (>7) | 14 | 12 | 12 |
| E/e’ lateral | ratio (<15) | 4.1 | 3.5 | 3.8 |
| E/e’ septal | ratio (<13) | 6.2 | 6.3 | 6.2 |
| Parameters | Units | Before the Run | 1 Day after the Run | 10 Days after the Run | Reference Values |
|---|---|---|---|---|---|
| Left atrium (4CH) | cm2 | 23 | 24 | 23 | 15–29 |
| LV ejection fraction | % | 73 | 73 | 73 | 57–75 |
| LV stroke volume | mL | 102 | 107 | 104 | 79–135 |
| LV end-diastolic volume index | mL/m2 | 79 | 83 | 80 | 66–101 |
| LV end-systolic volume index | mL/m2 | 22 | 23 | 22 | 18–39 |
| LV systolic volume index | mL/m2 | 57.6 | 60 | 58.5 | 43–67 |
| LV end-diastolic volume | mL | 140 | 148 | 142 | 121–204 |
| LV end-systolic volume | mL | 38 | 41 | 38 | 33–78 |
| LV mass | g | 182 | 182 | 182 | 109–185 |
| Interventricular septal end diastole | mm | 10 | 10 | 10 | 6.0–10.4 |
| Cardiac output | L/min | 7.1 | 7.2 | 7.3 | 2.8–8.8 |
| Right atrium (4CH) | cm2 | 25 | 28 | 25 | 14–30 |
| RV ejection fraction | % | 60 | 63 | 62 | 50–76 |
| RV stroke volume | mL | 101 | 107 | 105 | 74–142 |
| RV end-diastolic volume index | mL/m2 | 96 | 96.4 | 95.5 | 65–111 |
| RV end-systolic volume index | mL/m2 | 39 | 36 | 36 | 18–47 |
| RV systolic volume index | mL/m2 | 57.5 | 60.5 | 59 | 39–71 |
| RV end-diastolic volume | mL | 170 | 171 | 170 | 121–221 |
| RV end-systolic volume | mL | 69 | 64 | 65 | 34–94 |
| Heart rate | bpm | 70 | 67 | 70 | 60–90 |
| Myocardial contraction disorders | / | no | no | no | / |
| Perfusion disorders | / | no | no | no | / |
| Delayed myocardial enhancement | / | no | no | no | / |
| Tricuspid valve regurgitation | / | + mild | + mild | + mild | / |
| Myocardial edema on T2-weighted magnetic resonance imaging | / | no | no | no | / |
| Parameters | Before Run, Middle Part, IVS | Before Run, Anteromedial Segments, IVS | Before Run, Apical Part, IVS | Before Run, Average Measurements |
| PCr | 5.22 | 6.27 | 7.34 | 6.27 |
| ATP | 4.22 | 4.46 | 4.89 | 4.38 |
| PCr/ATP | 1.24 | 1.4 | 1.5 | 1.43 |
| Parameters | 2 Days after, Middle Part, IVS | 2 Days after, Anteromedial Segments, IVS | 2 Days after, Apical Part, IVS | 2 Days after, Average Measurements |
| PCr | 6.4 | 4.24 | 3.99 | 4.87 |
| ATP | 4.42 | 4.53 | 2.58 | 3.84 |
| PCr/ATP | 1.45 | 0.94 | 1.54 | 1.26 |
| Parameters | 11 Days after, Middle Part, IVS | 11 Days after, Anteromedial Segments, IVS | 11 Days after, Apical Part, IVS | 11 Days after, Average Measurements |
| PCr | 5.06 | 6.44 | 5.02 | 5.49 |
| ATP | 3.55 | 3.36 | 3.05 | 3.32 |
| PCr/ATP | 1.43 | 1.91 | 1.64 | 1.65 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajda, R.; Klisiewicz, A.; Matsibora, V.; Piotrowska-Kownacka, D.; Biernacka, E.K. Heart of the World’s Top Ultramarathon Runner—Not Necessarily Much Different from Normal. Diagnostics 2020, 10, 73. https://doi.org/10.3390/diagnostics10020073
Gajda R, Klisiewicz A, Matsibora V, Piotrowska-Kownacka D, Biernacka EK. Heart of the World’s Top Ultramarathon Runner—Not Necessarily Much Different from Normal. Diagnostics. 2020; 10(2):73. https://doi.org/10.3390/diagnostics10020073
Chicago/Turabian StyleGajda, Robert, Anna Klisiewicz, Vadym Matsibora, Dorota Piotrowska-Kownacka, and Elżbieta Katarzyna Biernacka. 2020. "Heart of the World’s Top Ultramarathon Runner—Not Necessarily Much Different from Normal" Diagnostics 10, no. 2: 73. https://doi.org/10.3390/diagnostics10020073
APA StyleGajda, R., Klisiewicz, A., Matsibora, V., Piotrowska-Kownacka, D., & Biernacka, E. K. (2020). Heart of the World’s Top Ultramarathon Runner—Not Necessarily Much Different from Normal. Diagnostics, 10(2), 73. https://doi.org/10.3390/diagnostics10020073






