Assessing the Costs of Screening for Ovarian Cancer in the United States: An Evolving Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Subjects
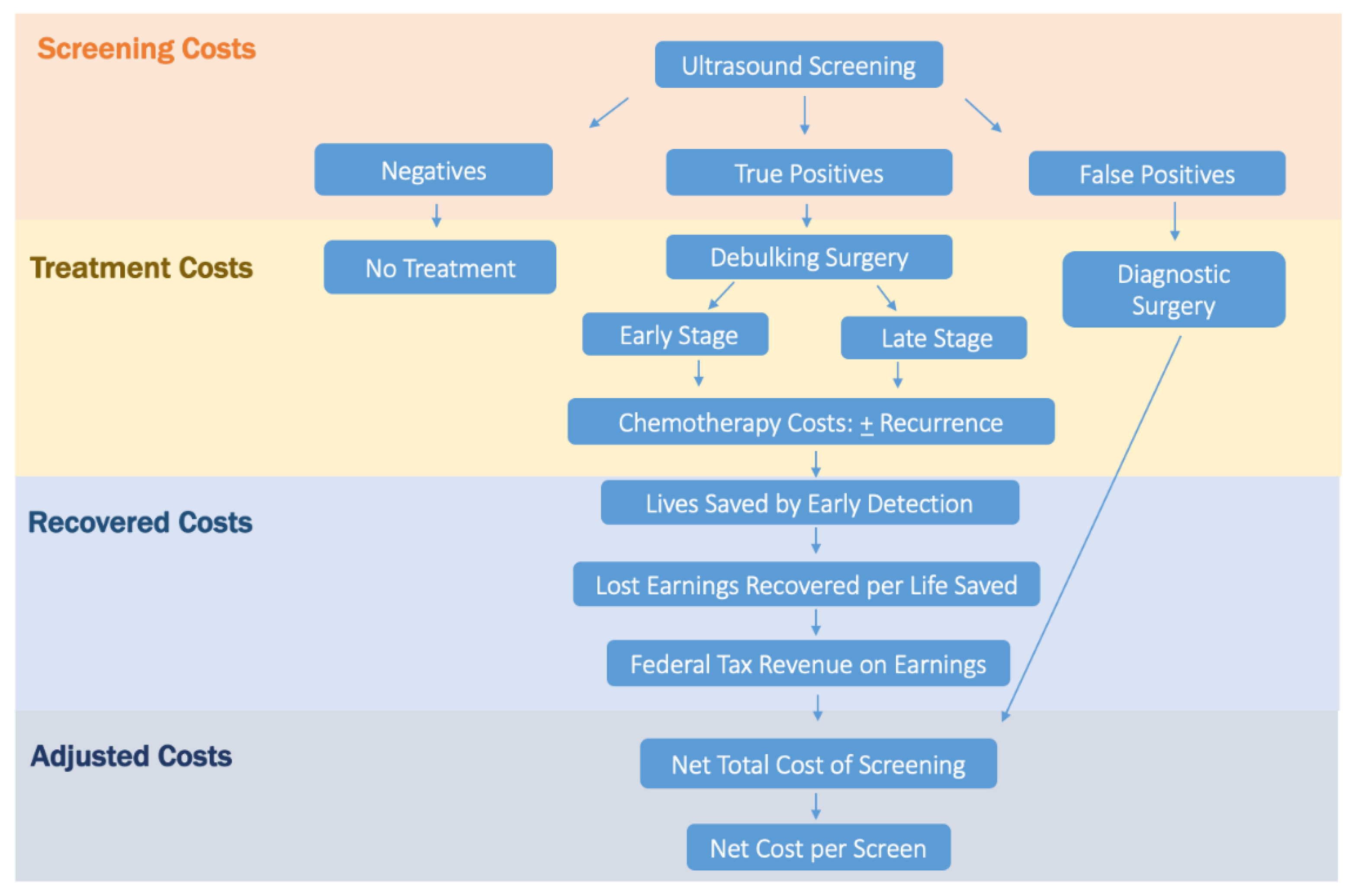
2.2. Cost Analysis
2.3. Screening and False-Positive Costs
2.4. Stage-Specific Disease Treatment Costs
2.5. Life-Years Gained Due to Screening
2.6. Adjusted Screening Cost per Life-Year Gained
2.7. Recovered Earnings and Tax Dollars
2.8. Adjusted Net Total Screening Cost
2.9. Adjusted Cost per Screen
3. Results
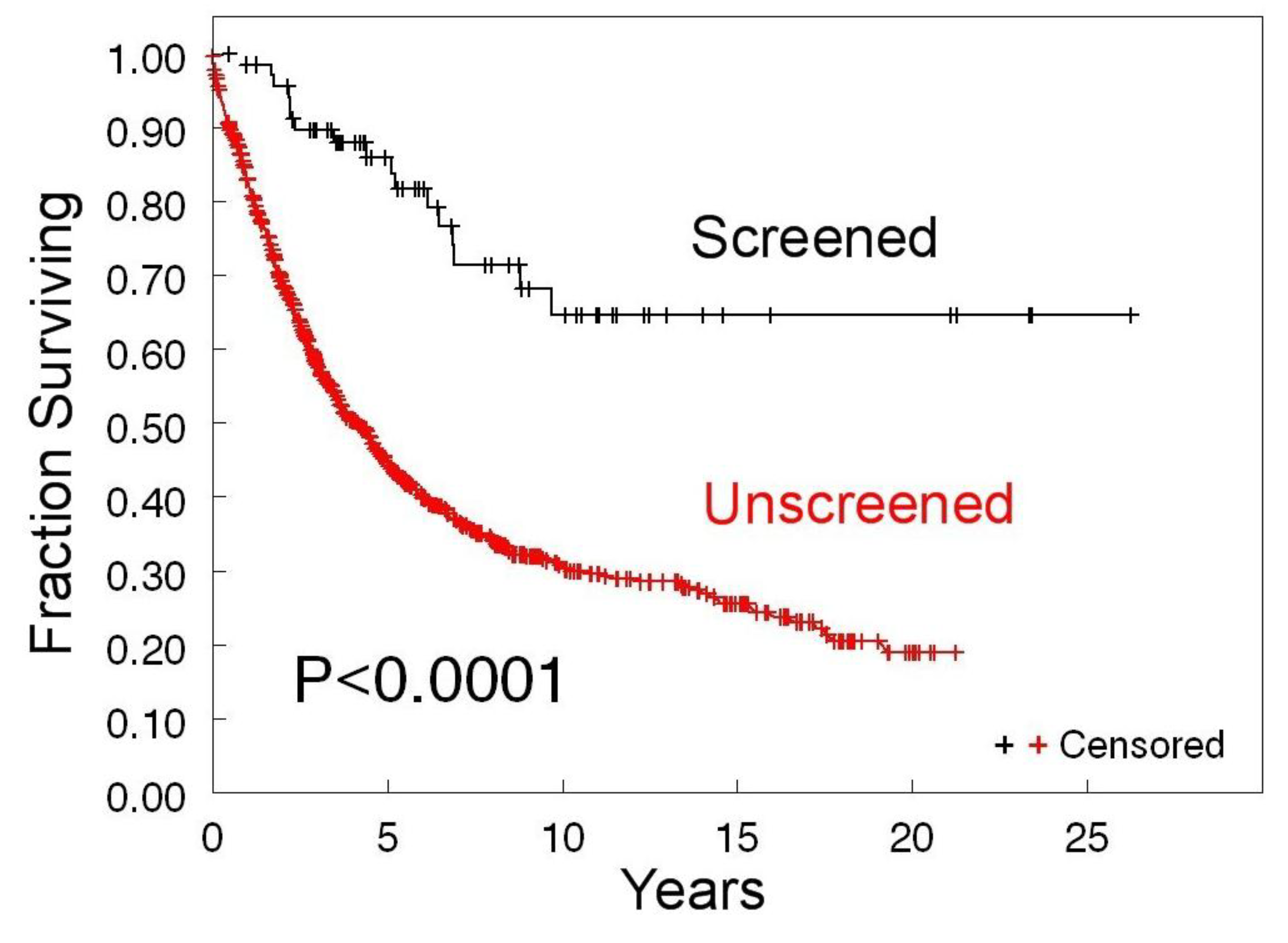
3.1. Survival of Screen-Detected versus Clinically Detected Ovarian Cancer Patients
3.2. Cost of Screening and False-Positive Surgeries
3.3. Treatment Cost Advantage of Screening
3.4. Life-Years Gained Due to Screening and Associated Cost per Life-Year
3.5. Lost Earnings and Federal Tax Dollars Recovered
3.6. Adjusted Net Total Screening Program Cost
3.7. Adjusted Cost per Screen
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Rauh-Hain, J.A.; Krivak, T.C.; Del Carmen, M.G.; Olawaive, A.B. Ovarian cancer screening and early detection in the general population. Rev. Obstet. Gynecol. 2011, 4, 15–21. [Google Scholar] [PubMed]
- Cannistra, S.A. Cancer of the ovary. N. Engl. J. Med. 2004, 351, 2519–2529. [Google Scholar] [CrossRef] [PubMed]
- van Nagell, J.R., Jr.; Miller, R.W.; DeSimone, C.P.; Ueland, J.R.; Podzielinski, I.; Goodrich, S.T.; Elder, J.W.; Huang, B.; Kryscio, R.J.; Pavlik, E.J. Long-term survival of women with epithelial ovarian cancer detected by ultrasonographic screening. Obstet. Gynecol. 2011, 118, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Buys, S.S.; Partridge, E.; Black, A.; Johnson, C.C.; Lamerato, L.; Isaacs, C.; Reding, D.J.; Greenlee, R.T.; Yokochi, L.A.; Kessel, B.; et al. PLCO Project Team. Effect of screening on ovarian cancer mortality: The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011, 305, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Menon, U.; Gentry-Maharaj, A.; Hallett, R.; Ryan, A.; Burnell, M.; Sharma, A.; Lewis, S.; Davies, S.; Philpott, S.; Lopes, A.; et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: Results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009, 10, 327–340. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yamada, Y.; Sado, T.; Sakata, M.; Yoshida, S.; Kawaguchi, R.; Kanayama, S.; Shigtomi, H.; Haruta, S.; Tsuji, Y.; et al. A randomized study of screening for ovarian cancer: A multicenter study in Japan. Int. J. Gynecol. Cancer 2008, 18, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, E.J.; van Nagell, J.R., Jr. Early detection of ovarian tumors using ultrasound. Womens Health (Lond.) 2013, 9, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, E.J. Ovarian cancer screening effectiveness: A realization from the UK Collaborative Trial of Ovarian Cancer Screening. Womens Health (Lond.) 2016, 12, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Yabroff, K.R.; Lamont, E.B.; Mariotto, A.; Warren, J.L.; Topor, M.; Meekins, A.; Brown, M.L. Cost of care for elderly cancer patients in the United States. J. Natl. Cancer Inst. 2008, 100, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Van Nagell, J.R., Jr.; Burgess, B.T.; Miller, R.W.; Baldwin, L.; DeSimone, C.P.; Ueland, F.R.; Huang, B.; Chen, Q.; Kryscio, R.J.; Pavlik, E.J. Survival of Women with Type I and II Epithelial Ovarian Cancer Detected by Ultrasound Screening. Obstet. Gynecol. 2018, 132, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Menon, U.; McGuire, A.J.; Raikou, M.; Ryan, A.; Davies, S.K.; Burnell, M.; Gentry-Maharaj, A.; Kalsi, J.K.; Singh, N.; Amso, N.N.; et al. The cost-effectiveness of screening for ovarian cancer: Results from the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Br. J. Cancer 2017, 117, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Moss, H.A.; Berchuck, A.; Neely, M.L.; Myers, E.R.; Havrilesky, L.J. Estimating Cost-effectiveness of a Multimodal Ovarian Cancer Screening Program in the United States: Secondary Analysis of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). JAMA Oncol. 2018, 4, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Committee on the State of the Science in Ovarian Cancer Research; Board on Health Care Services; Institute of Medicine; National Academies of Sciences, Engineering, and Medicine. Ovarian Cancers: Evolving Paradigms in Research and Care; National Academies Press (US): Washington, DC, USA, April 2016.
- Truven Health Analytics. Available online: http://truvenhealth.com/your-healthcare-focus/life-sciences/marketscan-databases-and-online-tools (accessed on 28 January 2017).
- Bercow, A.S.; Chen, L.; Chatterjee, S.; Tergas, A.I.; Hou, J.Y.; Burke, W.M.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. Cost of Care for the Initial Management of Ovarian Cancer. Obstet. Gynecol. 2017, 130, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Miller, K.D.; Siegel, R.L.; Zheng, Z.; Zhao, J.; Han, X.; Ma, J.; Jemal, A.; Yabroff, K.R. National and State Estimates of Lost Earnings from Cancer Deaths in the United States. JAMA Oncol. 2019, e191460. [Google Scholar] [CrossRef] [PubMed]
- Brady, D. Who Pays Income Taxes? [National Tax Payers Union Foundation Website]. 25 October 2019. Available online: https://www.ntu.org/library/doclib/2019/10/2017-who-pays-income-taxes-2.pdf (accessed on 1 December 2019).
- Transvaginal Ultrasound>Lexington, Kentucky HealthCost. Available online: https://www.healthcost.com/search/76830/Lexington,%20KY,%20USA/38.0405837,-84.50371640000003 (accessed on 12 December 2019).
- Wiggins, A.T.; Pavlik, E.J.; Andrykowski, M.A. Psychological Response to a False Positive Ovarian Cancer Screening Test Result: Distinct Distress Trajectories and Their Associated Characteristics. Diagnostics (Basel) 2019, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Network National Comprehensive Cancer. Ovarian cancer: Including fallopian tube cancer and primary peritoneal cancer. NCCN Clin. Pract. Guid. Oncol. 2018, 1, 1–115. [Google Scholar]
| Cost of Screens and False-Positive Cases | |
|---|---|
| A. Cost/screen | $56 |
| B. No. of screens | 326,998 |
| C. Cost of screens * | $18,311,888 |
| D. No. of true-positive results | 127 |
| E. No. of false-positive results | 381 |
| F. Positive Predictive Value (PPV) | 25% |
| G. Surgical cost/false-positive case | $7954 |
| H. Total surgical cost of false-positive cases ** | $3,030,474 |
| I. Total cost of screens and false-positive cases *** | $21,342,362 |
| A. Treatment Costs: Kentucky | ||||
| i. Screen-Detected Stage I–II (N = 48) | ii. Screen-Detected Stage III–IV (N = 28) | iii. Clinically Detected Stage I–II (N = 22) | iv. Clinically Detected Stage III–IV (N = 54) | |
| a. Primary Treatment Cost + | $1,606,272 | $936,992 | $736,208 | $1,807,056 |
| Recurrence (N = 7) | Recurrence (N = 23) | Recurrence (N = 3) | Recurrence (N = 52) | |
| b. Recurrence Treatment Cost # | $1,124,613 | $3,695,157 | $481,977 | $8,354,268 |
| c. Total Treatment Cost by Stage * | $2,730,885 | $4,632,149 | $1,218,185 | $10,161,324 |
| d. Treatment Cost All Stages ** | $7,363,034 | $11,379,509 | ||
| e. Treatment Cost Advantage of Screening *** $4,016,475 | ||||
| B. Treatment Costs: National (U.S.) | ||||
| i. Screen-Detected Stage I–II (N = 48) | ii. Screen-Detected Stage III–IV (N = 28) | iii. Clinically Detected Stage I–II (N = 22) | iv. Clinically Detected Stage III–IV (N = 54) | |
| a. Primary Treatment Cost + | $4,494,336 ($2,991,312–$3,923,920) | $2,621,696 ($1,744,932–$3,923,920) | $2,059,904 ($1,371,018–$3,083,080) | $5,056,128 ($3,365,226–$7,567,560) |
| Recurrence (N = 7) | Recurrence (N = 23) | Recurrence (N = 3) | Recurrence (N = 52) | |
| b. Recurrence Treatment Cost # | $427,014 ($203,196–$927,542) | $1,403,046 ($667,664–$3,047,638) | $183,006 ($87,084–$397,518) | $3,172,104 ($1,509,456–$6,890,312) |
| c. Total Treatment Cost by Stage * | $4,921,350 ($3,194,508–$7,654,262) | $4,024,742 ($2,412,576–$6,971,558) | $2,242,910 ($1,458,102–$3,480,598) | $8,228,232 ($4,874,682–$14,457,872) |
| d. Treatment Cost All Stages ** | $8,946,092 ($5,607,084–$14,625,820) | $10,471,142 ($6,332,784–$17,938,470) | ||
| e. Treatment Cost Advantage of Screening *** $1,525,050 ($725,700–$3,312,650) | ||||
| Lives Saved and Life-Years Gained Due to Screening | |
|---|---|
| A. The 10-year survival screen-detected cases (N = 76) | 52 (68%) |
| B. The 10-year survival clinically detected cases (N = 76) | 26 (34%) |
| C. Patient lives saved * | 26 |
| D. Mean age screen-detected women (years) | 65 |
| E. Mean age female life expectancy, United States (years) | 81 |
| F. Mean life-years gained/life saved ** | 16 |
| G. Total life-years gained *** | 416 |
| Adjusted Kentucky Trial Screening Program Cost per Life-Year Gained | ||
|---|---|---|
| Kentucky | National (U.S.) | |
| A. Base cost of screens/life-year gained * | $44,019 | $44,019 |
| B. Surgical cost of false-positive cases/life-year gained ** | $7285 | $7285 |
| C. Cost advantage of treating more early-stage disease/life-year gained *** | $9655 | $3666 ($1,744–$7,963) |
| D. Adjusted screening cost/life-year gained **** | $41,649 | $47,638 ($43,341–$49,560) |
| Total Earnings Gained and Recovered Federal Tax Dollars Due to Screening | |
|---|---|
| A. Lost earnings/death [17] | $136,500 ($132,518–$140,643) |
| B. Patient lives saved | 26 |
| C. Total earnings gained due to screening * | $3,549,000 ($3,445,468–$3,656,718) |
| D. Average federal income tax rate on earnings gained | 0.108 |
| E. Total federal income tax on earnings ** | $383,292 ($372,111–$394,926) |
| Adjusted Net Total Screening Cost | ||
|---|---|---|
| Kentucky | National (U.S.) | |
| A. Cost of screens (Table 1) | $18,311,888 | $18,311,888 |
| B. Total surgical cost of false-positive cases (Table 1) | $3,030,474 | $3,030,474 |
| C. Total cost advantage of treating earlier detected cases (Table 2) | $4,016,475 | $1,525,050 ($725,700–$3,312,650) |
| D. Total earnings gained due to screening (Table 4 row C) | $3,549,000 ($3,445,468–$3,656,718) | $3,549,000 ($3,445,468–$3,656,718) |
| E. Total federal income tax dollars recovered on earnings gained (Table 4 row E) | $383,292 ($372,111–$394,926) | $383,292 ($372,111–$394,926) |
| F. Adjusted total net screening cost * | $13,393,595 ($13,508,308–$13,274,243) | $15,885,020 ($13,978,068–$16,799,083) |
| Adjusted Cost per Screen | ||
|---|---|---|
| Kentucky | National (U.S.) | |
| Screens alone (Table 1 row A) | $56.00 | $56.00 |
| Screens and surgical cost of false-positive cases * | $65.27 | $65.27 |
| Screens and cost advantage of treating earlier detected cases ** | $43.72 | $51.34 ($45.87–$53.78) |
| Screens and recovered earnings and federal tax dollars *** | $43.97 ($43.61–$44.33) | $43.97 ($43.61–$44.33) |
| Adjusted net cost **** | $40.96 ($40.59–$41.31) | $48.58 ($42.75–$51.37) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorski, J.W.; Quattrone, M.; van Nagell, J.R.; Pavlik, E.J. Assessing the Costs of Screening for Ovarian Cancer in the United States: An Evolving Analysis. Diagnostics 2020, 10, 67. https://doi.org/10.3390/diagnostics10020067
Gorski JW, Quattrone M, van Nagell JR, Pavlik EJ. Assessing the Costs of Screening for Ovarian Cancer in the United States: An Evolving Analysis. Diagnostics. 2020; 10(2):67. https://doi.org/10.3390/diagnostics10020067
Chicago/Turabian StyleGorski, Justin W., McKell Quattrone, John R. van Nagell, and Edward J. Pavlik. 2020. "Assessing the Costs of Screening for Ovarian Cancer in the United States: An Evolving Analysis" Diagnostics 10, no. 2: 67. https://doi.org/10.3390/diagnostics10020067
APA StyleGorski, J. W., Quattrone, M., van Nagell, J. R., & Pavlik, E. J. (2020). Assessing the Costs of Screening for Ovarian Cancer in the United States: An Evolving Analysis. Diagnostics, 10(2), 67. https://doi.org/10.3390/diagnostics10020067







