Coronary Atherosclerosis Imaging
Abstract
1. Introduction
2. Pathology
3. Non-invasive imaging
3.1. Plaque Morphology
3.2. Computed Tomography (CT)
3.3. Computed Tomography Coronary Angiography (CTCA)
3.4. Cardiac Magnetic Resonance (CMR)
4. Disease Activity Imaging
Positron Emission Tomography (PET)
5. Intravascular Imaging
5.1. Angiography
5.2. Intravascular Ultrasound (IVUS) and Optical Coherence Tomography (OCT)
5.3. Near-Infrared Spectroscopy (NIRS)
5.4. Risk of Complications Associated with Coronary Atherosclerosis Imaging
6. Conclusions
Declaration
Funding
Conflicts of Interest
References
- Libby, P.; Theroux, P. Pathophysiology of Coronary Artery Disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, S.; Bentzon, J.F.; Daemen, M.; Falk, E.; Garcia-Garcia, H.M.; Herrmann, J.; Hoefer, I.; Jukema, J.W.; Krams, R.; Kwak, B.R.; et al. Stabilisation of atherosclerotic plaques. Position paper of the European Society of Cardiology (ESC) Working Group on atherosclerosis and vascular biology. Thromb. Haemost. 2011, 106, 1. [Google Scholar] [CrossRef]
- Libby, P. Current Concepts of the Pathogenesis of the Acute Coronary Syndromes. Circulation 2001, 104, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, K.; Nakano, M.; Otsuka, F.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathophysiology of Atherosclerosis Plaque Progression. HeartLung Circ. 2013, 22, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Butany, J. Pathogenesis of atherosclerosis. Diagn. Histopathol. 2017, 23, 473–478. [Google Scholar] [CrossRef]
- Plasschaert, H.; Heeneman, S.; Daemen, M.J. Progression in atherosclerosis: Histological features and pathophysiology of atherosclerotic lesions. Top. Magn. Reson. Imaging 2009, 20, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Ikeda, K.; Souma, Y.; Akakabe, Y.; Kitamura, Y.; Matsuo, K.; Shimoda, Y.; Ueyama, T.; Matoba, S.; Yamada, H.; Okigaki, M.; et al. Macrophages play a unique role in the plaque calcification by enhancing the osteogenic signals exerted by vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2012, 425, 39–44. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the Vulnerable Plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary Artery Calcification and its Progression: What Does It Really Mean? JACC Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Joner, M.; Prati, F.; Virmani, R.; Narula, J. Clinical classification of plaque morphology in coronary disease. Nat. Rev. Cardiol. 2014, 11, 379–389. [Google Scholar] [CrossRef]
- Tian, J.; Hou, J.; Xing, L.; Kim, S.-J.; Yonetsu, T.; Kato, K.; Lee, H.; Zhang, S.; Yu, B.; Jang, I.-K. Significance of intraplaque neovascularisation for vulnerability: Optical coherence tomography study. Heart 2012, 98, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Parma, L.; Baganha, F.; Quax, P.H.A.; de Vries, M.R. Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis. Eur. J. Pharmacol. 2017, 816, 107–115. [Google Scholar] [CrossRef]
- Varnava, A.M.; Davies, M.J. Relation between coronary artery remodelling (compensatory dilatation) and stenosis in human native coronary arteries. Heart 2001, 86, 207–211. [Google Scholar] [CrossRef][Green Version]
- Schulte, D.M.; Paulsen, K.; TüRk, K.; Brandt, B.; Freitag-Wolf, S.; Hagen, I.; Zeuner, R.; SchröDer, J.O.; Lieb, W.; Franke, A.; et al. Small dense LDL cholesterol in human subjects with different chronic inflammatory diseases. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Tabas, I.; Fredman, G.; Fisher, E.A. Inflammation and its Resolution as Determinants of Acute Coronary Syndromes. Circ. Res. 2014, 114, 1867–1879. [Google Scholar] [CrossRef] [PubMed]
- Falk, E.; Nakano, M.; Bentzon, J.F.; Finn, A.V.; Virmani, R. Update on acute coronary syndromes: The pathologists’ view. Eur. Heart J. 2013, 34, 719–728. [Google Scholar] [CrossRef]
- Kolodgie, D.F.; Burke, P.A.; Farb, K.A.; Gold, V.H.; Yuan, V.J.; Narula, V.J.; Finn, V.A.; Virmani, V.R. The thin-cap fibroatheroma: A type of vulnerable plaque: The major precursor lesion to acute coronary syndromes. Curr. Opin. Cardiol. 2001, 16, 285–292. [Google Scholar] [CrossRef]
- Yonetsu, T.; Kakuta, T.; Lee, T.; Takahashi, K.; Kawaguchi, N.; Yamamoto, G.; Koura, K.; Hishikari, K.; Iesaka, Y.; Fujiwara, H.; et al. In vivo critical fibrous cap thickness for rupture-prone coronary plaques assessed by optical coherence tomography. Eur. Heart J. 2011, 32, 1251–1259. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Kolodgie, F.D.; Farb, A. Pathology of the Thin-Cap Fibroatheroma. J. Interv. Cardiol. 2003, 16, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Nicholls, S.J.; Ellis, S.G.; Tuzcu, E.M.; Kapadia, S.R. High-Risk Coronary Atheroma: The Interplay Between Ischemia, Plaque Burden, and Disease Progression. J. Am. Coll. Cardiol. 2014, 63, 1134–1140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hutcheson, J.D.; Maldonado, N.; Aikawa, E. Small entities with large impact: Microcalcifications and atherosclerotic plaque vulnerability. Curr. Opin. Lipidol. 2014, 25, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Sakakura, K.; Yahagi, K.; Joner, M.; Virmani, R. Has Our Understanding of Calcification in Human Coronary Atherosclerosis Progressed? Arterioscler. Thromb. Vasc. Biol. 2014, 34, 724–736. [Google Scholar] [CrossRef]
- Hsu, J.J.; Lim, J.; Tintut, Y.; Demer, L.L. Cell-matrix mechanics and pattern formation in inflammatory cardiovascular calcification. Heart 2016, 102, 1710–1715. [Google Scholar] [CrossRef]
- Shioi, A.; Ikari, Y. Plaque Calcification during Atherosclerosis Progression and Regression. J. Atheroscler. Thromb. 2018, 25, 294–303. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons From Sudden Coronary Death. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Schaar, J.A.; Muller, J.E.; Falk, E.; Virmani, R.; Fuster, V.; Serruys, P.W.; Colombo, A.; Stefanadis, C.; Ward Casscells, S.; Moreno, P.R.; et al. Terminology for high-risk and vulnerable coronary artery plaques. Eur. Heart J. 2004, 25, 1077–1082. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Nakano, M.; Virmani, R.; Fuster, V. Acute Coronary Events. Circulation 2012, 125, 1147–1156. [Google Scholar] [CrossRef]
- Partida, R.A.; Libby, P.; Crea, F.; Jang, I.-K. Plaque erosion: A new in vivo diagnosis and a potential major shift in the management of patients with acute coronary syndromes. Eur. Heart J. 2018, 39, 2070–2076. [Google Scholar] [CrossRef]
- Fleg, J.L.; Stone, G.W.; Fayad, Z.A.; Granada, J.F.; Hatsukami, T.S.; Kolodgie, F.D.; Ohayon, J.; Pettigrew, R.; Sabatine, M.S.; Tearney, G.J.; et al. Detection of High-Risk Atherosclerotic Plaque: Report of the NHLBI Working Group on Current Status and Future Directions. JACC Cardiovasc. Imaging 2012, 5, 941–955. [Google Scholar] [CrossRef]
- Kubo, T.; Maehara, A.; Mintz, G.S.; Doi, H.; Tsujita, K.; Choi, S.-Y.; Katoh, O.; Nasu, K.; Koenig, A.; Pieper, M.; et al. The Dynamic Nature of Coronary Artery Lesion Morphology Assessed by Serial Virtual Histology Intravascular Ultrasound Tissue Characterization. J. Am. Coll. Cardiol. 2010, 55, 1590–1597. [Google Scholar] [CrossRef]
- Tian, J.; Ren, X.; Vergallo, R.; Xing, L.; Yu, H.; Jia, H.; Soeda, T.; McNulty, I.; Hu, S.; Lee, H.; et al. Distinct Morphological Features of Ruptured Culprit Plaque for Acute Coronary Events Compared to Those With Silent Rupture and Thin-Cap Fibroatheroma: A Combined Optical Coherence Tomography and Intravascular Ultrasound Study. J. Am. Coll. Cardiol. 2014, 63, 2209–2216. [Google Scholar] [CrossRef]
- Nicoll, R.; Henein, M. Arterial calcification: A new perspective? Int. J. Cardiol. 2017, 228, 11–22. [Google Scholar] [CrossRef]
- Motreff, P.; Rioufol, G.; Finet, G. Seventy-Four Month Follow-Up of Coronary Vulnerable Plaques by Serial Gray-Scale Intravascular Ultrasound. Circulation 2012, 126, 2878–2879. [Google Scholar] [CrossRef]
- Bom, M.J.; Heijden, D.J.v.d.; Kedhi, E.; Heyden, J.v.d.; Meuwissen, M.; Knaapen, P.; Timmer, S.A.J.; Royen, N.v. Early Detection and Treatment of the Vulnerable Coronary Plaque. Circ. Cardiovasc. Imaging 2017, 10, e005973. [Google Scholar] [CrossRef]
- Nicoll, R.; Henein, M. Extensive Coronary Calcification: A Clinically Unrecognised Condition. Curr. Vasc. Pharmacol. 2010, 8, 701–705. [Google Scholar] [CrossRef]
- Goldstein, J.A. Multifocal coronary plaque instability. Prog. Cardiovasc. Dis. 2002, 44, 449–454. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Fuster, V. The Myth of the “Vulnerable Plaque”: Transitioning From a Focus on Individual Lesions to Atherosclerotic Disease Burden for Coronary Artery Disease Risk Assessment. J. Am. Coll. Cardiol. 2015, 65, 846–855. [Google Scholar] [CrossRef]
- Libby, P.; Pasterkamp, G. Requiem for the ‘vulnerable plaque’. Eur. Heart J. 2015, 36, 2984–2987. [Google Scholar] [CrossRef]
- Mauriello, A.; Servadei, F.; Zoccai, G.B.; Giacobbi, E.; Anemona, L.; Bonanno, E.; Casella, S. Coronary calcification identifies the vulnerable patient rather than the vulnerable Plaque. Atherosclerosis 2013, 229, 124–129. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Villagra, R.; Gibson, R.; Donders, R.; Warlow, C.P. Evidence of a chronic systemic cause of instability of atherosclerotic plaques. Lancet 2000, 355, 19–24. [Google Scholar] [CrossRef]
- Nissen, S.E. The Vulnerable Plaque “Hypothesis”: Promise, but Little Progress⁎⁎Editorials published in JACC: Cardiovascular Imaging reflect the views of the authors and do not necessarily represent the views of JACC: Cardiovascular Imaging or the American College of Cardiology. JACC Cardiovasc. Imaging 2009, 2, 483–485. [Google Scholar] [CrossRef]
- Sandfort, A.C.V.; Lima, A.J.; Bluemke, A.D. Noninvasive Imaging of Atherosclerotic Plaque Progression: Status of Coronary Computed Tomography Angiography. Circ. Cardiovasc. Imaging 2015, 8, e003316. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Mahabadi, A.A.; Möhlenkamp, S.; Lehmann, N.; Kälsch, H.; Dykun, I.; Pundt, N.; Moebus, S.; Jöckel, K.-H.; Erbel, R. CAC Score Improves Coronary and CV Risk Assessment Above Statin Indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC Cardiovasc. Imaging 2017, 10, 143–153. [Google Scholar] [CrossRef]
- Youssef, G.; Kalia, N.; Darabian, S.; Budoff, M.J. Coronary Calcium: New Insights, Recent Data, and Clinical Role. Curr. Cardiol. Rep. 2013, 15, 325. [Google Scholar] [CrossRef]
- Nicoll, R.; Wiklund, U.; Zhao, Y.; Diederichsen, A.; Mickley, H.; Ovrehus, K.; Zamorano, P.; Gueret, P.; Schmermund, A.; Maffei, E.; et al. The coronary calcium score is a more accurate predictor of significant coronary stenosis than conventional risk factors in symptomatic patients: Euro-CCAD study. Int. J. Cardiol. 2016, 207, 13–19. [Google Scholar] [CrossRef]
- Sandfort, V.; Bluemke, D.A. CT calcium scoring. History, current status and outlook. Diagn. Interv. Imaging 2017, 98, 3–10. [Google Scholar] [CrossRef]
- McClelland, R.L.; Jorgensen, N.W.; Budoff, M.; Blaha, M.J.; Post, W.S.; Kronmal, R.A.; Bild, D.E.; Shea, S.; Liu, K.; Watson, K.E.; et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J. Am. Coll. Cardiol. 2015, 66, 1643–1653. [Google Scholar] [CrossRef]
- Carr, J.J.; Jacobs, D.R., Jr.; Terry, J.G.; Shay, C.M.; Sidney, S.; Liu, K.; Schreiner, P.J.; Lewis, C.E.; Shikany, J.M.; Reis, J.P.; et al. Association of Coronary Artery Calcium in Adults Aged 32 to 46 Years With Incident Coronary Heart Disease and DeathCoronary Artery Calcium and Incident Coronary Heart Disease and DeathCoronary Artery Calcium and Incident Coronary Heart Disease and Death. JAMA Cardiol. 2017, 2, 391–399. [Google Scholar] [CrossRef]
- Detrano, R.; Guerci, A.D.; Carr, J.J.; Bild, D.E.; Burke, G.; Folsom, A.R.; Liu, K.; Shea, S.; Szklo, M.; Bluemke, D.A.; et al. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. N. Engl. J. Med. 2008, 358, 1336–1345. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Hecht, H.; Blaha, M.J.; Berman, D.S.; Nasir, K.; Budoff, M.; Leipsic, J.; Blankstein, R.; Narula, J.; Rumberger, J.; Shaw, L.J. Clinical indications for coronary artery calcium scoring in asymptomatic patients: Expert consensus statement from the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2017, 11, 157–168. [Google Scholar] [CrossRef]
- Greenland, P.; Bonow, R.O.; Brundage, B.H.; Budoff, M.J.; Eisenberg, M.J.; Grundy, S.M. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain. J. Am. Coll. Cardiol. 2007, 49, 378–402. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef]
- Sarwar, A.; Shaw, L.J.; Shapiro, M.D.; Blankstein, R.; Hoffman, U.; Cury, R.C.; Abbara, S.; Brady, T.J.; Budoff, M.J.; Blumenthal, R.S.; et al. Diagnostic and Prognostic Value of Absence of Coronary Artery Calcification. JACC Cardiovasc. Imaging 2009, 2, 675–688. [Google Scholar] [CrossRef]
- Gottlieb, I.; Miller, J.M.; Arbab-Zadeh, A.; Dewey, M.; Clouse, M.E.; Sara, L.; Niinuma, H.; Bush, D.E.; Paul, N.; Vavere, A.L.; et al. The Absence of Coronary Calcification Does Not Exclude Obstructive Coronary Artery Disease or the Need for Revascularization in Patients Referred for Conventional Coronary Angiography. J. Am. Coll. Cardiol. 2010, 55, 627–634. [Google Scholar] [CrossRef]
- Drosch, T.; Brodoefel, H.; Reimann, A.; Thomas, C.; Tsiflikas, I.; Heuschmid, M.; Schroeder, S.; Burgstahler, C. Prevalence and Clinical Characteristics of Symptomatic Patients with Obstructive Coronary Artery Disease in the Absence of Coronary Calcifications. Acad. Radiol. 2010, 17, 1254–1258. [Google Scholar] [CrossRef]
- Martin, S.S.; Blaha, M.J.; Blankstein, R.; Agatston, A.; Rivera, J.J.; Virani, S.S.; Ouyang, P.; Jones, S.R.; Blumenthal, R.S.; Budoff, M.J.; et al. Dyslipidemia, Coronary Artery Calcium, and Incident Atherosclerotic Cardiovascular Disease. Circulation 2014, 129, 77–86. [Google Scholar] [CrossRef]
- Shaw, L.J.; Giambrone, A.E.; Blaha, M.J.; Knapper, J.T.; Berman, D.S.; Bellam, N.; Quyyumi, A.; Budoff, M.J.; Callister, T.Q.; Min, J.K. Long-Term Prognosis After Coronary Artery Calcification Testing in Asymptomatic Patients: A Cohort Study. Ann. Intern. Med. 2015, 163, 14–21. [Google Scholar] [CrossRef]
- Nasir, J.K.; Rubin, J.J.; Blaha, J.M.; Shaw, N.L.; Blankstein, A.R.; Rivera, R.J.; Khan, S.A.; Berman, J.D.; Raggi, J.P.; Callister, J.T.; et al. Interplay of Coronary Artery Calcification and Traditional Risk Factors for the Prediction of All-Cause Mortality in Asymptomatic Individuals. Circ. Cardiovasc. Imaging 2012, 5, 467–473. [Google Scholar] [CrossRef]
- Criqui, M.H.; Denenberg, J.O.; Ix, J.H.; McClelland, R.L.; Wassel, C.L.; Rifkin, D.E.; Carr, J.J.; Budoff, M.J.; Allison, M.A. Calcium Density of Coronary Artery Plaque and Risk of Incident Cardiovascular Events. JAMA 2014, 311, 271–278. [Google Scholar] [CrossRef]
- Halon, D.A.; Lavi, I.; Barnett-Griness, O.; Rubinshtein, R.; Zafrir, B.; Azencot, M.; Lewis, B.S. Plaque Morphology as Predictor of Late Plaque Events in Patients With Asymptomatic Type 2 Diabetes: A Long-Term Observational Study. JACC Cardiovasc. Imaging 2019, 12, 1353–1363. [Google Scholar] [CrossRef]
- Criqui, M.H.; Knox, J.B.; Denenberg, J.O.; Forbang, N.I.; McClelland, R.L.; Novotny, T.E.; Sandfort, V.; Waalen, J.; Blaha, M.J.; Allison, M.A. Coronary Artery Calcium Volume and Density: Potential Interactions and Overall Predictive Value: The Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc. Imaging 2017, 10, 845–854. [Google Scholar] [CrossRef]
- Yoon, H.-C.; Emerick, A.M.; Hill, J.A.; Gjertson, D.W.; Goldin, J.G. Calcium Begets Calcium: Progression of Coronary Artery Calcification in Asymptomatic Subjects. Radiology 2002, 224, 236–241. [Google Scholar] [CrossRef]
- Baron, K.B.; Choi, A.D.; Chen, M.Y. Low Radiation Dose Calcium Scoring: Evidence and Techniques. Curr. Cardiovasc. Imaging Rep. 2016, 9, 12. [Google Scholar] [CrossRef]
- Thomsen, C.; Abdulla, J. Characteristics of high-risk coronary plaques identified by computed tomographic angiography and associated prognosis: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 120–129. [Google Scholar] [CrossRef]
- Thomas, I.C.; Forbang, N.I.; Criqui, M.H. The evolving view of coronary artery calcium and cardiovascular disease risk. Clin. Cardiol. 2018, 41, 144–150. [Google Scholar] [CrossRef]
- Saremi, F.; Achenbach, S. Coronary Plaque Characterization Using CT. Am. J. Roentgenol. 2015, 204, W249–W260. [Google Scholar] [CrossRef]
- Taylor, A.J.; Cerqueira, M.; Hodgson, J.M.; Mark, D.; Min, J.; O’Gara, P.; Rubin, G.D. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J. Am. Coll. Cardiol. 2010, 56, 1864–1894. [Google Scholar] [CrossRef]
- Gilard, M.; Le Gal, G.; Cornily, J.-C.; Vinsonneau, U.; Joret, C.; Pennec, P.-Y.; Mansourati, J.; Boschat, J. Midterm Prognosis of Patients With Suspected Coronary Artery Disease and Normal Multislice Computed Tomographic Findings: A Prospective Management Outcome Study. Arch. Intern. Med. 2007, 167, 1686–1689. [Google Scholar] [CrossRef]
- Min, J.K.; Shaw, L.J.; Devereux, R.B.; Okin, P.M.; Weinsaft, J.W.; Russo, D.J.; Lippolis, N.J.; Berman, D.S.; Callister, T.Q. Prognostic Value of Multidetector Coronary Computed Tomographic Angiography for Prediction of All-Cause Mortality. J. Am. Coll. Cardiol. 2007, 50, 1161–1170. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, J.; Lv, X.; Cai, W. Prognostic Value of Cardiac Computed Tomography Angiography in Patients with Suspected Coronary Artery Disease: A Meta-Analysis. Cardiology 2014, 128, 304–312. [Google Scholar] [CrossRef]
- Fischer, C.; Hulten, E.; Belur, P.; Smith, R.; Voros, S.; Villines, T.C. Coronary CT angiography versus intravascular ultrasound for estimation of coronary stenosis and atherosclerotic plaque burden: A meta-analysis. J. Cardiovasc. Comput. Tomogr. 2013, 7, 256–266. [Google Scholar] [CrossRef]
- Voros, S.; Rinehart, S.; Qian, Z.; Joshi, P.; Vazquez, G.; Fischer, C.; Belur, P.; Hulten, E.; Villines, T.C. Coronary Atherosclerosis Imaging by Coronary CT Angiography: Current Status, Correlation With Intravascular Interrogation and Meta-Analysis. JACC Cardiovasc. Imaging 2011, 4, 537–548. [Google Scholar] [CrossRef]
- Bittencourt, S.M.; Hulten, P.E.; Ghoshhajra, A.B.; O’leary, L.D.; Christman, J.M.; Montana, W.P.; Truong, J.Q.; Steigner, F.M.; Murthy, F.V.; Rybicki, F.F.; et al. Prognostic Value of Nonobstructive and Obstructive Coronary Artery Disease Detected by Coronary Computed Tomography Angiography to Identify Cardiovascular Events. Circ. Cardiovasc. Imaging 2014, 7, 282–291. [Google Scholar] [CrossRef]
- Butler, J.; Shapiro, M.; Reiber, J.; Sheth, T.; Ferencik, M.; Kurtz, E.G.; Nichols, J.; Pena, A.; Cury, R.C.; Brady, T.J.; et al. Extent and distribution of coronary artery disease: A comparative study of invasive versus noninvasive angiography with computed angiography. Am. Heart J. 2007, 153, 378–384. [Google Scholar] [CrossRef]
- Pathan, F.; Negishi, K. Prediction of cardiovascular outcomes by imaging coronary atherosclerosis. Cardiovasc. Diagn. Ther. 2016, 6, 322–339. [Google Scholar] [CrossRef]
- de Knegt, M.C.; Linde, J.J.; Fuchs, A.; Pham, M.H.C.; Jensen, A.K.; Nordestgaard, B.G.; Kelbæk, H.; Køber, L.V.; Heitmann, M.; Fornitz, G.; et al. Relationship between patient presentation and morphology of coronary atherosclerosis by quantitative multidetector computed tomography. Eur. Heart J. Cardiovasc. Imaging 2018, 20, 1221–1230. [Google Scholar] [CrossRef]
- Shishikura, D. Noninvasive imaging modalities to visualize atherosclerotic plaques. Cardiovasc. Diagn. Ther. 2016, 6, 340–353. [Google Scholar] [CrossRef]
- Xie, Y.; Kim, Y.-J.; Pang, J.; Kim, J.-S.; Yang, Q.; Wei, J.; Nguyen, C.T.; Deng, Z.; Choi, B.W.; Fan, Z.; et al. Coronary Atherosclerosis T1-Weighed Characterization with Integrated Anatomical Reference: Comparison with High-Risk Plaque Features Detected by Invasive Coronary Imaging. JACC Cardiovasc. Imaging 2017, 10, 637–648. [Google Scholar] [CrossRef]
- Andrews, J.P.M.; Fayad, Z.A.; Dweck, M.R. New methods to image unstable atherosclerotic plaques. Atherosclerosis 2018, 272, 118–128. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ehara, S.; Hasegawa, T.; Sakaguchi, M.; Otsuka, K.; Yoshikawa, J.; Shimada, K. Localization of Coronary High-Intensity Signals on T1-Weighted MR Imaging: Relation to Plaque Morphology and Clinical Severity of Angina Pectoris. JACC Cardiovasc. Imaging 2015, 8, 1143–1152. [Google Scholar] [CrossRef]
- Jansen, C.H.P.; Perera, D.; Wiethoff, A.J.; Phinikaridou, A.; Razavi, R.M.; Rinaldi, A.; Marber, M.S.; Greil, G.F.; Nagel, E.; Maintz, D.; et al. Contrast-enhanced magnetic resonance imaging for the detection of ruptured coronary plaques in patients with acute myocardial infarction. PLoS ONE 2017, 12, e0188292. [Google Scholar] [CrossRef]
- Hamdan, A.; Asbach, P.; Wellnhofer, E.; Klein, C.; Gebker, R.; Kelle, S.; Kilian, H.; Huppertz, A.; Fleck, E. A Prospective Study for Comparison of MR and CT Imaging for Detection of Coronary Artery Stenosis. JACC Cardiovasc. Imaging 2011, 4, 50–61. [Google Scholar] [CrossRef]
- Sakuma, H.; Ichikawa, Y.; Chino, S.; Hirano, T.; Makino, K.; Takeda, K. Detection of Coronary Artery Stenosis with Whole-Heart Coronary Magnetic Resonance Angiography. J. Am. Coll. Cardiol. 2006, 48, 1946–1950. [Google Scholar] [CrossRef]
- Kato, S.; Kitagawa, K.; Ishida, N.; Ishida, M.; Nagata, M.; Ichikawa, Y.; Katahira, K.; Matsumoto, Y.; Seo, K.; Ochiai, R.; et al. Assessment of Coronary Artery Disease Using Magnetic Resonance Coronary Angiography: A National Multicenter Trial. J. Am. Coll. Cardiol. 2010, 56, 983–991. [Google Scholar] [CrossRef]
- Robson, P.M.; Dweck, M.R.; Trivieri, M.G.; Abgral, R.; Karakatsanis, N.A.; Contreras, J.; Gidwani, U.; Narula, J.P.; Fuster, V.; Kovacic, J.C.; et al. Coronary Artery PET/MR Imaging: Feasibility, Limitations, and Solutions. JACC Cardiovasc. Imaging 2017, 10, 1103–1112. [Google Scholar] [CrossRef]
- Wurster, T.; Landmesser, U.; Engel, L.-C.; Bigalke, B.; Makowski, M. Coronary Vessel Wall Imaging: State of the Art and Future Directions. Curr. Cardiovasc. Imaging Rep. 2019, 12, 16. [Google Scholar] [CrossRef]
- Evans, N.R.; Tarkin, J.M.; Chowdhury, M.M.; Warburton, E.A.; Rudd, J.H.F. PET Imaging of Atherosclerotic Disease: Advancing Plaque Assessment from Anatomy to Pathophysiology. Curr. Atheroscler. Rep. 2016, 18. [Google Scholar] [CrossRef]
- Joshi, N.V.; Vesey, A.T.; Williams, M.C.; Shah, A.S.V.; Calvert, P.A.; Craighead, F.H.M.; Yeoh, S.E.; Wallace, W.; Salter, D.; Fletcher, A.M.; et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet 2014, 383, 705. [Google Scholar] [CrossRef]
- Robson, P.M.; Dey, D.; Newby, D.E.; Berman, D.; Li, D.; Fayad, Z.A.; Dweck, M.R. MR/PET Imaging of the Cardiovascular System. JACC Cardiovasc. Imaging 2017, 10, 1165–1179. [Google Scholar] [CrossRef]
- Ben-Haim, S.; Kupzov, E.; Tamir, A.; Israel, O. Evaluation of 18F-FDG Uptake and Arterial Wall Calcifications Using 18F-FDG PET/CT. J. Nucl. Med. 2004, 45, 1816–1821. [Google Scholar]
- Adamson, P.D.; Vesey, A.T.; Joshi, N.V.; Newby, D.E.; Dweck, M.R. Salt in the wound: (18)F-fluoride positron emission tomography for identification of vulnerable coronary plaques. Cardiovasc. Diagn. Ther. 2015, 5, 150–155. [Google Scholar] [CrossRef]
- Fiz, F.; Morbelli, S.; Piccardo, A.; Bauckneht, M.; Ferrarazzo, G.; Pestarino, E.; Cabria, M.; Democrito, A.; Riondato, M.; Villavecchia, G.; et al. F-18-NaF Uptake by Atherosclerotic Plaque on PET/CT Imaging: Inverse Correlation Between Calcification Density and Mineral Metabolic Activity. J. Nucl. Med. 2015, 56, 1019–1023. [Google Scholar] [CrossRef]
- Dweck, M.R.; Aikawa, E.; Newby, D.E.; Tarkin, J.M.; Rudd, J.H.F.; Narula, J.; Fayad, Z.A. Noninvasive Molecular Imaging of Disease Activity in Atherosclerosis. Circ. Res. 2016, 119, 330–340. [Google Scholar] [CrossRef]
- Kwiecinski, J.; Dey, D.; Cadet, S.; Lee, S.-E.; Tamarappoo, B.; Otaki, Y.; Huynh, P.T.; Friedman, J.D.; Dweck, M.R.; Newby, D.E.; et al. Predictors of 18F-sodium fluoride uptake in patients with stable coronary artery disease and adverse plaque features on computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 58–66. [Google Scholar] [CrossRef]
- Kitagawa, T.; Yamamoto, H.; Toshimitsu, S.; Sasaki, K.; Senoo, A.; Kubo, Y.; Tatsugami, F.; Awai, K.; Hirokawa, Y.; Kihara, Y. 18F-sodium fluoride positron emission tomography for molecular imaging of coronary atherosclerosis based on computed tomography analysis. Atherosclerosis 2017, 263, 385–392. [Google Scholar] [CrossRef]
- Mintz, G.S. Intravascular Imaging of Coronary Calcification and Its Clinical Implications. JACC Cardiovasc. Imaging 2015, 8, 461–471. [Google Scholar] [CrossRef]
- Meijboom, W.B.; Van Mieghem, C.A.G.; van Pelt, N.; Weustink, A.; Pugliese, F.; Mollet, N.R.; Boersma, E.; Regar, E.; van Geuns, R.J.; de Jaegere, P.J.; et al. Comprehensive Assessment of Coronary Artery Stenoses: Computed Tomography Coronary Angiography Versus Conventional Coronary Angiography and Correlation With Fractional Flow Reserve in Patients With Stable Angina. J. Am. Coll. Cardiol. 2008, 52, 636–643. [Google Scholar] [CrossRef]
- Nicoll, R.; Henein, M.Y. Arterial calcification: Friend or foe? Int. J. Cardiol. 2013, 167, 322–327. [Google Scholar] [CrossRef]
- Giavarini, A.; Kilic, I.D.; Redondo Diéguez, A.; Longo, G.; Vandormael, I.; Pareek, N.; Kanyal, R.; De Silva, R.; Di Mario, C. Intracoronary Imaging. Heart 2017, 103, 708–725. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Kataoka, Y.; Kanaya, T.; Noguchi, T.; Ogawa, H.; Yasuda, S. Characterization of coronary atherosclerosis by intravascular imaging modalities. Cardiovasc. Diagn. Ther. 2016, 6, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Mintz, G.S.; Biro, S.; Lee, J.-B.; Sum, S.T.; Madden, S.P.; Burke, A.P.; Zhang, P.; He, B.; Goldstein, J.A. Insights into echo-attenuated plaques, echolucent plaques, and plaques with spotty calcification: Novel findings from comparisons among intravascular ultrasound, near-infrared spectroscopy, and pathological histology in 2,294 human coronary artery segments. J. Am. Coll. Cardiol. 2014, 63, 2220–2233. [Google Scholar] [CrossRef]
- Van Soest, G.; Marcu, L.; Bouma, B.E.; Regar, E. Intravascular imaging for characterization of coronary atherosclerosis. Curr. Opin. Biomed. Eng. 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Cheng, J.M.; Garcia-Garcia, H.M.; de Boer, S.P.M.; Kardys, I.; Heo, J.H.; Akkerhuis, K.M.; Oemrawsingh, R.M.; van Domburg, R.T.; Ligthart, J.; Witberg, K.T.; et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: Results of the ATHEROREMO-IVUS study. Eur. Heart J. 2014, 35, 639–647. [Google Scholar] [CrossRef]
- Calvert, P.A.; Obaid, D.R.; O’Sullivan, M.; Shapiro, L.M.; McNab, D.; Densem, C.G.; Schofield, P.M.; Braganza, D.; Clarke, S.C.; Ray, K.K.; et al. Association Between IVUS Findings and Adverse Outcomes in Patients with Coronary Artery Disease: The VIVA (VH-IVUS in Vulnerable Atherosclerosis) Study. JACC Cardiovasc. Imaging 2011, 4, 894–901. [Google Scholar] [CrossRef]
- Nasu, K.; Tsuchikane, E.; Katoh, O.; Vince, D.G.; Virmani, R.; Surmely, J.-F.; Murata, A.; Takeda, Y.; Ito, T.; Ehara, M.; et al. Accuracy of In Vivo Coronary Plaque Morphology Assessment: A Validation Study of In Vivo Virtual Histology Compared With In Vitro Histopathology. J. Am. Coll. Cardiol. 2006, 47, 2405–2412. [Google Scholar] [CrossRef]
- Lowe, H.C.; Narula, J.; Fujimoto, J.G.; Jang, I.-K. Intracoronary Optical Diagnostics: Current Status, Limitations, and Potential. JACC Cardiovasc. Interv. 2011, 4, 1257–1270. [Google Scholar] [CrossRef]
- Mintz, G.S.; Guagliumi, G. Intravascular imaging in coronary artery disease. Lancet 2017, 390, 793–809. [Google Scholar] [CrossRef]
- Suh, M.W.; Seto, H.A.; Margey, J.P.R.; Cruz-Gonzalez, J.P.I.; Jang, J.P.I.-K. Intravascular Detection of the Vulnerable Plaque. Circ. Cardiovasc. Imaging 2011, 4, 169–178. [Google Scholar] [CrossRef]
- Tearney, G.J.; Yabushita, H.; Houser, S.L.; Aretz, H.T.; Jang, I.-K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Halpern, E.F.; Bouma, B.E. Quantification of Macrophage Content in Atherosclerotic Plaques by Optical Coherence Tomography. Circulation 2003, 107, 113–119. [Google Scholar] [CrossRef]
- Sinclair, H.; Bourantas, C.; Bagnall, A.; Mintz, G.S.; Kunadian, V. OCT for the Identification of Vulnerable Plaque in Acute Coronary Syndrome. JACC Cardiovasc. Imaging 2015, 8, 198–209. [Google Scholar] [CrossRef]
- Habara, M.; Otsuka, F.; Tsuchikane, E.; Terashima, M.; Nasu, K.; Kinoshita, Y.; Murata, A.; Suzuki, Y.; Kawase, Y.; Okubo, M.; et al. In vivo tissue characterization of human atherosclerotic plaques by optical coherence tomography: A directional coronary atherectomy study with histopathologic confirmation. Int. J. Cardiol. 2018, 268, 1–10. [Google Scholar] [CrossRef]
- Burgmaier, M.; Milzi, A.; Dettori, R.; Burgmaier, K.; Marx, N.; Reith, S. Co-localization of plaque macrophages with calcification is associated with a more vulnerable plaque phenotype and a greater calcification burden in coronary target segments as determined by OCT. PLoS ONE 2018, 13, e0205984. [Google Scholar] [CrossRef]
- Reith, S.; Milzi, A.; Dettori, R.; Marx, N.; Burgmaier, M. Predictors for target lesion microcalcifications in patients with stable coronary artery disease: An optical coherence tomography study. Clin. Res. Cardiol. 2018, 107, 763–771. [Google Scholar] [CrossRef]
- Bourantas, C.V.; Jaffer, F.A.; Gijsen, F.J.; van Soest, G.; Madden, S.P.; Courtney, B.K.; Fard, A.M.; Tenekecioglu, E.; Zeng, Y.; van der Steen, A.F.W.; et al. Hybrid intravascular imaging: Recent advances, technical considerations, and current applications in the study of plaque pathophysiology. Eur. Heart J. 2017, 38, 400–412. [Google Scholar] [CrossRef]
- Fujii, K.; Hao, H.; Shibuya, M.; Imanaka, T.; Fukunaga, M.; Miki, K.; Tamaru, H.; Sawada, H.; Naito, Y.; Ohyanagi, M. Accuracy of OCT, grayscale IVUS, and their combination for the diagnosis of coronary TCFA: An ex vivo validation study. JACC Cardiovasc. Imaging 2015, 8, 451–460. [Google Scholar] [CrossRef]
- Xing, L.; Higuma, T.; Wang, Z.; Aguirre, A.; Zhang, S.; Yu, B.; Lee, H.; Fujimoto, J.; Fuster, V.; Jang, I.-K. Prevalence and clinical significance of lipid-rich plaque detected by optical coherence tomography: A four-year follow-up study. J. Am. Coll. Cardiol. 2017, 69, 975. [Google Scholar] [CrossRef]
- Osborn, E.A.; Jaffer, F.A. Imaging Atherosclerosis and Risk of Plaque Rupture. Curr. Atheroscler. Rep. 2013, 15, 359. [Google Scholar] [CrossRef]
- Brugaletta, S.; Sabaté, M. Assessment of Plaque Composition by Intravascular Ultrasound and Near-Infrared Spectroscopy—From PROSPECT I to PROSPECT II. Circ. J. 2014, 78, 1531–1539. [Google Scholar] [CrossRef]
- Gardner, C.M.; Tan, H.; Hull, E.L.; Lisauskas, J.B.; Sum, S.T.; Meese, T.M.; Jiang, C.; Madden, S.P.; Caplan, J.D.; Burke, A.P.; et al. Detection of Lipid Core Coronary Plaques in Autopsy Specimens with a Novel Catheter-Based Near-Infrared Spectroscopy System. JACC Cardiovasc. Imaging 2008, 1, 638–648. [Google Scholar] [CrossRef]
- Waksman, R.; Di Mario, C.; Torguson, R.; Ali, Z.A.; Singh, V.; Skinner, W.H.; Artis, A.K.; Cate, T.T.; Powers, E.; Kim, C.; et al. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: A prospective, cohort study. Lancet 2019. [Google Scholar] [CrossRef]
- Madder, R.D.; Husaini, M.; Davis, A.T.; VanOosterhout, S.; Khan, M.; Wohns, D.; McNamara, R.F.; Wolschleger, K.; Gribar, J.; Collins, J.S.; et al. Large lipid-rich coronary plaques detected by near-infrared spectroscopy at non-stented sites in the target artery identify patients likely to experience future major adverse cardiovascular events. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 393–399. [Google Scholar] [CrossRef]
- Kang, S.-J.; Mintz, G.S.; Pu, J.; Sum, S.T.; Madden, S.P.; Burke, A.P.; Xu, K.; Goldstein, J.A.; Stone, G.W.; Muller, J.E.; et al. Combined IVUS and NIRS Detection of Fibroatheromas: Histopathological Validation in Human Coronary Arteries. JACC Cardiovasc. Imaging 2015, 8, 184–194. [Google Scholar] [CrossRef]
- Gandhi, S.; Mosleh, W.; Abdel-Qadir, H.; Farkouh, M.E. Statins and Contrast-induced Acute Kidney Injury with Coronary Angiography. Am. J. Med. 2014, 127, 987–1000. [Google Scholar] [CrossRef]
- Tavakol, M.; Ashraf, S.; Brener, S.J. Risks and complications of coronary angiography: A comprehensive review. Glob. J. Health Sci. 2012, 4, 65–93. [Google Scholar] [CrossRef]
- La Manna, G.; Pancaldi, L.G.; Capecchi, A.; Maska, E.; Comai, G.; Cappuccilli, M.L.; Carretta, E.; Lombardi, A.; Colì, L.; Stefoni, S. Risk for Contrast Nephropathy in Patients Undergoing Coronarography. Artif. Organs 2010, 34, E193–E199. [Google Scholar] [CrossRef]
- Raposeiras-Roubín, S.; Aguiar-Souto, P.; Barreiro-Pardal, C.; Otero, D.L.; Teja, J.E.; Sanchez, R.O.; Álvarez, B.C.; Nouche, R.T.; Maceiras, M.V.R.; Abu-Assi, E.; et al. GRACE Risk Score Predicts Contrast-Induced Nephropathy in Patients With Acute Coronary Syndrome and Normal Renal Function. Angiology 2013, 64, 31–39. [Google Scholar] [CrossRef]
- Nough, H.; Eghbal, F.; Soltani, M.; Nejafi, F.; Falahzadeh, H.; Fazel, H.; Sheikhvatan, M. Incidence and Main Determinants of Contrast-Induced Nephropathy following Coronary Angiography or Subsequent Balloon Angioplasty. Cardiorenal Med. 2013, 3, 128–135. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Huang, B.; Zhao, Q.; Yu, G.; Xuan, C.; Wei, M.; Wang, N. Clinical survey on contrast-induced nephropathy after coronary angiography. Ren. Fail. 2013, 35, 1255–1259. [Google Scholar] [CrossRef]
- McCullough, P.A.; Adam, A.; Becker, C.R.; Davidson, C.; Lameire, N.; Stacul, F.; Tumlin, J. Epidemiology and Prognostic Implications of Contrast-Induced Nephropathy. Am. J. Cardiol. 2006, 98, 5–13. [Google Scholar] [CrossRef]
- Caspi, O.; Habib, M.; Cohen, Y.; Kerner, A.; Roguin, A.; Abergel, E.; Boulos, M.; Kapeliovich, M.R.; Beyar, R.; Nikolsky, E.; et al. Acute Kidney Injury After Primary Angioplasty: Is Contrast-Induced Nephropathy the Culprit? J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- James, M.T.; Samuel, S.M.; Manning, M.A.; Tonelli, M.; Ghali, W.A.; Faris, P.; Knudtson, M.L.; Pannu, N.; Hemmelgarn, B.R. Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: A systematic review and meta-analysis. Circ. Cardiovasc. Interv. 2013, 6, 37–43. [Google Scholar] [CrossRef]
- Maioli, M.; Toso, A.; Leoncini, M.; Gallopin, M.; Musilli, N.; Bellandi, F. Persistent Renal Damage after Contrast-Induced Acute Kidney Injury. Circulation 2012, 125, 3099–3107. [Google Scholar] [CrossRef]
- Lencioni, R.; Fattori, R.; Morana, G.; Stacul, F. Contrast-induced nephropathy in patients undergoing computed tomography (CONNECT)—A clinical problem in daily practice? A multicenter observational study. Acta Radiol. 2010, 51, 741–750. [Google Scholar] [CrossRef]
- Wichmann, L.J.; Katzberg, W.R.; Litwin, E.S.; Zwerner, L.P.; De Cecco, N.C.; Vogl, J.T.; Costello, J.P.; Schoepf, J.U. Contrast-Induced Nephropathy. Circulation 2015, 132, 1931–1936. [Google Scholar] [CrossRef]
- Pedersen, C.; Thomsen, C.F.; Hosbond, S.E.; Thomassen, A.; Mickley, H.; Diederichsen, A.C.P. Coronary computed tomography angiography—Tolerability of β-blockers and contrast media, and temporal changes in radiation dose. Scand. Cardiovasc. J. 2014, 48, 271–277. [Google Scholar] [CrossRef]
- Sun, Z. Coronary CT angiography with prospective ECG-triggering: An effective alternative to invasive coronary angiography. Cardiovasc. Diagn. Ther. 2012, 2, 28–37. [Google Scholar] [CrossRef]
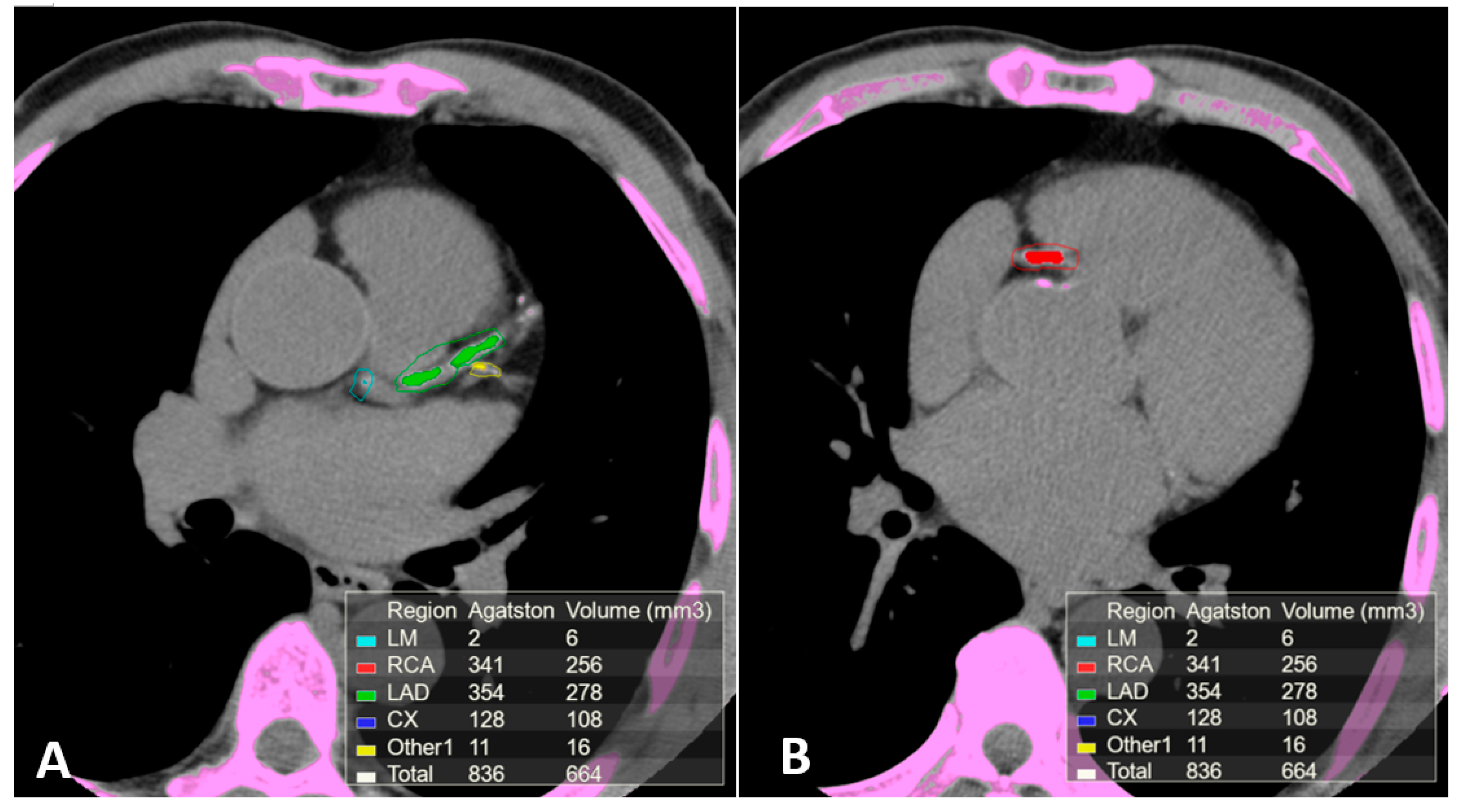
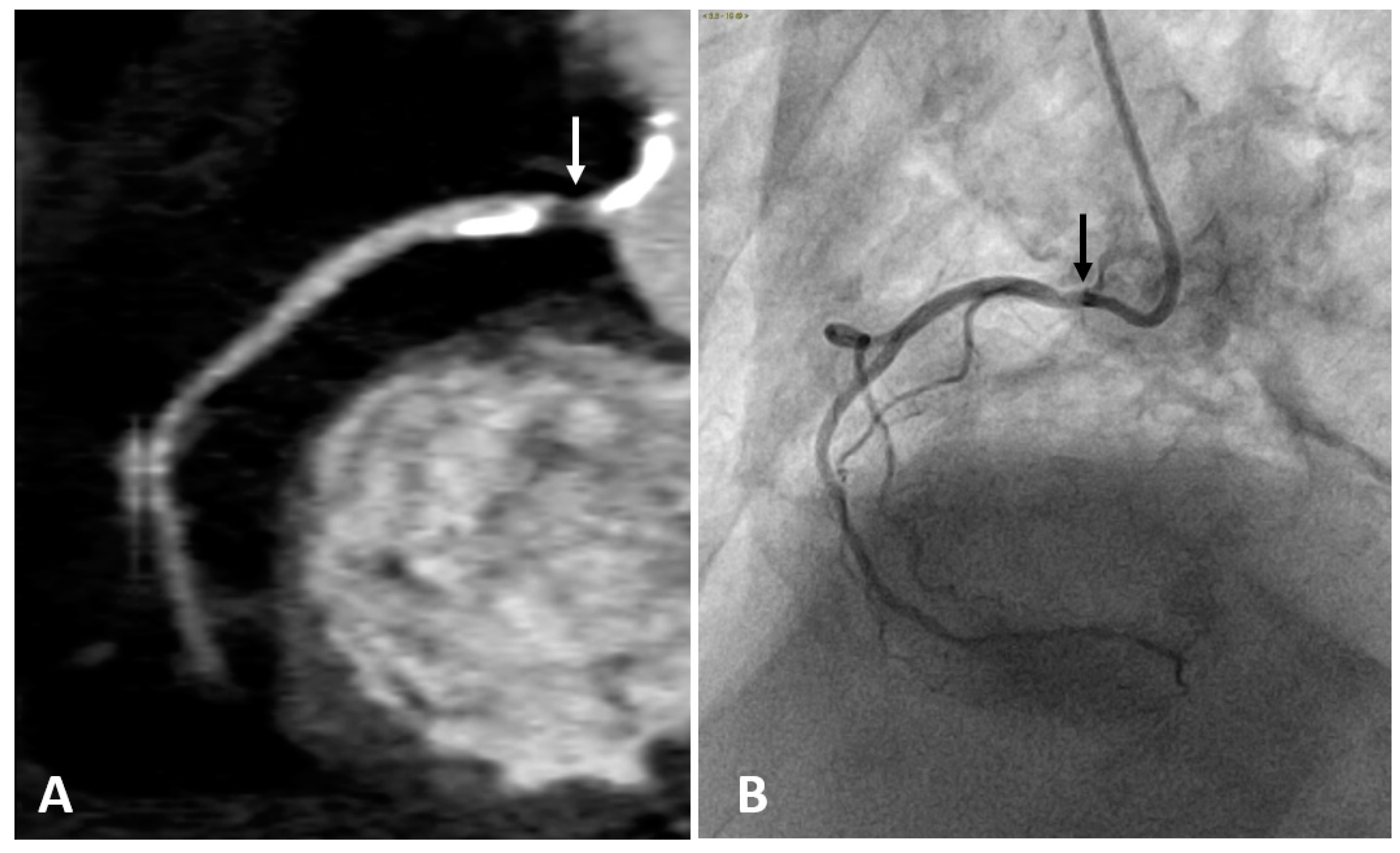

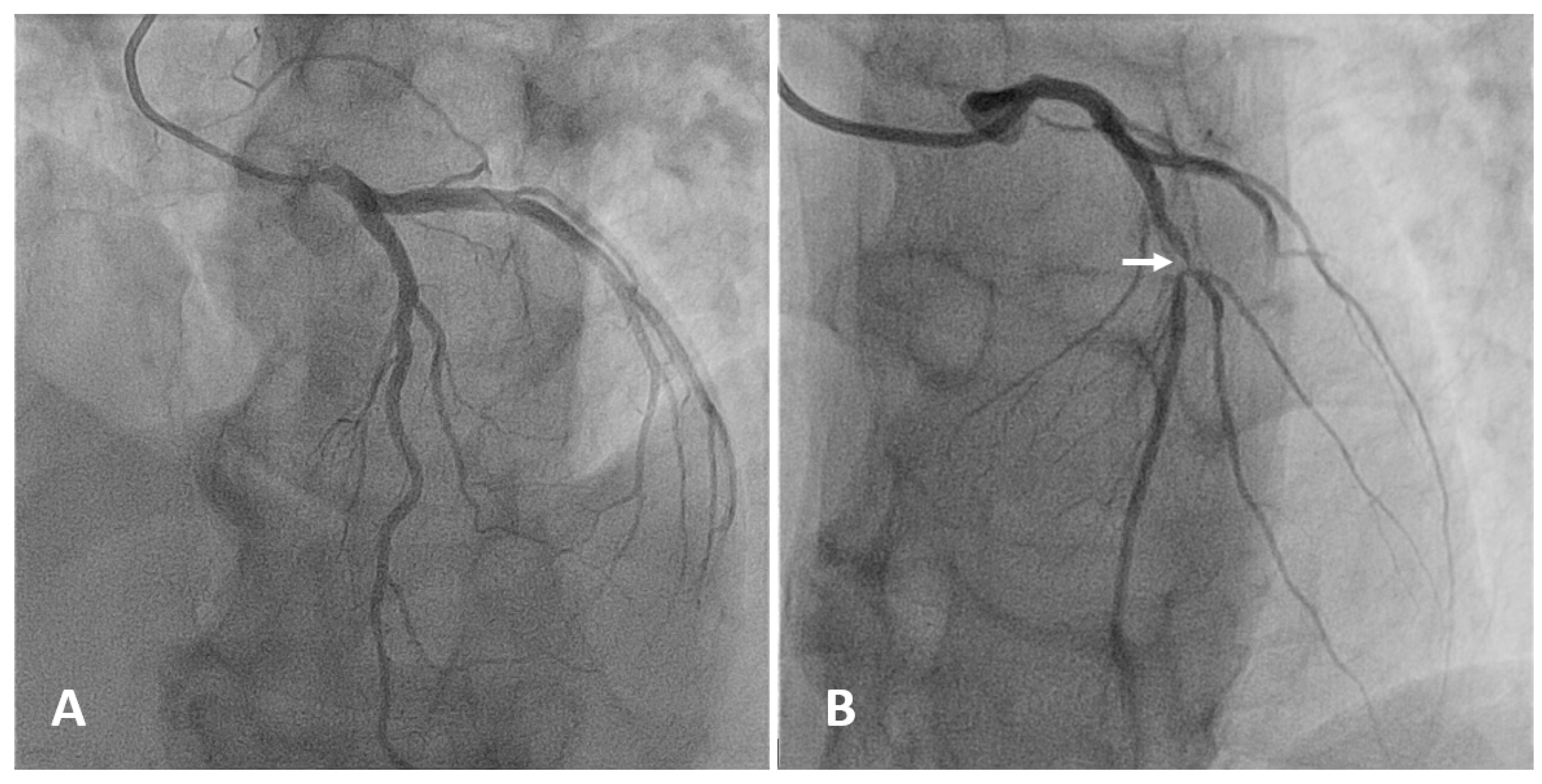

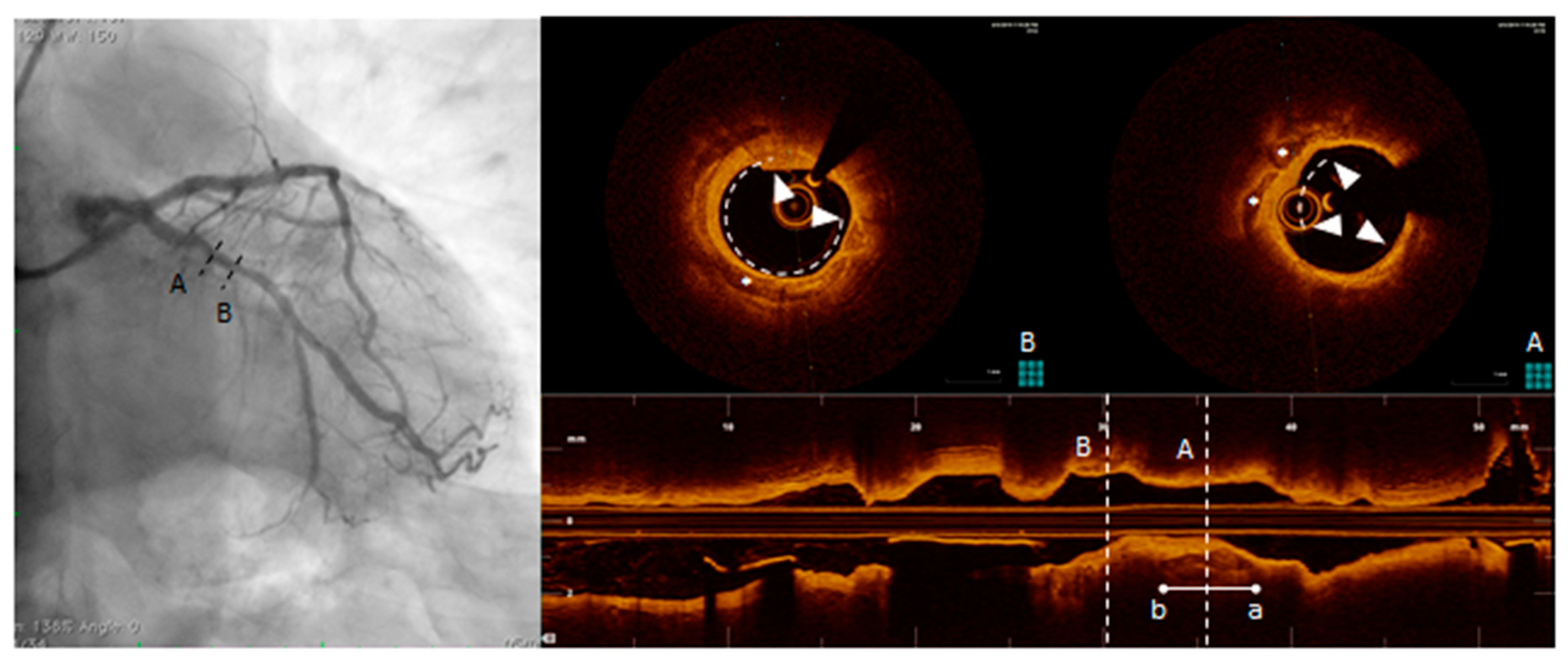
| Angiography | CT | CMR | IVUS | OCT | CT/MR PET | |
|---|---|---|---|---|---|---|
| Detection of coronary calcium | + | +++ | ++ | +++ | +++ | + |
| Quantification of coronary calcium | + | +++ | + | ++ | +++ | + |
| Microcalcifications | ++ | ++ | ++ | |||
| Spatial resolution | 1 mm | 500 µm | <2 mm | 200 µm | 20 µm | 2 µm |
| Depth | no limit | no limit | no limit | 10 mm | 2 mm | no limit |
| Inflammatory plaque activity | + | ++ | ++ | +++ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henein, M.Y.; Vancheri, S.; Bajraktari, G.; Vancheri, F. Coronary Atherosclerosis Imaging. Diagnostics 2020, 10, 65. https://doi.org/10.3390/diagnostics10020065
Henein MY, Vancheri S, Bajraktari G, Vancheri F. Coronary Atherosclerosis Imaging. Diagnostics. 2020; 10(2):65. https://doi.org/10.3390/diagnostics10020065
Chicago/Turabian StyleHenein, Michael Y., Sergio Vancheri, Gani Bajraktari, and Federico Vancheri. 2020. "Coronary Atherosclerosis Imaging" Diagnostics 10, no. 2: 65. https://doi.org/10.3390/diagnostics10020065
APA StyleHenein, M. Y., Vancheri, S., Bajraktari, G., & Vancheri, F. (2020). Coronary Atherosclerosis Imaging. Diagnostics, 10(2), 65. https://doi.org/10.3390/diagnostics10020065






