Skeletal Muscle Metastasis in Papillary Thyroid Microcarcinoma Evaluated by F18-FDG PET/CT
Abstract
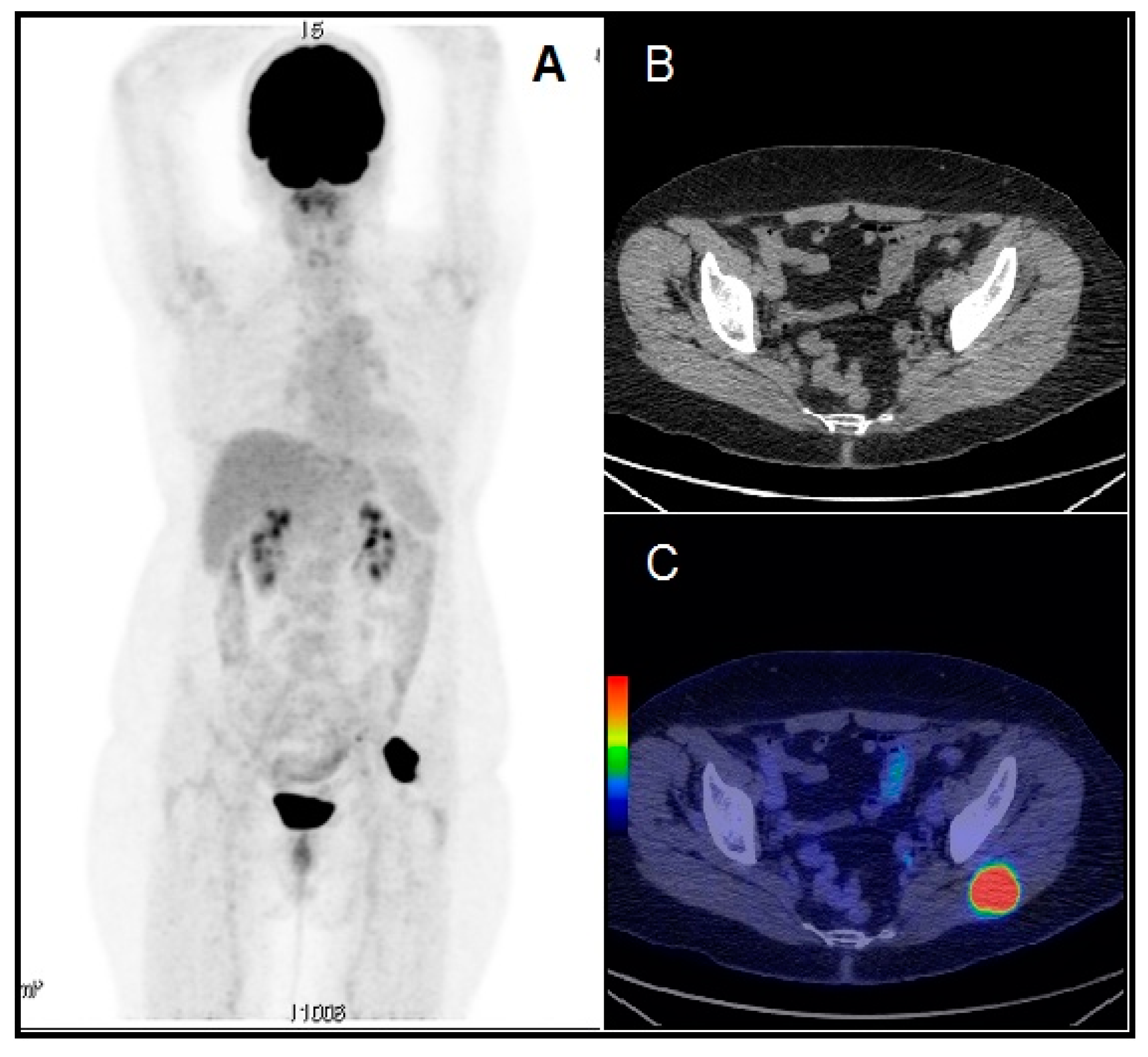
1. Introduction
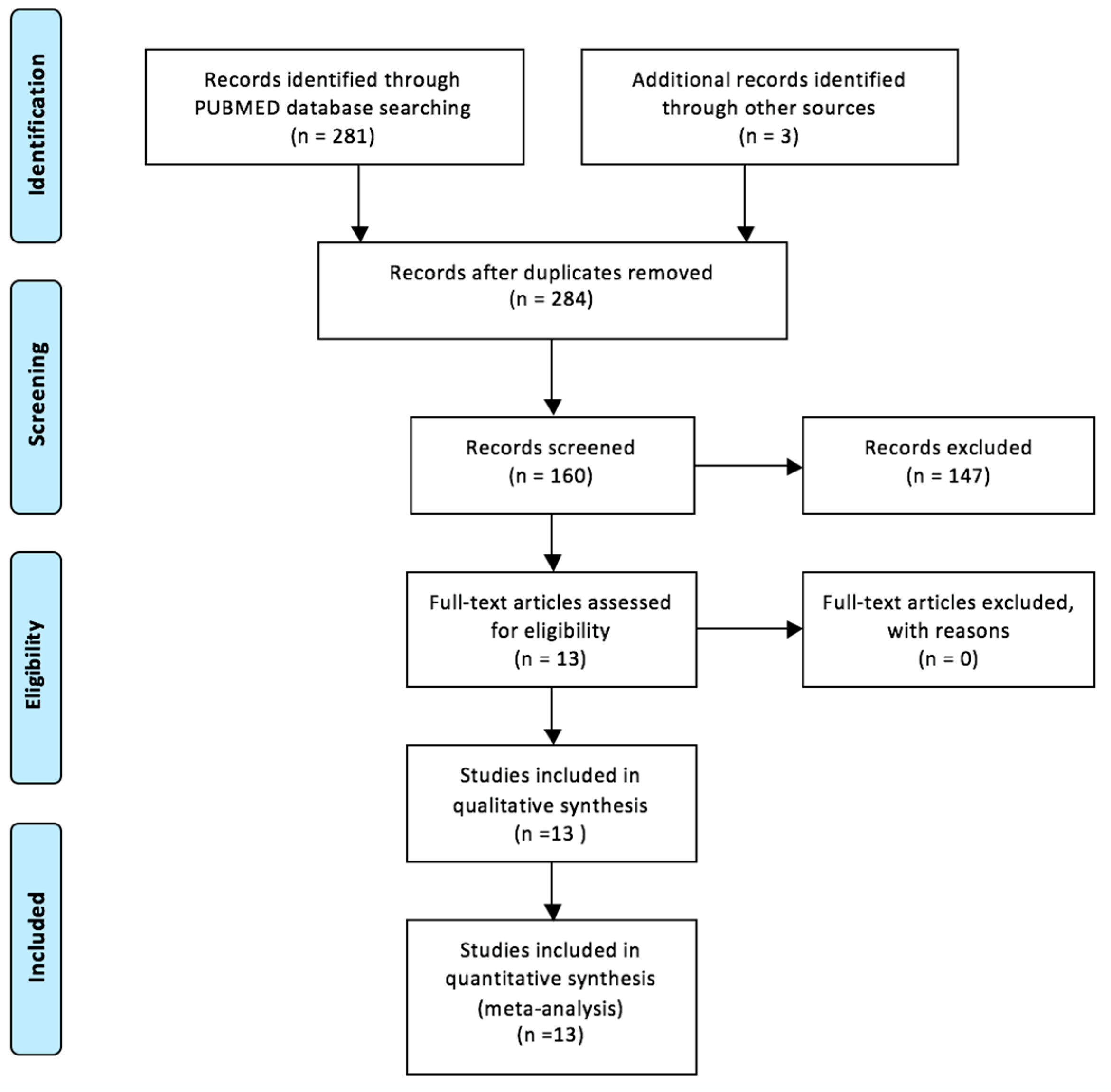
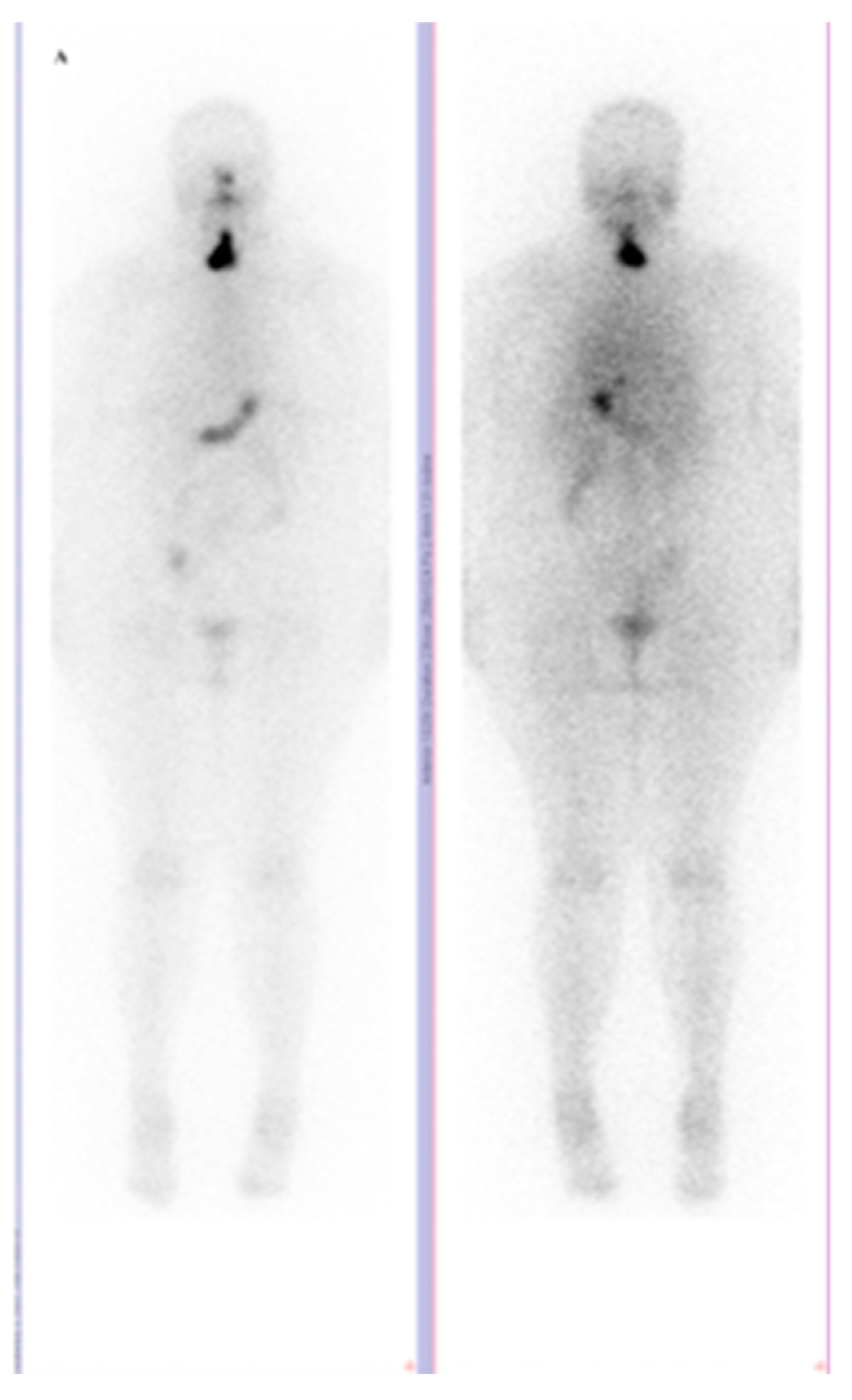
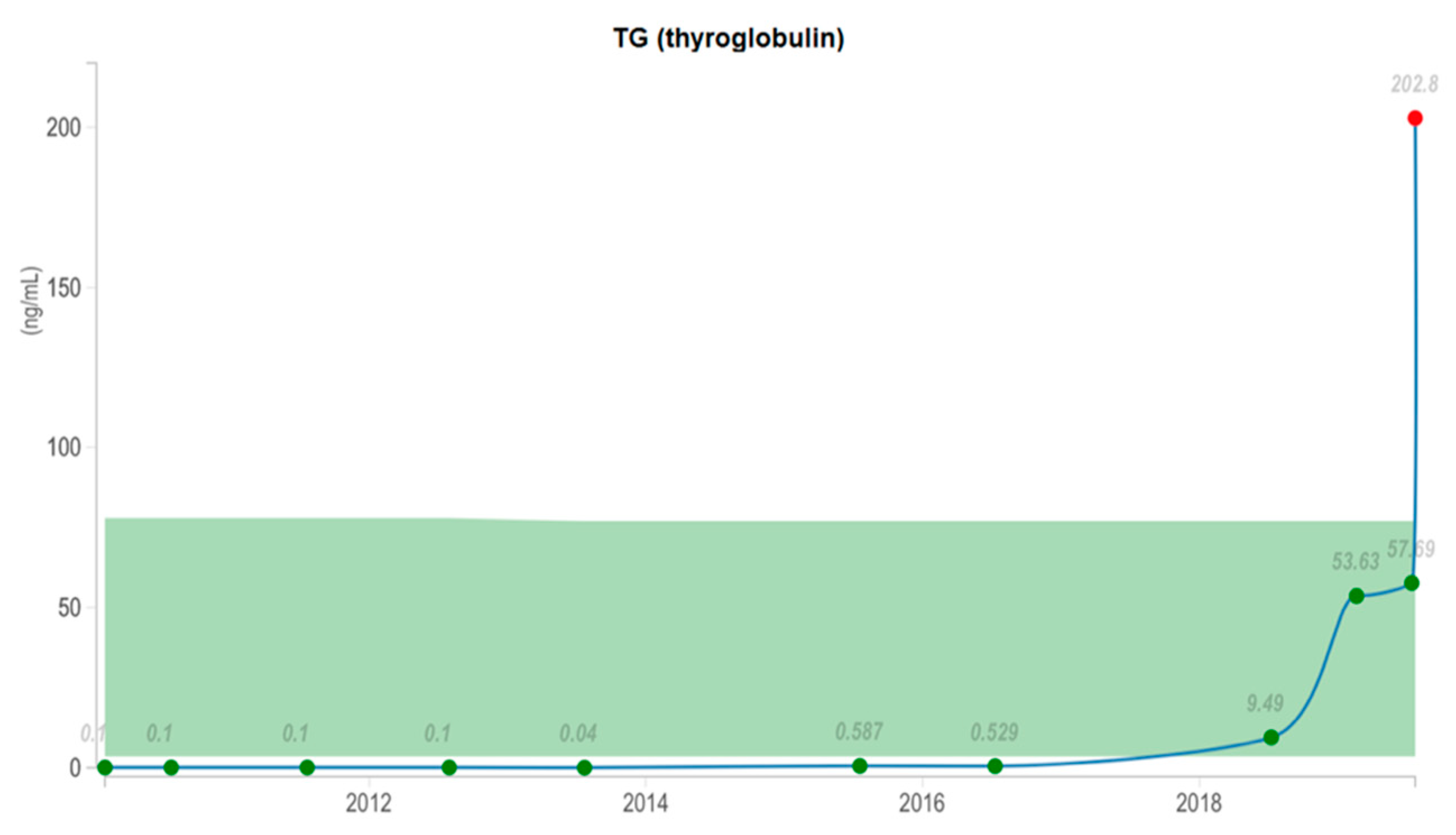
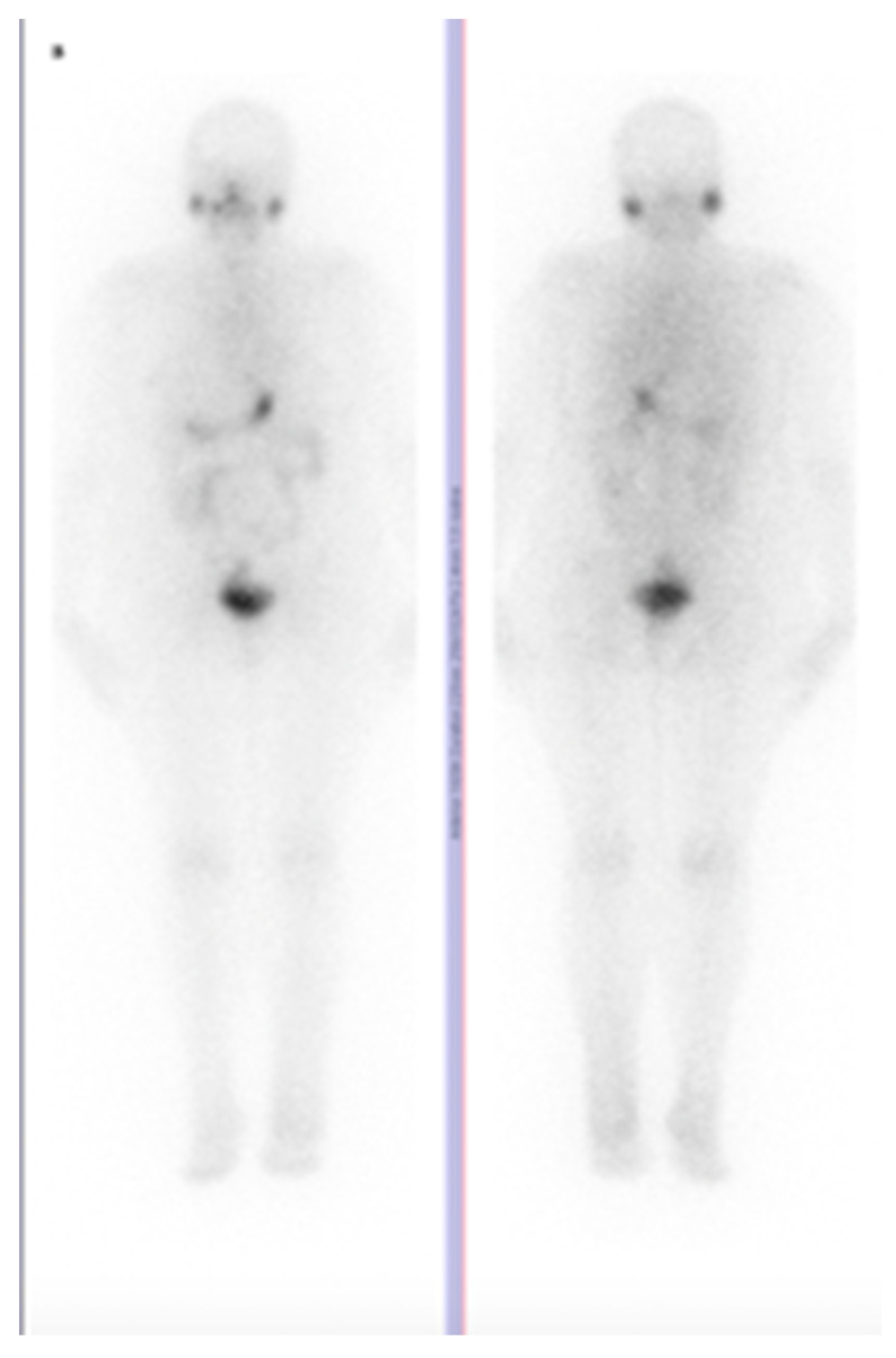
2. The Available Evidence of the Existing Cases
3. Conclusions
Funding
Conflicts of Interest
References
- Piciu, D.; Irimie, A. Investigation of thyroid carcinoma over 40 years, using the database of the Ion Chiricuta Institute of oncology Cluj-Napoca. J. Buon. 2014, 19, 524–529. [Google Scholar]
- Fugazzola, L.; Rossella, E. 2019 European Thyroid Association Guidelines for the Treatment and Follow-Up of Advanced Radioiodine-Refractory Thyroid Cancer. Eur. Thyroid J. 2019, 8, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.; Alexander, E. 2015 American Thyroid Association Management guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Muntean, V.; Domsa, I. Incidental papillary thyroid microcarcinoma: Is completion surgery required? Chirurgia (Bucur) 2013, 108, 490–497. [Google Scholar] [PubMed]
- Piciu, D.; Irimie, A. Diagnostic and treatment guidelines in thyroid carcinoma. American and European consensus, adapted to Romania. Acta. Endo. 2007, 3, 103–115. [Google Scholar] [CrossRef]
- Filleti, S.; Durante, C. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 23, 1–88. [Google Scholar] [CrossRef] [PubMed]
- Sarma, M.; Sonik, B. Isolated skeletal muscle metastatic deposit in a patient with micropapillary carcinoma thyroid identified by 18F FDG PET CT. J. Egypt Natl. Canc. Inst. 2015, 1, 47–50. [Google Scholar] [CrossRef]
- Haddad, R.; Nasr, C. NCCN Guidelines Insights Thyroid Carcinoma, Version 2.2018. J. Natl. Compr. Canc. Netw. 2018, 16, 1429–1440. [Google Scholar] [CrossRef]
- Yang, J.; Li, L. Unusual synchronous skeletal muscle and lung metastasis in papillary thyroid cancer: A case report and review of the literature. Oncol. Lett. 2015, 9, 727–730. [Google Scholar] [CrossRef]
- Bae, S.; Lee, S. Distant, solitary skeletal muscle metastasis in recurrent papillary thyroid carcinoma. Thyroid 2011, 21, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Bruglia, M.; Palmonella, G. Skin and thigh muscle metastasis from papillary thyroid cancer. Singapore Med. J. 2009, 50, 61–64. [Google Scholar]
- Caobelli, F.; Paghera, B. Two distant muscular metastases from papillary carcinoma of the thyroid demonstrated by 18F-FDG PET/CT and confirmed by biopsy. Nucl. Med. Mol. Imaging 2011, 45, 324–325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuscic, L.; Klancnik, M.; Paladin, L.; Kuna, S.K. Obstructive nephropathy caused by renal metastasis of papillary thyroid carcinoma: A case report. Endocr. Oncol. Metab. 2016, 2, 83–86. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z. Polysplenia syndrome with splenic and skeletal muscle metastases from thyroid carcinoma evaluated by FDG PET/CT: Case report and literature review: A care-compliant article. Medicine (Baltimore) 2016, 96, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Luo, Q.-Y. Localization of concomitant metastases to kidney and erector spinae from papillary thyroid carcinoma using 131 I—SPECT and CT. Thyroid 2008, 18, 663–664. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, T.; Arora, A. Coexisting iodine avid and iodine nonconcentrating lesions with multiple distant soft tissue metastasis in papillary thyroid cancer. Indian J. Nucl. Med. 2012, 27, 38–41. [Google Scholar] [CrossRef]
- Panoussopoulos, D.; Theodoropoulos, G. Distant solitary skeletal muscle metastasis from papillary thyroid carcinoma. Int. Surg. 2007, 92, 226–229. [Google Scholar]
- Pucci, A.; Suppo, M. Papillary thyroid carcinoma presenting as a solitary soft tissue arm metastasis in an elderly hyperthyroid patient. Case report and review of the literature. Virchows Arch. 2006, 448, 857–861. [Google Scholar] [CrossRef]
- Qiu, Z.L.; Luo, Q. Erector spinae metastases from differentiated thyroid cancer identified by I-131 SPECT/CT. Clin. Nucl. Med. 2009, 34, 137–140. [Google Scholar] [CrossRef]
- Zhao, L.; Li, L. Rectus abdominis muscle metastasis from papillary thyroid cancer identified by I-131 SPECT/CT. Clin. Nucl. Med. 2010, 35, 360–361. [Google Scholar] [CrossRef]
- Seely, S. Possible reasons for the high resistance of muscle to cancer. Med. Hypotheses 1980, 6, 133–137. [Google Scholar] [CrossRef]
- Piciu, D.; Irimie, A. Nuclear Endocrinology, 1st ed.; Springer: Berlin, Germany, 2012; pp. 211–215. [Google Scholar]
- Piciu, D.; Irimie, A. Highly aggressive pathology of non-functional parathyroid carcinoma. Orphanet J. Rare Dis. 2013, 115, 1–3. [Google Scholar] [CrossRef] [PubMed]
| Author/Origin | Year | Patient Sex/Age | Muscle Involved | Muscle Lesion (nr.) | Other Metastasis |
|---|---|---|---|---|---|
| Bae S Y, et al [10]. Seoul, Korea | 2011 | f/31 | vastus medialis (distal femur) | 1 | 0 |
| Bruglia M, et al [11]. Ancona, Italy | 2009 | m/44 | biceps femuris | 1 | lung, mediastinum, brain |
| Caobelli F, et al [12]. Brescia, Italy | 2011 | f/68 | right adductor longus; right iliopsoas | 2 | 0 |
| Kuscic L J, et al [13]. Split, Croatia | 2016 | m/68 | left thigh (medial muscle group) | 1 | kidney, lung |
| Li Z G, et al [14]. Tianjin, China | 2016 | m/84 | bilateral piriformis, left erector spinae, gluteus max. | 4 | spleen, bones, |
| Luo Q, et al [15]. Shanghai, China | 2008 | m/29 | erector spinae | 1 | kidney, lung |
| Mohapatra T, et al [16]. Pradesh, India | 2012 | m/42 | left gluteal, right erector spinae | 2 | liver |
| Panoussopoulos D [17], Athens, Greece | 2007 | f/69 | trapezoid | 1 | 0 |
| Pucci A, et al [18]. Torino, Italy | 2006 | m/77 | right biceps | 1 | 0 |
| Qiu ZL, et al [19]. Shanghai, China | 2009 | m/82 | erector spinae | 1 | manubrium sterni |
| Sarma M, et al [7]. Cochin, India | 2014 | m/66 | left deltoid | 1 | 0 |
| Yang J, et al [9]. Hangzhou, China | 2014 | m/31 | left gastrocnemius | 1 | lung |
| Zhao L, et al [20]. Chengdu, China | 2010 | f/53 | left rectus abdominis | 1 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hitu, L.; Cainap, C.; Apostu, D.; Gabora, K.; Bonci, E.-A.; Badan, M.; Mester, A.; Piciu, A. Skeletal Muscle Metastasis in Papillary Thyroid Microcarcinoma Evaluated by F18-FDG PET/CT. Diagnostics 2020, 10, 100. https://doi.org/10.3390/diagnostics10020100
Hitu L, Cainap C, Apostu D, Gabora K, Bonci E-A, Badan M, Mester A, Piciu A. Skeletal Muscle Metastasis in Papillary Thyroid Microcarcinoma Evaluated by F18-FDG PET/CT. Diagnostics. 2020; 10(2):100. https://doi.org/10.3390/diagnostics10020100
Chicago/Turabian StyleHitu, Liviu, Calin Cainap, Dragos Apostu, Katalin Gabora, Eduard-Alexandru Bonci, Marius Badan, Alexandru Mester, and Andra Piciu. 2020. "Skeletal Muscle Metastasis in Papillary Thyroid Microcarcinoma Evaluated by F18-FDG PET/CT" Diagnostics 10, no. 2: 100. https://doi.org/10.3390/diagnostics10020100
APA StyleHitu, L., Cainap, C., Apostu, D., Gabora, K., Bonci, E.-A., Badan, M., Mester, A., & Piciu, A. (2020). Skeletal Muscle Metastasis in Papillary Thyroid Microcarcinoma Evaluated by F18-FDG PET/CT. Diagnostics, 10(2), 100. https://doi.org/10.3390/diagnostics10020100







