Detection of Occult Metastases in Patients with T1 and T2 Stage Lower Lip Squamous Cell Carcinomas after Positive Lymphoscintigraphy
Abstract
:1. Introduction
2. Materials and Methods
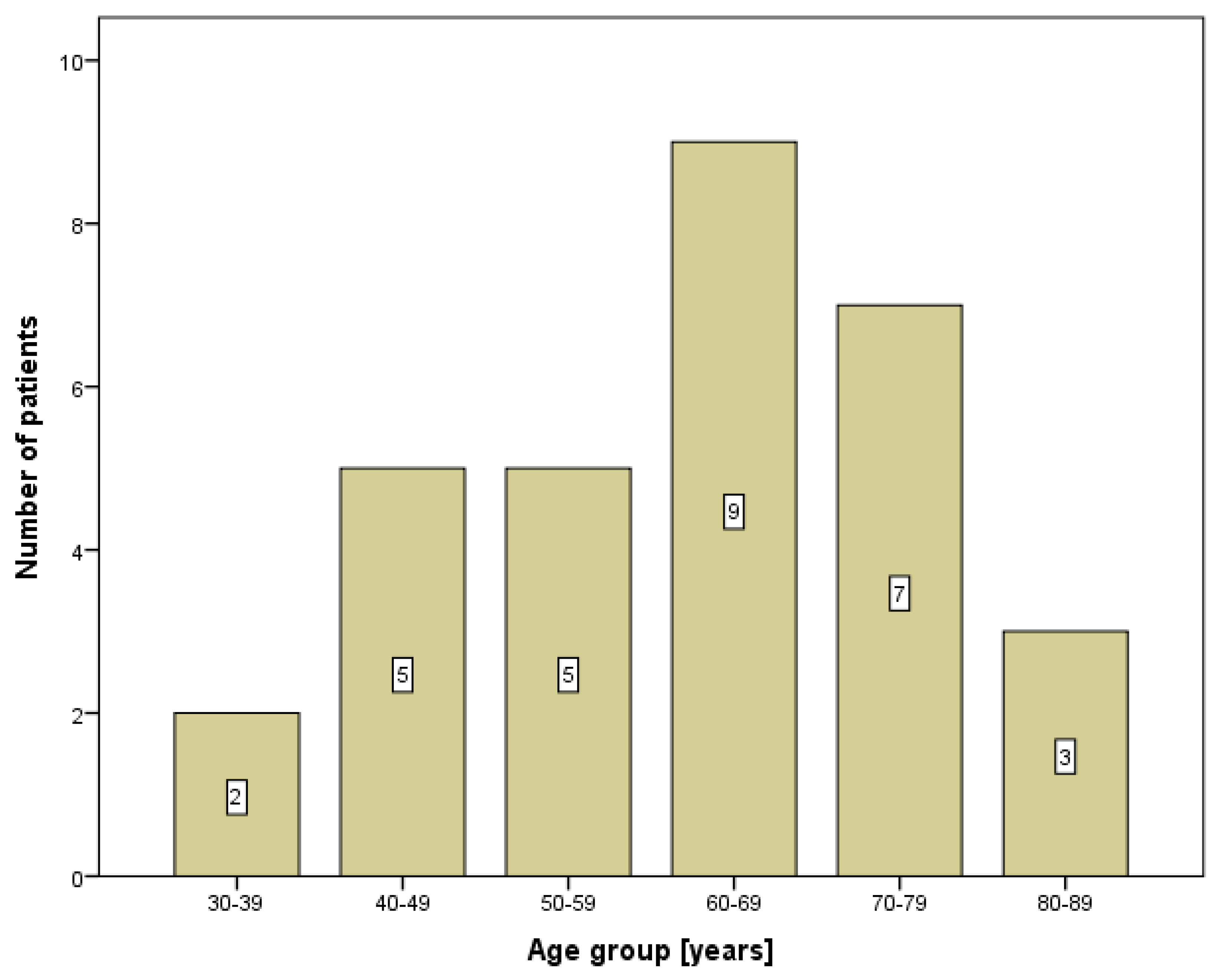
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Han, A.Y.; Kuan, E.C.; Mallen-St Clair, J.; Alonso, J.E.; Arshi, A.; St John, M.A. Epidemiology of Squamous Cell Carcinoma of the Lip in the United States: A Population-Based Cohort Analysis. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 1216–1223. [Google Scholar] [CrossRef]
- Zitsch, R.P., 3rd; Park, C.W.; Renner, G.J.; Rea, J.L. Outcome analysis for lip carcinoma. Otolaryngol. Head Neck Surg. 1995, 113, 589–596. [Google Scholar] [CrossRef]
- Neville, B.W.; Damm, D.D.; Allen, C.M.; Bouquot, J.E. Oral & Maxillofacial Pathology, 2nd ed.; W.B. Saunders: Philadelphia, PA, USA, 2002; pp. 337–369. [Google Scholar]
- Martinez, J.C.; Cook, J.L. High-risk cutaneous squamous cellcarcinoma without palpable lymphadenopathy: Is there a therapeutic role for elective neck dissection? Dermatol. Surg. 2007, 33, 410–420. [Google Scholar]
- Mourouzis, C.; Boynton, A.; Grant, J.; Umar, T.; Wilson, A.; Macpheson, D.; Pratt, C. Cutaneous head and neck SCCs and risk of nodal metastasis—UK experience. J. Craniomaxillofac. Surg. 2009, 37, 443–447. [Google Scholar] [CrossRef]
- Maruccia, M.; Onesti, M.G.; Parisi, P.; Cigna, E.; Troccola, A.; Scuderi, N. Lip cancer: A 10-year retrospective epidemiological study. Anticancer. Res. 2012, 32, 15436. [Google Scholar]
- Grimaldo-Carjevschi, M.; López-Labady, J.; Villarroel-Dorrego, M. Squamous cell carcinoma on the palate in a patient with systemic lupus erythematosus: Case report and review of literature. Lupus 2011, 20, 519–522. [Google Scholar] [CrossRef]
- Lydon, E.J.; Belmont, H.M. When rectal bleeding is serious: Anal squamous cell carcinoma in two intravenous cyclophosphamide treated systemic lupus erythematosus patients with human papilloma virus infection. Lupus 2013, 22, 1182–1184. [Google Scholar] [CrossRef]
- Takeda, A.; Akimoto, M.; Nemoto, M.; Kounoike, N.; Uchinuma, E. Preoperative risk factors of lymph node metastasis in cutaneous squamous cell carcinoma. J. Plast. Surg. Hand Surg. 2013, 47, 204–208. [Google Scholar] [CrossRef]
- Dediol, E.; Luksic, I.; Virag, M. Treatment of squamous cell carcinoma of the lip. Coll. Antropol. 2008, 32, 199–202. [Google Scholar]
- Morselli, P.; Masciotra, L.; Pinto, V.; Zollino, I.; Brunelli, G.; Carinci, F. Clinical parameters in T1N0M0 lower lip squamous cell carcinoma. J. Craniofac. Surg. 2007, 18, 1079–1082. [Google Scholar] [CrossRef]
- Gooris, P.J.; Vermey, A.; de Visscher, J.G.; Burlage, F.R.; Roodenburg, J.L. Supraomohyoid neck dissection in the management of cervical lymph node metastases of squamous cell carcinoma of the lower lip. Head Neck 2002, 24, 678–683. [Google Scholar] [CrossRef]
- Kornevs, E.; Skagers, A.; Tars, J.; Bigestans, A.; Lauskis, G.; Libermanis, O. 5-year experience with lower lip cancer. Stomatologija 2005, 7, 95–98. [Google Scholar]
- Szewczyk, M.; Pazdrowski, J.; Golusiński, P.; Dańczak-Pazdrowska, A.; Marszałek, S.; Golusiński, W. Analysis of selected risk factors for nodal metastases in head and neck cutaneous squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2015, 272, 3007–3012. [Google Scholar] [CrossRef] [Green Version]
- Hasson, O. Squamous cell carcinoma of the lower lip. J. Oral Maxillofac. Surg. 2008, 66, 1259–1262. [Google Scholar] [CrossRef]
- Kuscu, O.; Bajin, M.D.; Süslü, N.; Hosal, A.S. The role of suprahyoid neck dissection in the treatment of squamous cell carcinoma of the lower lip: 20 years’ experience at a Tertiary Center. J. Craniomaxillofac. Surg. 2016, 44, 1404–1407. [Google Scholar] [CrossRef]
- Rapoport, A.; Ortellado, D.K.; Amar, A.; Lehn, C.N.; Dedivitis, R.A.; Perez, R.S.; Rodrigues, H.M. Radical versus supraomohyoid neck dissection in the treatment of squamous cell carcinoma of the inferior level of the mouth. Braz. J. Otorhinolaryngol. 2007, 73, 641–646. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.H.; Ko, S.F.; Toh, C.H.; Chen, U.L. Imaging of neck metastases. Chang Gung Med. J. 2006, 29, 119–129. [Google Scholar]
- Altinyollar, H.; Berberoglu, U.; Celen, O. Lymphatic mapping and sentinel lymph node biopsy in squamous cell carcinoma of the lower lip. Eur. J. Surg. Oncol. 2002, 28, 72–74. [Google Scholar] [CrossRef]
- Morton, D.L. Overview and update of the phase III Multicenter Selective Lymphadenectomy Trials (MSLT-I and MSLT-II) in melanoma. Clin. Exp. Metastasis 2012, 29, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Dong, Z.M.; Wu, P.C. Sentinel lymph node biopsy for highrisk cutaneous squamous cell carcinoma: Clinical experience and review of literature. World J. Surg. Oncol. 2011, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.M.; Moore, B.A.; Schmalbach, C.E. Utility of head and neck cutaneous squamous cell carcinoma sentinel node biopsy: A systematic review. Otolaryngol. Head Neck Surg. 2014, 150, 180–187. [Google Scholar] [CrossRef]
- Tsujino, Y.; Mizumoto, K.; Matsuzaka, Y.; Niihara, H.; Morita, E. Fluorescence navigation with indocyanine green for detecting sentinel nodes in extramammary Paget’s disease and squamous cell carcinoma. J. Dermatol. 2009, 36, 90–94. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Nakamura, Y.; Kawachi, Y.; Otsuka, F. Indocyanine green fluorescence-navigated sentinel node biopsy showed higher sensitivity than the radioisotope or blue dye method, which may help to reduce false-negative cases in skin cancer. J. Surg. Oncol. 2012, 106, 41–45. [Google Scholar] [CrossRef]
- Hashibe, M.; Brennan, P.; Benhamou, S.; Castellsague, X.; Chen, C.; Curado, M.P. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers and the risk of head and neck cancer: Pooled analysis in the international head and neck cancer epidemiology consortium. J. Natl. Cancer Inst. 2007, 99, 777–789. [Google Scholar] [CrossRef]
- Petti, S. Lifestyle risk factors for oral cancer. Oral Oncol. 2009, 45, 340–350. [Google Scholar] [CrossRef]
- Galyon, S.W.; Frodel, J.L. Lip and perioral defects. Otolaryngol. Clin. N. Am. 2001, 34, 647–666. [Google Scholar] [CrossRef]
- Veness, M.J. High-risk cutaneous squamous cell carcinoma of the head andneck. J. Biomed. Biotechnol. 2007, 2007, 80572. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Nguyen, H.V.; Nguyen, H.X.; Le, Q.V. Lower lip squamous cell carcinoma: A Vietnamese case report of surgical treatment with reconstruction by local flap. Int. J. Surg. Case Rep. 2018, 53, 471–474. [Google Scholar] [CrossRef]
- Vukadinovic, M.; Jezdic, Z.; Petrovic, M.; Medenica, L.M.; Lens, M. Surgical management of squamous cell carcinoma of the lip: Analysis of a 10-year experience in 223 patients. J. Oral Maxillofac. Surg. 2007, 65, 675–679. [Google Scholar] [CrossRef]
- Dunne, A.A.; Budach, V.G.; Wagner, W.; Werner, J.A. Management of N0 neck in head and neck cancer: Current controversies. Onkologie 2004, 27, 363–367. [Google Scholar] [CrossRef]
- Bucur, A.; Stefanescu, L. Management of patients with squamous cell carcinoma of the lower lip and N0-neck. J. Craniomaxillofac. Surg. 2004, 32, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Chone, C.T.; Magalhes, R.S.; Etchehebere, E.; Camargo, E.; Altemani, A.; Crespo, A.N. Predictive value of sentinel node biopsy in head and neck cancer. Acta Otolaryngol. 2008, 128, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Civantos, F.J.; Zitsch, R.P.; Schuller, D.E.; Agrawal, A.; Smith, R.B.; Nason, R. Sentinel lymph node biopsy accurately stages the regional lymph nodes for T1-T2 oral squamous cell carcinomas: Results of a prospective multi-institutional trial. J. Clin. Oncol. 2010, 28, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, M.A.; Trivedi, N.P. Sentinel node biopsy in head and neck squamous cell carcinoma. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 100–110. [Google Scholar] [CrossRef]
- Stoeckli, S.J.; Alkureishi, L.W.; Ross, G.L. Sentinel node biopsy for early oral and oropharyngeal squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2009, 266, 787–793. [Google Scholar] [CrossRef]
- Alkureishi, L.W.; Ross, G.L.; Shoaib, T.; Soutar, D.S.; Robertson, A.G.; Thompson, R. Sentinel node biopsy in head and neck squamous cell cancer: 5-year follow-up of a European multicenter trial. Ann. Surg. Oncol. 2010, 17, 2459–2464. [Google Scholar] [CrossRef]
- Fasunla, A.J.; Greene, B.H.; Timmesfeld, N.; Wiegand, S.; Werner, J.A.; Sesterhenn, A.M. A meta-analysis of the randomized controlled trials on elective neck dissection versus therapeutic neck dissection in oral cavity cancers with clinically node-negative neck. Oral Oncol. 2011, 47, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.R.; Chen, F.J.; Yang, A.K.; Zhang, G.P.; Song, M.; Liu, W.W.; Chen, W.C.; Chen, Y.F.; Ouyang, D.; Li, Q.L. Elective neck dissection in clinical stage I squamous cell carcinoma of the tongue: Does it improve regional control or survival time? Oral Oncol. 2011, 47, 136–141. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Ashford, B.G.; Clark, J.R. Improved survival with elective neck dissection in thick early-stage oral squamous cell carcinoma. Head Neck 2012, 34, 709–716. [Google Scholar] [CrossRef]
- Sagheb, K.; Sagheb, K.; Rahimi-Nedjat, R.; Taylor, K.; Al-Nawas, B.; Walter, C. Sentinel lymph node biopsy in T1/T2 squamous cell carcinomas of the tongue: A prospective study. Oncol. Lett. 2016, 11, 600–604. [Google Scholar] [CrossRef] [Green Version]
- Nibu, K.; Ebihara, Y.; Ebihara, M.; Kawabata, K.; Onitsuka, T.; Fujii, T.; Saikawa, M. Quality of life after neck dissection: A multicenter longitudinal study by the Japanese clinical study group on standardization of treatment for lymph node metastasis of head and neck cancer. Int. J. Clin. Oncol. 2010, 15, 33–38. [Google Scholar] [CrossRef]
- Terrell, J.E.; Ronis, D.L.; Fowler, K.E.; Bradford, C.R.; Chepeha, D.B.; Prince, M.E.; Teknos, T.N.; Wolf, G.T.; Duffy, S.A. Clinical predictors of quality of life in patients with head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Ferlito, A.; Rinaldo, A.; Silver, C.E.; Gourin, C.G.; Shah, J.P.; Clayman, G.L. Elective and therapeutic selective neck dissection. Oral Oncol. 2006, 42, 14–25. [Google Scholar] [CrossRef]
- Murer, K.; Huber, G.F.; Haile, S.R.; Stoeckli, S.J. Comparison of morbidity between sentinel node biopsy and elective neck dissection for treatment of the n0 neck in patients with oral squamous cell carcinoma. Head Neck 2011, 33, 1260–1264. [Google Scholar] [CrossRef]
- Schiefke, F.; Akdemir, M.; Weber, A.; Akdemir, D.; Singer, S.; Frerich, B. Function, postoperative morbidity and quality of life after cervical sentinel node biopsy and after selective neck dissection. Head Neck 2009, 31, 503–512. [Google Scholar] [CrossRef]
| Patients (n = 31) | |
|---|---|
| Age (years) | 61.6 ± 13.4 |
| Gender | |
| M/F | 28 (90.3%)/3 (9.7%) |
| Profession | |
| Farmer | 14 (45.2%) |
| Housewife | 1 (3.2%) |
| Machinist | 3 (9.7%) |
| Pensioner | 6 (19.4%) |
| Physical worker | 6 (19.4%) |
| Police officer | 1 (3.2%) |
| Patients (n = 31) | |
|---|---|
| TNM classification | |
| Tc1Nc0M0 | 16 (51.6%) |
| Tc1Nc1M0 | 6 (19.4%) |
| Tc2Nc0M0 | 5 (16.1%) |
| Tc2Nc1M0 | 4 (12.9%) |
| Tumor size | |
| T1 | 22 (71.0%) |
| T2 | 9 (29.0%) |
| Lymph node enlargement | 10 (32.3%) |
| Risk factors | |
| Sun exposure | 15 (48.4%) |
| Smoking | 22 (71.0%) |
| Alcohol | 6 (19.4%) |
| Family history | 5 (16%) |
| Duration of pathology | |
| Under 1 year | 14 (45.2%) |
| Over 1 year | 17 (54.8%) |
| Positive echo | 13 (41.9%) |
| Positive MRI | 12 (38.7%) |
| Positive CT | 2 (6.5%) |
| Positive LSG | 21 (67.7%) |
| Submental region | 6 (28.6%) |
| Submandibular region | 2 (9.5%) |
| Both regions | 13 (61.9%) |
| No Enlargement (n = 21) | Lymph Node Enlargement (n = 10) | p-Value | |
|---|---|---|---|
| Age (years) | 60.2 ± 12.7 | 64.6 ± 15.1 | 0.407 |
| Gender | |||
| M/F | 20/1 | 8/2 | 0.180 |
| Profession | 0.960 | ||
| Farmer | 9 (42.9%) | 5 (50.0%) | |
| Housewife | 1 (4.8%) | 0 | |
| Machinist | 2 (9.5%) | 1 (10.0%) | |
| Pensioner | 4 (19.0%) | 2 (20.0%) | |
| Physical worker | 4 (19.0%) | 2 (20.0%) | |
| Police officer | 1 (4.8%) | 0 | |
| Tumor size | 0.353 | ||
| T1 | 16 (76.2%) | 6 (60.0%) | |
| T2 | 5 (23.8%) | 4 (40.0%) | |
| Sun exposure | 10 (47.6%) | 5 (50.0%) | 0.901 |
| Smoking | 16 (76.2%) | 6 (60.0%) | 0.353 |
| Alcohol | 5 (23.8%) | 1 (10.0%) | 0.363 |
| Family history | 4 (19.0%) | 1 (10.0%) | 0.522 |
| Duration of pathology | 0.052 | ||
| Under 1 year | 12 (57.1%) | 2 (20.0%) | |
| Over 1 year | 9 (42.9%) | 8 (80.0%) |
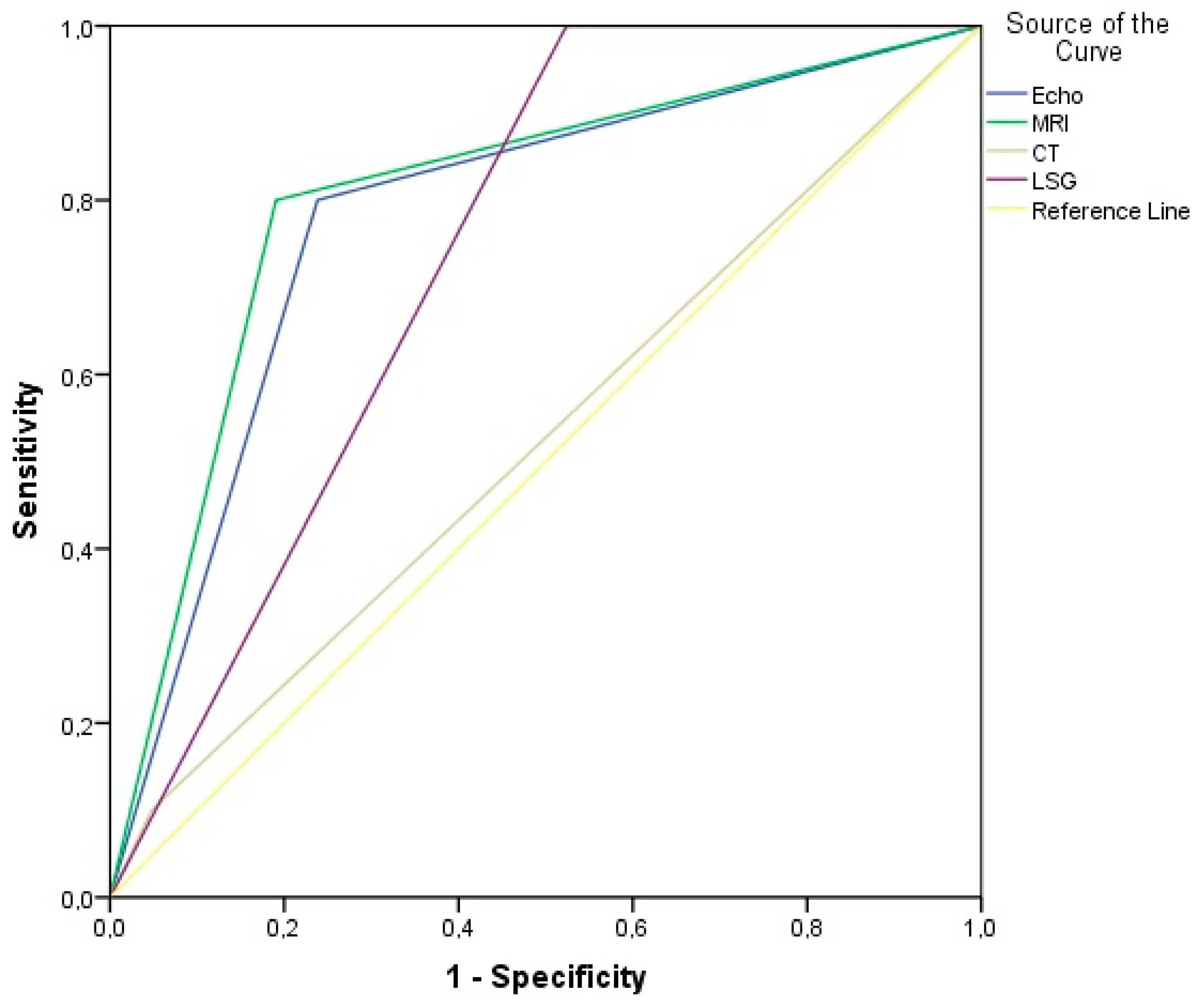
| Lymph Node Enlargement (n =10) | AUC (95% CI) | Sn | Sp | PPV | NPV | Accuracy | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Echo-ultrasound | 8 (80%) | 0.781 (0.600–0.962) | 80.0% | 86.2% | 61.5% | 88.9% | 77.4% | 0.013 |
| MRI | 8 (80%) | 0.805 (0.629–0.980) | 80.0% | 81.0% | 66.7% | 89.5% | 80.7% | 0.007 |
| CT | 1 (10%) | 0.526 (0.302–0.750) | 10.0% | 95.2% | 50.0% | 69.0% | 67.7% | 0.816 |
| LSG | 10 (100%) | 0.738 (0.567–0.909) | 100% | 47.6% | 47.6% | 100% | 64.5% | 0.035 |
| No Metastases (n = 23) | Metastases (n = 8) | B | OR * | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|---|
| Age | 61.6 ± 13.4 | 63.6 ± 14.7 | 0.138 | 1.148 | 1.030 | 1.280 | 0.013 |
| Gender M/F | 22/1 (95.7%/4.3%) | 6/2 (75.0%/25.0%) | −1.992 | 0.136 | 0.010 | 1.772 | 0.128 |
| Profession | −0.109 | 0.896 | 0.559 | 1.436 | 0.649 | ||
| Farmer | 10 (43.5%) | 4 (50.0%) | |||||
| Housewife | 1 (4.3%) | 0 | |||||
| Machinist | 2 (8.7%) | 1 (12.5%) | |||||
| Pensioner | 4 (17.4%) | 2 (25.0%) | |||||
| Physical worker | 5 (21.7%) | 1 (12.5%) | |||||
| Police officer | 1 (4.3%) | 0 | |||||
| TNM | 0.377 | 1.458 | 0.711 | 2.990 | 0.303 | ||
| Tc1Nc0M0 | 14 (60.9%) | 2 (25.0%) | |||||
| Tc1Nc1M0 | 2 (8.7%) | 4 (50.0%) | |||||
| Tc2Nc0M0 | 5 (21.7%) | 0 | |||||
| Tc2Nc1M0 | 2 (8.7%) | 2 (25.0%) | |||||
| Tumor size | −0.272 | 0.762 | 0.122 | 4.751 | 0.771 | ||
| T1 | 16 (69.6%) | 6 (75.0%) | |||||
| T2 | 7 (30.4%) | 2 (25.0%) | |||||
| Lymph node enlargement | 2 (8.7%) | 6 (75.0%) | 2.657 | 14.250 | 2.069 | 98.140 | 0.007 |
| Risk factors | |||||||
| Sun exposure | 10 (43.5%) | 5 (62.5%) | 0.773 | 2.167 | 0.415 | 11.302 | 0.359 |
| Smoking | 18 (78.3%) | 4 (50.0%) | −1.281 | 0.278 | 0.051 | 1.526 | 0.141 |
| Alcohol | 5 (21.7%) | 1 (12.5%) | −0.665 | 0.514 | 0.051 | 5.221 | 0.574 |
| Family history | 3 (13.0%) | 2 (25.0%) | 0.799 | 2.222 | 0.298 | 16.558 | 0.436 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prekazi Loxha, M.; Stubljar, D.; Jukic, T.; Rusinovci, S. Detection of Occult Metastases in Patients with T1 and T2 Stage Lower Lip Squamous Cell Carcinomas after Positive Lymphoscintigraphy. Diagnostics 2020, 10, 97. https://doi.org/10.3390/diagnostics10020097
Prekazi Loxha M, Stubljar D, Jukic T, Rusinovci S. Detection of Occult Metastases in Patients with T1 and T2 Stage Lower Lip Squamous Cell Carcinomas after Positive Lymphoscintigraphy. Diagnostics. 2020; 10(2):97. https://doi.org/10.3390/diagnostics10020097
Chicago/Turabian StylePrekazi Loxha, Mergime, David Stubljar, Tomislav Jukic, and Sinan Rusinovci. 2020. "Detection of Occult Metastases in Patients with T1 and T2 Stage Lower Lip Squamous Cell Carcinomas after Positive Lymphoscintigraphy" Diagnostics 10, no. 2: 97. https://doi.org/10.3390/diagnostics10020097
APA StylePrekazi Loxha, M., Stubljar, D., Jukic, T., & Rusinovci, S. (2020). Detection of Occult Metastases in Patients with T1 and T2 Stage Lower Lip Squamous Cell Carcinomas after Positive Lymphoscintigraphy. Diagnostics, 10(2), 97. https://doi.org/10.3390/diagnostics10020097





