Pericapillary Edema Assessment by Means of the Nailfold Capillaroscopy and Laser Scanning Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Volunteers
2.2. Wide-Field Capillaroscopy Measurements
2.3. Confocal Laser-Scanning Microscopy Measurements
2.4. Short-Term Edema Induction Protocols
2.4.1. Histamine Application Protocol
2.4.2. Venous Occlusion Protocol
2.5. Image Processing Algorithms
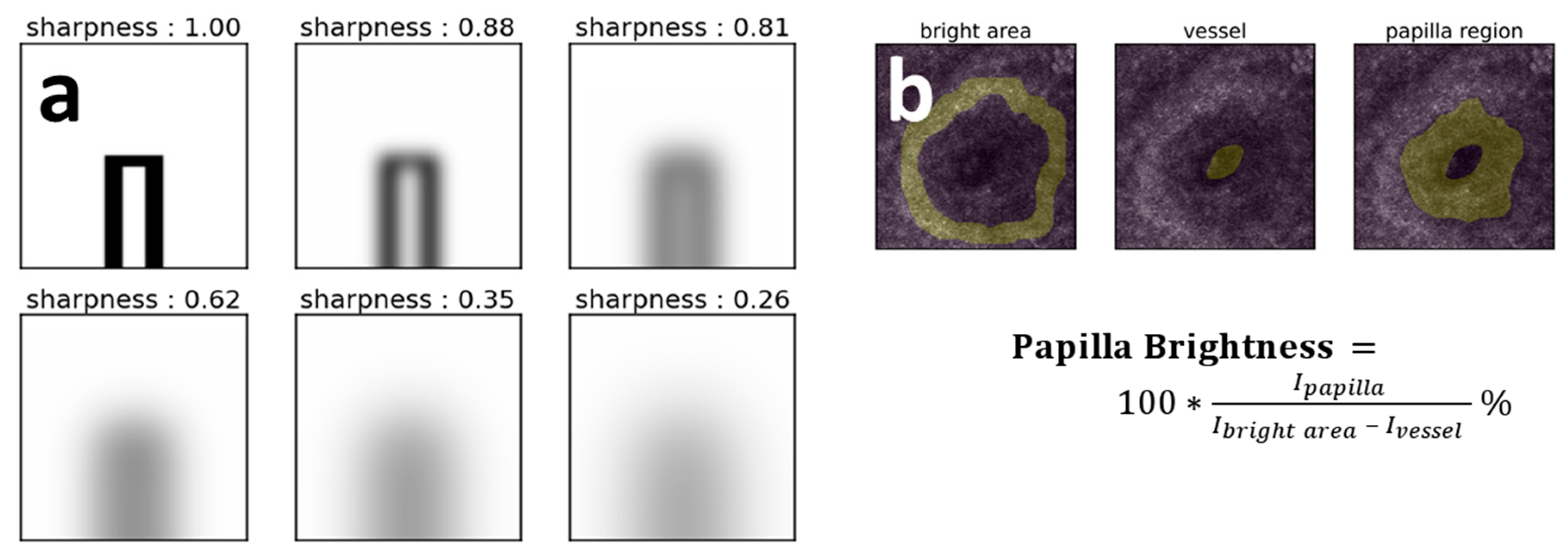
2.5.1. Capillaries’ Image Sharpness Assessment Procedure
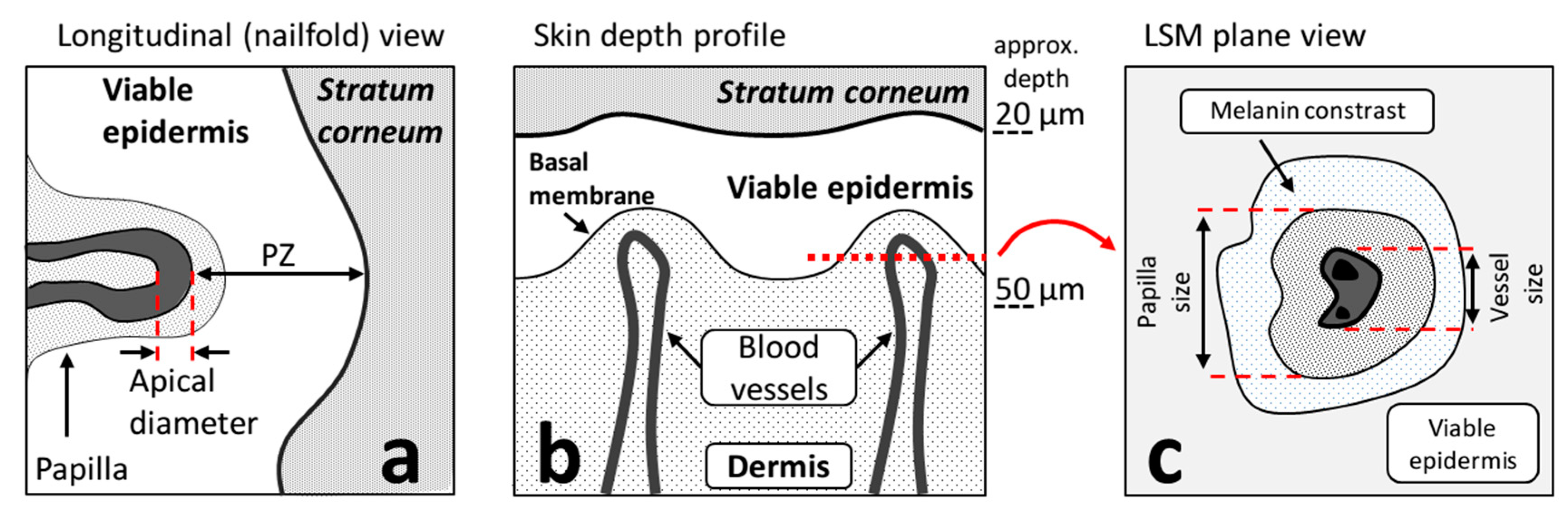
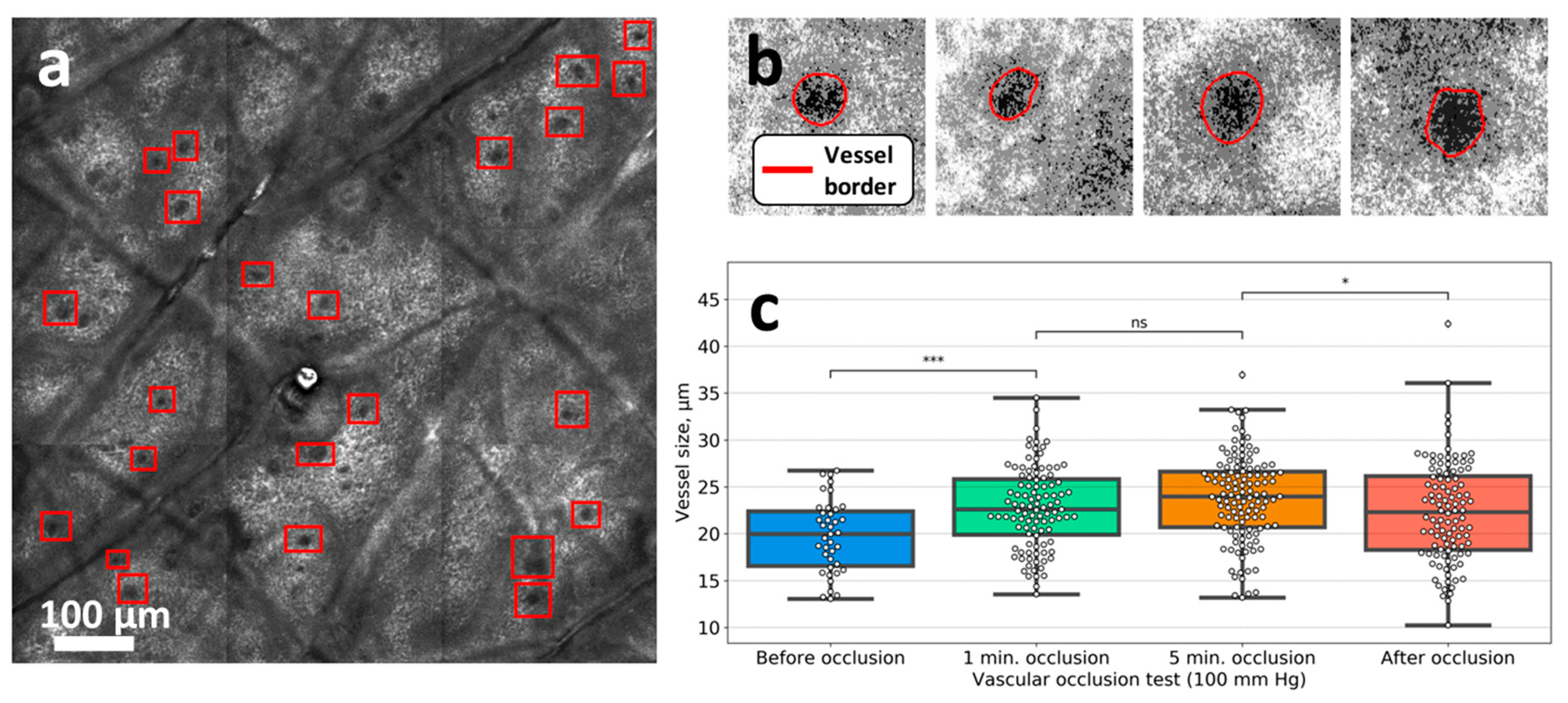
2.5.2. Vessel Size Estimation in the CLSM Images
2.5.3. Papilla Brightness Estimation in the CLSM Images
3. Results
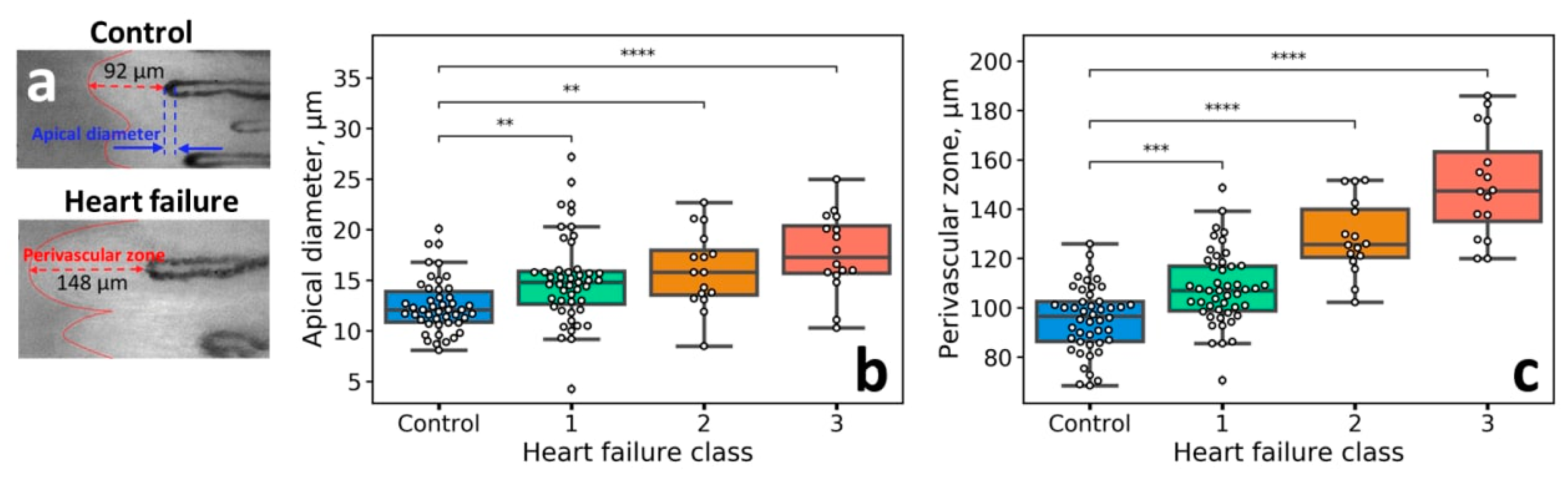
3.1. Capillaroscopic Assessment of Patients with HF
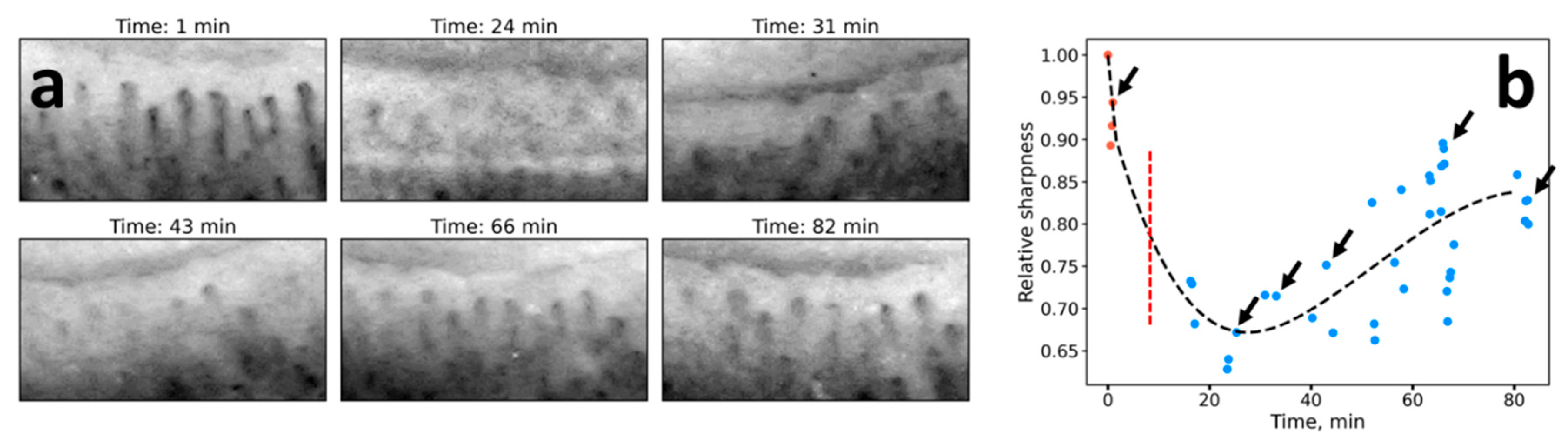
3.2. Short-Term Edema Models
3.3. Venous Occlusion
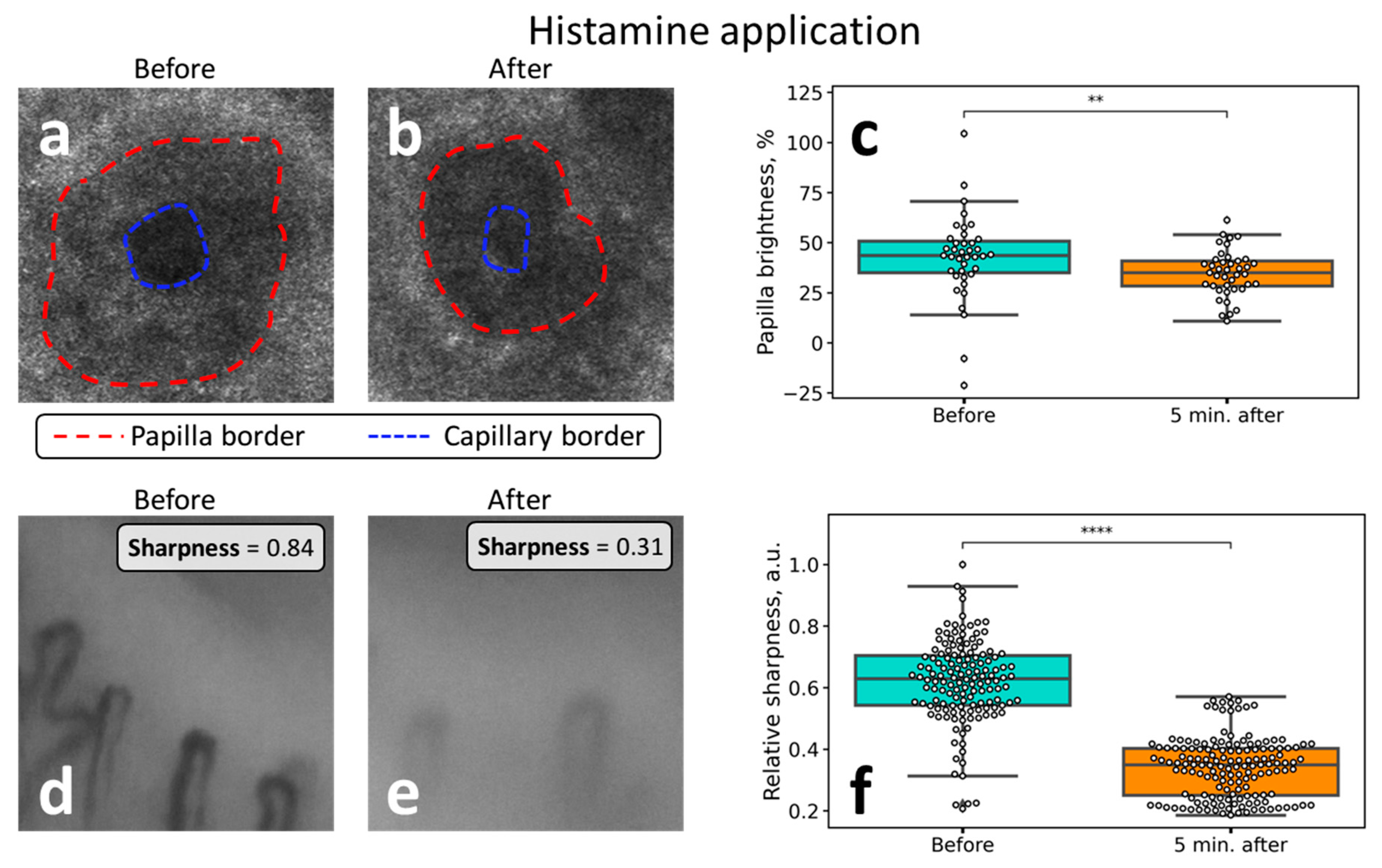
3.4. Histamine Application
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Navas, J.P.; Martinez-Maldonado, M. Pathophysiology of edema in congestive heart failure. Heart Dis. Stroke 1993, 2, 325–329. [Google Scholar] [PubMed]
- Warren, A.G.; Brorson, H.; Borud, L.J.; Slavin, S.A. Lymphedema: A comprehensive review. Ann. Plast. Surg. 2007, 59, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Brain, S.D.; Williams, T.J. Inflammatory oedema induced by synergism between calcitonin gene-related peptide (CGRP) and mediators of increased vascular permeability. Br. J. Pharmacol. 1985, 86, 855–860. [Google Scholar] [CrossRef]
- Brodowicz, K.G.; McNaughton, K.; Uemura, N.; Meininger, G.; Girman, C.J.; Yale, S.H. Reliability and feasibility of methods to quantitatively assess peripheral edema. Clin. Med. Res. 2009, 7, 21–31. [Google Scholar] [CrossRef]
- Stamatas, G.N.; Southall, M.; Kollias, N. In vivo monitoring of cutaneous edema using spectral imaging in the visible and near infrared. J. Investig. Dermatol. 2006, 126, 1753–1760. [Google Scholar] [CrossRef]
- Perkov, S.A.; Gorin, D.A.; Esenaliev, R.O. Optoacoustic Monitoring of Water Content in Tissue Phantoms and Human Skin. J. Biophotonics 2020, e202000363. [Google Scholar] [CrossRef]
- Yakimov, B.P.; Davydov, D.A.; Fadeev, V.V.; Budylin, G.S.; Shirshin, E.A. Comparative analysis of the methods for quantitative determination of water content in skin from diffuse reflectance spectroscopy data. Quantum Electron. 2020, 50, 41–46. [Google Scholar] [CrossRef]
- Eikje, N.S.; Ozaki, Y.; Aizawa, K.; Arase, S. Fiber optic near-infrared Raman spectroscopy for clinical noninvasive determination of water content in diseased skin and assessment of cutaneous edema. J. Biomed. Opt. 2005, 10, 014013. [Google Scholar] [CrossRef][Green Version]
- Bajwa, N.; Sung, S.; Ennis, D.B.; Fishbein, M.C.; Nowroozi, B.N.; Ruan, D.; MacCabi, A.; Alger, J.; John, M.A.S.; Grundfest, W.S.; et al. Terahertz Imaging of Cutaneous Edema: Correlation with Magnetic Resonance Imaging in Burn Wounds. IEEE Trans. Biomed. Eng. 2017, 64, 2682–2694. [Google Scholar] [CrossRef]
- Kistenev, Y.V.; Nikolaev, V.V.; Kurochkina, O.S.; Borisov, A.V.; Vrazhnov, D.A.; Sandykova, E.A. Application of multiphoton imaging and machine learning to lymphedema tissue analysis. Biomed. Opt. Express 2019, 10, 3353–3368. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Herrick, A.L.; Ingegnoli, F.; Damjanov, N.; De Angelis, R.; Denton, C.P.; Distler, O.; Espejo, K.; Foeldvari, I.; Frech, T.; et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun. Rev. 2020, 19, 102458. [Google Scholar] [CrossRef] [PubMed]
- Shirshin, E.A.; Gurfinkel, Y.I.; Matskeplishvili, S.T.; Sasonko, M.L.; Omelyanenko, N.P.; Yakimov, B.P.; Lademann, J.; Darvin, M.E. In vivo optical imaging of the viable epidermis around the nailfold capillaries for the assessment of heart failure severity in humans. J. Biophotonics 2018, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kaznacheev, V.P.; Dzizinsky, A.A. Clinical Pathology of Transcapillary Metabolism; Izdatelstvo Meditsina: Moscow, Russia, 1975. [Google Scholar]
- Natalello, G.; De Luca, G.; Gigante, L.; Campochiaro, C.; De Lorenzis, E.; Verardi, L.; Paglionico, A.; Petricca, L.; Martone, A.M.; Calvisi, S.; et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: Broadening the spectrum of COVID-19 microvascular involvement. Microvasc. Res. 2020, 133, 104071. [Google Scholar] [CrossRef] [PubMed]
- Monticone, G.; Colonna, L.; Palermi, G.; Bono, R.; Puddu, P. Quantitative nailfold capillary microscopy findings in patients with acrocyanosis compared with patients having systemic sclerosis and control subjects. J. Am. Acad. Dermatol. 2000, 42, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Lambova, S.; Mueller-Ladner, U. Capillaroscopic findings in systemic sclerosis—Are they associated with disease duration and presence of digital ulcers? Discov. Med. 2011, 12, 413–418. [Google Scholar] [PubMed]
- Parisi, S.; Ditto, M.C. Videocapillaroscopy in connective tissue diseases. Syst. Scler. 2017. [Google Scholar] [CrossRef][Green Version]
- Ilie, M.A.; Caruntu, C.; Lixandru, D.; Tampa, M.; Georgescu, S.R.; Constantin, M.M.; Constantin, C.; Neagu, M.; Zurac, S.A.; Boda, D. In vivo confocal laser scanning microscopy imaging of skin inflammation: Clinical applications and research directions (review). Exp. Ther. Med. 2019, 17, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Batani, A.; Brănișteanu, D.E.; Ilie, M.A.; Boda, D.; Ianosi, S.; Ianosi, G.; Caruntu, C. Assessment of dermal papillary and microvascular parameters in psoriasis vulgaris using in vivo reflectance confocal microscopy. Exp. Ther. Med. 2018, 15, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Mathura, K.R.; Vollebregt, K.C.; Boer, K.; De Graaff, J.C.; Ubbink, D.T.; Ince, C. Comparison of OPS imaging and conventional capillary microscopy to study the human microcirculation. J. Appl. Physiol. 2001, 91, 74–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tuchin, V.V. Optical Biomedical Diagnostics; Fizmatlit: Moscow, Russia, 2006. [Google Scholar]
- Bashkatov, A.N.; Berezin, K.V.; Dvoretskiy, K.N.; Chernavina, M.L.; Genina, E.A.; Genin, V.D.; Kochubey, V.I.; Lazareva, E.N.; Pravdin, A.B.; Shvachkina, M.E.; et al. Measurement of tissue optical properties in the context of tissue optical clearing. J. Biomed. Opt. 2018, 23, 1–31. [Google Scholar] [CrossRef] [PubMed]

| Application | NVC | CLSM |
|---|---|---|
| Venous occlusion | • no changes | • a 10–15% increase in the capillary diameter after occlusion |
| Histamine application | • a twofold decrease of the capillaries’ sharpness after histamine application | • a 20% decrease in the papillae brightness (hyporefractivity) following histamine application |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakimov, B.P.; Gurfinkel, Y.I.; Davydov, D.A.; Allenova, A.S.; Budylin, G.S.; Vasiliev, V.Y.; Soldatova, V.Y.; Kamalov, A.A.; Matskeplishvili, S.T.; Priezzhev, A.V.; et al. Pericapillary Edema Assessment by Means of the Nailfold Capillaroscopy and Laser Scanning Microscopy. Diagnostics 2020, 10, 1107. https://doi.org/10.3390/diagnostics10121107
Yakimov BP, Gurfinkel YI, Davydov DA, Allenova AS, Budylin GS, Vasiliev VY, Soldatova VY, Kamalov AA, Matskeplishvili ST, Priezzhev AV, et al. Pericapillary Edema Assessment by Means of the Nailfold Capillaroscopy and Laser Scanning Microscopy. Diagnostics. 2020; 10(12):1107. https://doi.org/10.3390/diagnostics10121107
Chicago/Turabian StyleYakimov, Boris P., Yury I. Gurfinkel, Denis A. Davydov, Anastasia S. Allenova, Gleb S. Budylin, Vladimir Yu. Vasiliev, Vera Yu. Soldatova, Armais A. Kamalov, Simon T. Matskeplishvili, Alexander V. Priezzhev, and et al. 2020. "Pericapillary Edema Assessment by Means of the Nailfold Capillaroscopy and Laser Scanning Microscopy" Diagnostics 10, no. 12: 1107. https://doi.org/10.3390/diagnostics10121107
APA StyleYakimov, B. P., Gurfinkel, Y. I., Davydov, D. A., Allenova, A. S., Budylin, G. S., Vasiliev, V. Y., Soldatova, V. Y., Kamalov, A. A., Matskeplishvili, S. T., Priezzhev, A. V., & Shirshin, E. A. (2020). Pericapillary Edema Assessment by Means of the Nailfold Capillaroscopy and Laser Scanning Microscopy. Diagnostics, 10(12), 1107. https://doi.org/10.3390/diagnostics10121107





