Heart Rate Influence on the QT Variability Risk Factors
Abstract
1. Introduction
2. Methods
2.1. Investigated Population and Electrocardiographic Recordings
2.2. Beat-to-Beat QT Interval Measurements
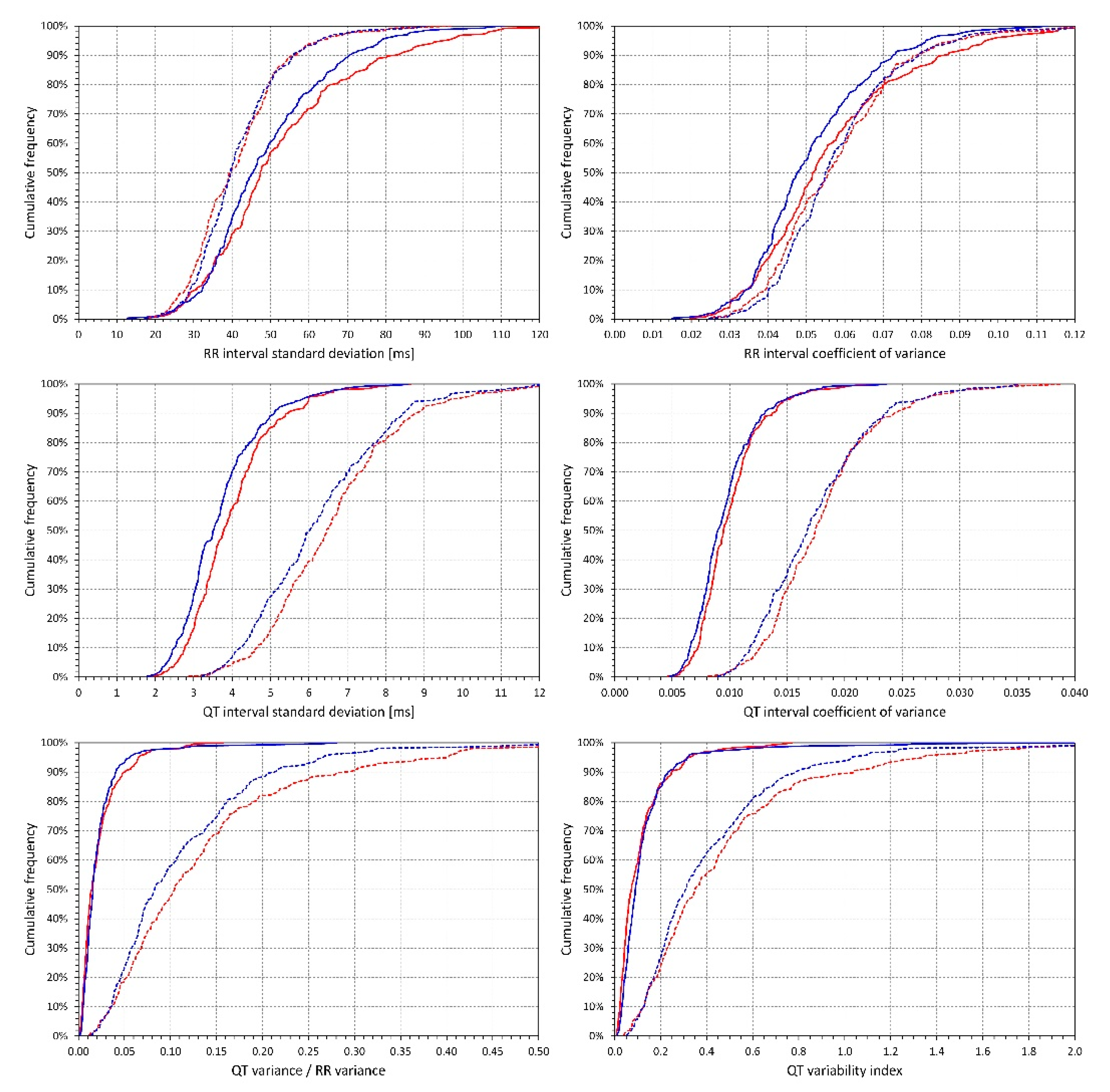
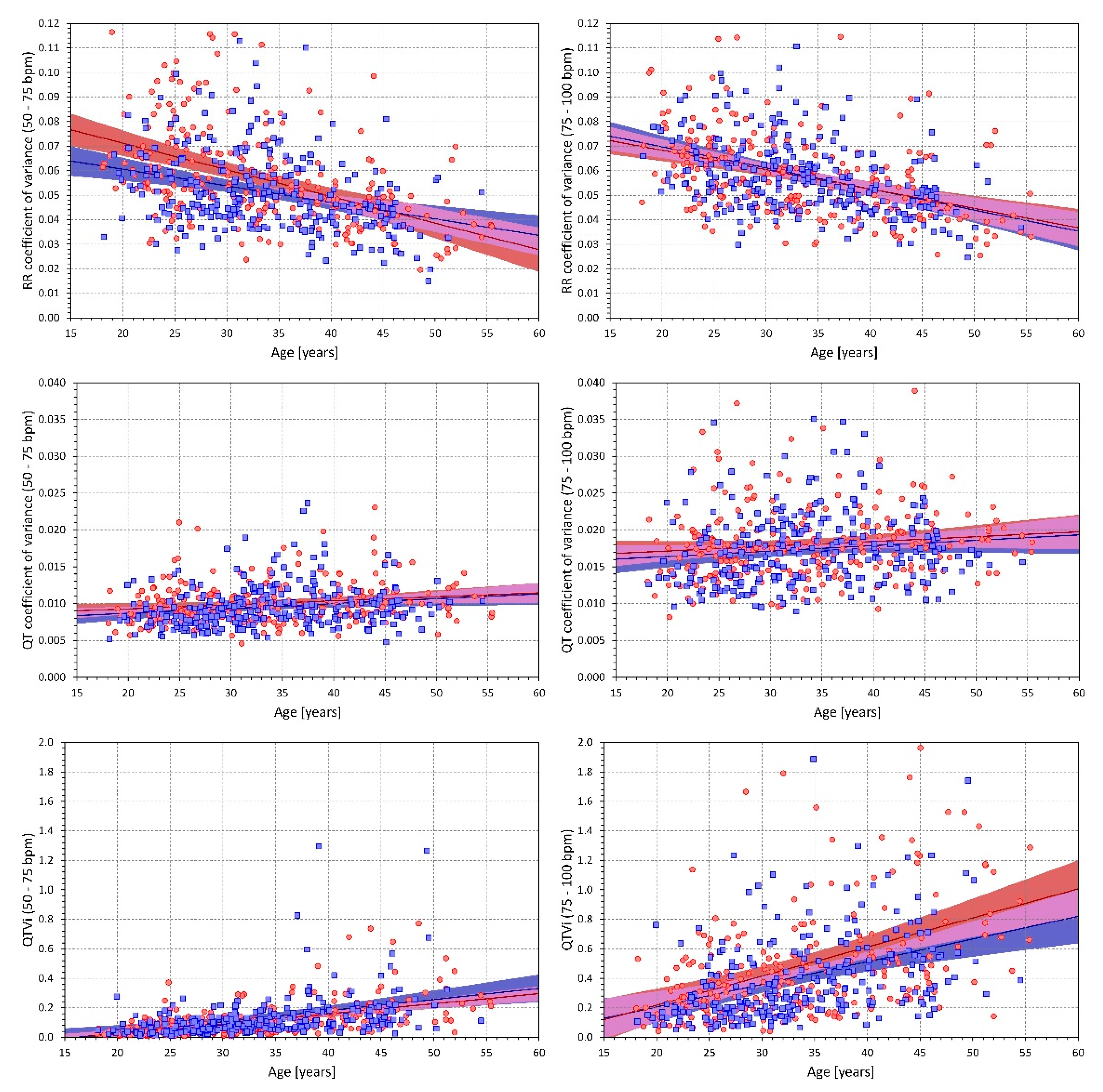
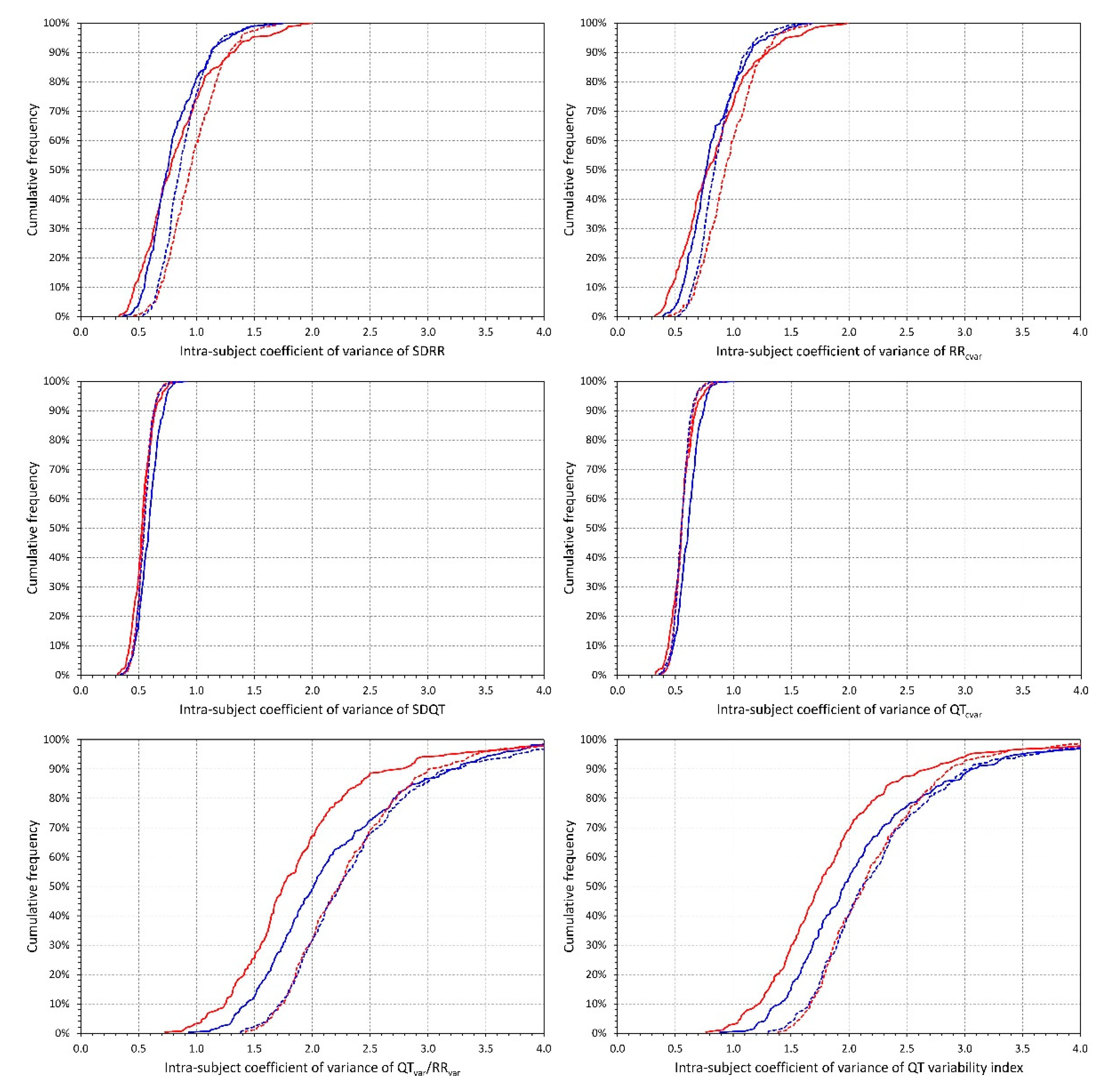
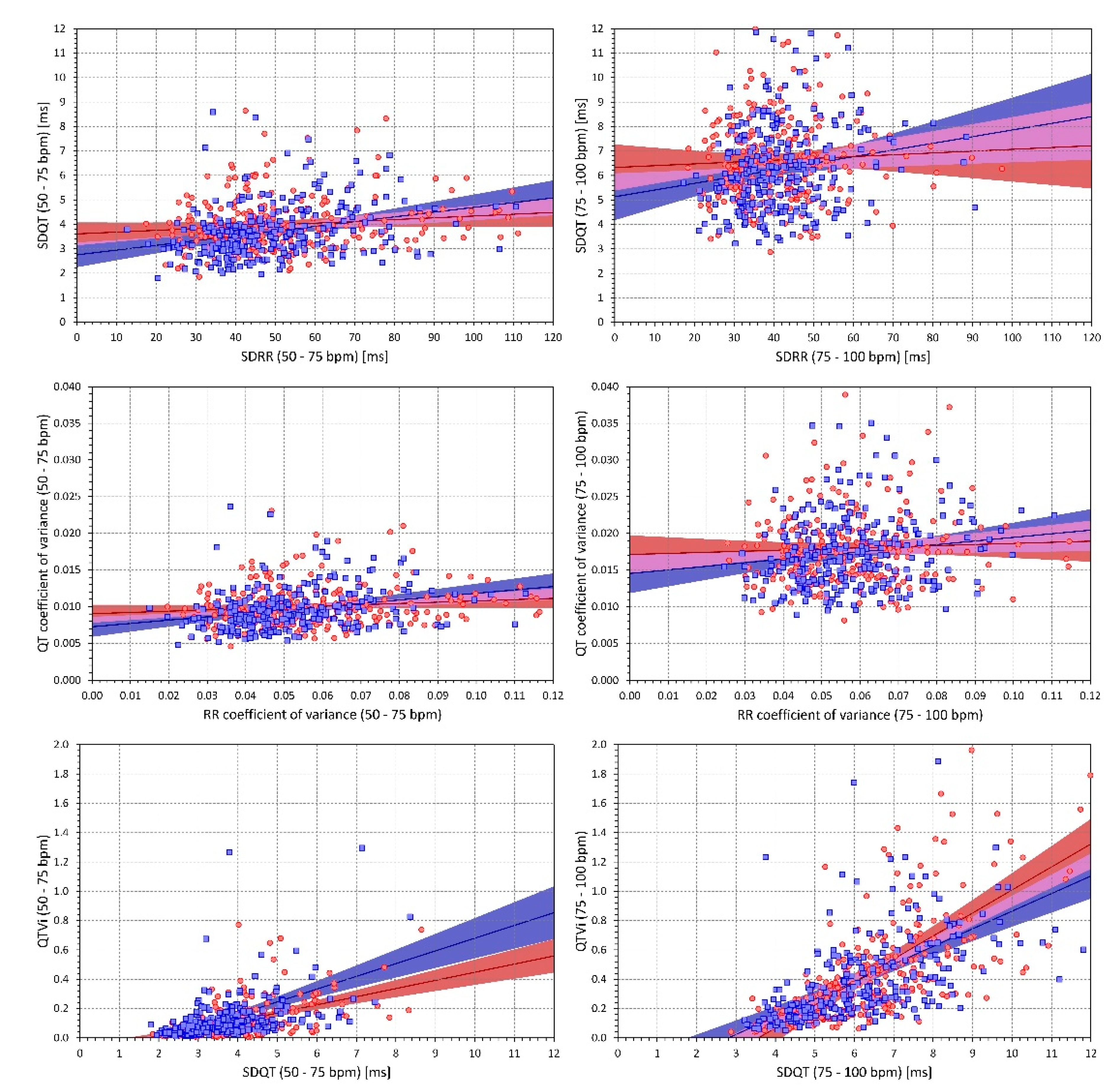
2.3. QT Interval Variability Expressions
- Standard deviation of all RR intervals (SDRR),
- Coefficient of variance of RR intervals (RRcvar = SDRR/, where is the average of all RR intervals),
- Standard deviation of all QT intervals in individual beats (SDQT),
- Coefficient of variance of QT intervals (QTcvar = SDQT/, where is the average of all QT intervals),
- Proportion of QT and RR interval variances (QTvar/RRvar = SDQT2/SDRR2),
- QT variability index (QTVi = ).
2.4. Data Investigations
2.4.1. Effects of Heart Rate
2.4.2. Influence of Age
2.4.3. Intra-Subject Variability
2.4.4. Intra-Subject and Inter-Subject Relationship between the Indices
2.5. Statistics and Data Presentation
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- US Preventive Services Task Force; Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Kemper, A.R.; et al. Screening for cardiovascular disease risk with electrocardiography: US Preventive Services Task Force recommendation statement. JAMA 2018, 319, 2308–2314. [Google Scholar] [PubMed]
- Somsen, G.A. The role of ECG screening in primary care; a call for collaboration between general practitioner and cardiologist. Neth. Heart J. 2020, 28, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.; Zhou, X.; Han, C.; Abrishami, H.; Webster, G.; Miyake, C.Y.; Sower, C.T.; Anderson, J.B.; Knilans, T.K.; Czosek, R.J. Pilot study analyzing automated ECG screening of hypertrophic cardiomyopathy. Heart Rhythm 2017, 14, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.M.; Kraemer, J.F.; Dhingra, R.; Keenan, B.T.; Wessel, N.; Glos, M.; Penzel, T.; Gurubhagavatula, I. Screening for obstructive sleep apnea in commercial drivers using EKG-derived respiratory power index. J. Clin. Sleep Med. 2019, 15, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; McCoy, R.G.; Friedman, P.A.; Shah, N.D.; Barry, B.A.; Behnken, E.M.; Inselman, J.W.; Attia, Z.I.; Noseworthy, P.A. ECG AI-guided screening for low ejection fraction (EAGLE): Rationale and design of a pragmatic cluster randomized trial. Am. Heart J. 2020, 219, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Farcomeni, A.; Goethals, P.; Scavee, C.; Vijgen, J.; Blankoff, I.; Vandekerckhove, Y.; Lip, G.Y.; Mairesse, G.H. Belgian Heart Rhythm Week Investigators. Cost-effectiveness and screening performance of ECG handheld machine in a population screening programme: The Belgian Heart Rhythm Week screening programme. Eur. J. Prev. Cardiol. 2019, 26, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Baumert, M.; Porta, A.; Vos, M.A.; Malik, M.; Couderc, J.P.; Laguna, P.; Piccirillo, G.; Smith, G.L.; Tereshchenko, L.G.; Volders, P.G. QT interval variability in body surface ECG: Measurement, physiological basis, and clinical value: Position statement and consensus guidance endorsed by the European Heart Rhythm Association jointly with the ESC Working Group on Cardiac Cellular Electrophysiology. Europace 2016, 18, 925–944. [Google Scholar]
- Fischer, C.; Seeck, A.; Schroeder, R.; Goernig, M.; Schirdewan, A.; Figulla, H.R.; Baumert, M.; Voss, A. QT variability improves risk stratification in patients with dilated cardiomyopathy. Physiol. Meas. 2015, 36, 699–713. [Google Scholar] [CrossRef]
- Orosz, A.; Baczkó, I.; Nagy, V.; Gavallér, H.; Csanády, M.; Forster, T.; Papp, J.G.; Varró, A.; Lengyel, C.; Sepp, R. Short-term beat-to-beat variability of the QT interval is increased and correlates with parameters of left ventricular hypertrophy in patients with hypertrophic cardiomyopathy. Can. J. Physiol. Pharmacol. 2015, 93, 765–772. [Google Scholar] [CrossRef]
- Porta, A.; Girardengo, G.; Bari, V.; George, A.L., Jr.; Brink, P.A.; Goosen, A.; Crotti, L.; Schwartz, P.J. Autonomic control of heart rate and QT interval variability influences arrhythmic risk in long QT syndrome type 1. J. Am. Coll. Cardiol. 2015, 65, 367–374. [Google Scholar] [CrossRef]
- Seethala, S.; Singh, P.; Shusterman, V.; Ribe, M.; Haugaa, K.H.; Němec, J. QT adaptation and intrinsic QT variability in congenital long QT syndrome. J. Am. Heart Assoc. 2015, 4, e002395. [Google Scholar] [CrossRef] [PubMed]
- Monasterio, V.; Martínez, J.P.; Laguna, P.; McNitt, S.; Polonsky, S.; Moss, A.J.; Haigney, M.; Zareba, W.; Couderc, J.P. Prognostic value of average T-wave alternans and QT variability for cardiac events in MADIT-II patients. J. Electrocardiol. 2013, 46, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Smoczyńska, A.; Loen, V.; Sprenkeler, D.J.; Tuinenburg, A.E.; Ritsema van Eck, H.J.; Malik, M.; Schmidt, G.; Meine, M.; Vos, M.A. Short-term variability of the QT interval can be used for the prediction of imminent ventricular arrhythmias in patients with primary prophylactic implantable cardioverter defibrillators. J. Am. Heart Assoc. 2020, 9, e018133. [Google Scholar] [CrossRef] [PubMed]
- Orosz, A.; Csajbók, É.; Czékus, C.; Gavallér, H.; Magony, S.; Valkusz, Z.; Várkonyi, T.T.; Nemes, A.; Baczkó, I.; Forster, T.; et al. Increased short-term beat-to-beat variability of QT interval in patients with acromegaly. PLoS ONE 2015, 10, e0125639. [Google Scholar] [CrossRef] [PubMed]
- Viigimae, M.; Karai, D.; Pilt, K.; Pirn, P.; Huhtala, H.; Polo, O.; Meigas, K.; Kaik, J. QT interval variability index and QT interval duration during different sleep stages in patients with obstructive sleep apnea. Sleep Med. 2017, 37, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Nussinovitch, U.; Rubin, S.; Levy, Y.; Lidar, M.; Livneh, A. QT variability index in patients with systemic sclerosis. Eur. J. Rheumatol. 2018, 6, 179–183. [Google Scholar] [PubMed]
- Niemeijer, M.N.; van den Berg, M.E.; Eijgelsheim, M.; van Herpen, G.; Stricker, B.H.; Kors, J.A.; Rijnbeek, P.R. Short-term QT variability markers for the prediction of ventricular arrhythmias and sudden cardiac death: A systematic review. Heart 2014, 100, 1831–1836. [Google Scholar] [CrossRef]
- Feeny, A.; Han, L.; Tereshchenko, L.G. Repolarization lability measured on 10-second ECG by spatial TT’ angle: Reproducibility and agreement with QT variability. J. Electrocardiol. 2014, 47, 708–715. [Google Scholar] [CrossRef]
- van den Berg, M.E.; Kors, J.A.; van Herpen, G.; Bots, M.L.; Hillege, H.; Swenne, C.A.; Stricker, B.H.; Rijnbeek, P.R. Normal values of QT variability in 10-s electrocardiograms for all ages. Front. Physiol. 2019, 10, 1272. [Google Scholar] [CrossRef]
- Bazett, J.C. An analysis of time relations of electrocardiograms. Heart 1920, 7, 353–367. [Google Scholar] [CrossRef]
- Fridericia, L.S. Die Systolendauer im Elekrokardiogramm bei normalen Menschen und bei Herzkranken. Acta Med. Scand. 1920, 53, 469–486. [Google Scholar] [CrossRef]
- ICH Guideline. Safety pharmacology studies for human pharmaceuticals S7A. Fed. Regist. 2001, 66, 36791–36792. [Google Scholar]
- Malik, M.; Andreas, J.-O.; Hnatkova, K.; Hoeckendorff, J.; Cawello, W.; Middle, M.; Horstmann, R.; Braun, M. Thorough QT/QTc Study in patients with advanced Parkinson’s disease: Cardiac safety of rotigotine. Clin. Pharmacol. Ther. 2008, 84, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; van Gelderen, E.M.; Lee, J.H.; Kowalski, D.L.; Yen, M.; Goldwater, R.; Mujais, S.K.; Schaddelee, M.P.; de Koning, P.; Kaibara, A.; et al. Proarrhythmic safety of repeat doses of mirabegron in healthy subjects: A randomized, double-blind, placebo-, and active-controlled thorough QT study. Clin. Pharm. Ther. 2012, 92, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Hnatkova, K.; Smetana, P.; Toman, O.; Bauer, A.; Schmidt, G.; Malik, M. Systematic comparisons of electrocardiographic morphology increase the precision of QT interval measurement. Pacing Clin. Electrophysiol. 2009, 32, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Malik, M. Errors and misconceptions in ECG measurement used for the detection of drug induced QT interval prolongation. J. Electrocardiol. 2004, 37, 25–33. [Google Scholar] [CrossRef]
- Xue, J.Q. Robust QT interval estimation—From algorithm to validation. Ann. Noninvasive Electrocardiol. 2009, 14 (Suppl. 1), S35–S41. [Google Scholar] [CrossRef]
- Berger, R.D.; Kasper, E.K.; Baughman, K.L.; Marban, E.; Calkins, H.; Tomaselli, G.F. Beat-to-beat QT interval variability: Novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation 1997, 96, 1557–1565. [Google Scholar] [CrossRef]
- Baumert, M.; Lambert, G.W.; Dawood, T.; Lambert, E.A.; Esler, M.D.; McGrane, M.; Barton, D.; Nalivaiko, E. QT interval variability and cardiac norepinephrine spillover in patients with depression and panic disorder. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H962–H968. [Google Scholar] [CrossRef]
- Malik, M. Beat-to-beat QT variability and cardiac autonomic regulation. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H923–H925. [Google Scholar] [CrossRef][Green Version]
- Guldenring, D.; Finlay, D.D.; Strauss, D.G.; Galeotti, L.; Nugent, C.D.; Donnelly, M.P.; Bond, R.R. Transformation of the Mason-Likar 12-lead electrocardiogram to the Frank vectorcardiogram. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2012, 2012, 677–680. [Google Scholar] [PubMed]
- Hiromoto, K.; Shimizu, H.; Mine, T.; Masuyama, T.; Ohyanagi, M. Correlation between beat-to-beat QT interval variability and impaired left ventricular function in patients with previous myocardial infarction. Ann Noninvasive Ann. Noninvasive Electrocardiol. 2006, 11, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Oosterhoff, P.; Tereshchenko, L.G.; van der Heyden, M.A.; Ghanem, R.N.; Fetics, B.J.; Berger, R.D.; Vos, M.A. Short-term variability of repolarization predicts ventricular tachycardia and sudden cardiac death in patients with structural heart disease: A comparison with QT variability index. Heart Rhythm 2011, 8, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Haigney, M.C.; Zareba, W.; Gentlesk, P.J.; Goldstein, R.E.; Illovsky, M.; McNitt, S.; Andrews, M.L.; Moss, A., Jr. Multicenter Automatic Defibrillator Implantation Trial II investigators. QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J. Am. Coll. Cardiol. 2004, 44, 1481–1487. [Google Scholar] [CrossRef]
- Dobson, C.P.; Kim, A.; Haigney, M. QT Variability Index. Prog. Cardiovasc. Dis. 2013, 56, 186–194. [Google Scholar] [CrossRef]
- Heravi, A.S.; Etzkorn, L.H.; Urbanek, J.K.; Crainiceanu, C.M.; Punjabi, N.M.; Ashikaga, H.; Brown, T.T.; Budoff, M.J.; D’Souza, G.; Magnani, J.W.; et al. HIV infection is associated with variability in ventricular repolarization: The Multicenter AIDS Cohort Study (MACS). Circulation 2020, 141, 176–187. [Google Scholar] [CrossRef]
- Copie, X.; Hnatkova, K.; Staunton, A.; Fei, L.; Camm, A.J.; Malik, M. Predictive power of increased heart rate versus depressed left ventricular ejection fraction and heart rate variability for risk stratification after myocardial infarction. Results of a two-year follow-up study. J. Am. Coll. Cardiol. 1996, 27, 270–276. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Heart rate as cardiovascular risk factor. Postgrad. Med. 2020, 132, 358–367. [Google Scholar] [CrossRef]
- Malik, M.; Hnatkova, K.; Huikuri, H.V.; Lombardi, F.; Schmidt, G.; Zabel, M. CrossTalk proposal: Heart rate variability is a valid measure of cardiac autonomic responsiveness. J. Physiol. 2019, 597, 2595–2598. [Google Scholar] [CrossRef]
- Goldenberg, I.; Goldkorn, R.; Shlomo, N.; Einhorn, M.; Levitan, J.; Kuperstein, R.; Klempfner, R.; Johnson, B. Heart rate variability for risk assessment of myocardial ischemia in patients without known coronary artery disease: The HRV-DETECT (Heart Rate Variability for the Detection of Myocardial Ischemia) study. J. Am. Heart Assoc. 2019, 8, e014540. [Google Scholar] [CrossRef]
- Dobson, C.P.; La Rovere, M.T.; Pinna, G.D.; Goldstein, R.; Olsen, C.; Bernardinangeli, M.; Veniani, M.; Midi, P.; Tavazzi, L.; Haigney, M. GISSI-HF Investigators. QT variability index on 24-hour Holter independently predicts mortality in patients with heart failure: Analysis of Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca (GISSI-HF) trial. Heart Rhythm 2011, 8, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- ESC/NASPE Task Force. Heart rate variability—Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Schmidt, G.; Malik, M.; Barthel, P.; Schneider, R.; Ulm, K.; Rolnitzky, L.; Camm, A.J.; Bigger, J.T., Jr.; Schömig, A. Heart rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet 1999, 353, 1390–1396. [Google Scholar] [CrossRef]
- Bauer, A.; Malik, M.; Schmidt, G.; Barthel, P.; Bonnemeier, H.; Cygankiewicz, I.; Guzik, P.; Lombardi, F.; Müller, A.; Oto, A.; et al. Heart rate turbulence: Standards of measurement, physiological interpretation, and clinical use. International Society for Holter and Noninvasive Electrophysiology Consensus. J. Am. Coll. Cardiol. 2008, 52, 1353–1365. [Google Scholar] [CrossRef]
- Bauer, A.; Kantelhardt, J.W.; Barthel, P.; Schneider, R.; Mäkikallio, T.; Ulm, K.; Hnatkova, K.; Schömig, A.; Huikuri, H.; Bunde, A.; et al. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: Cohort study. Lancet 2006, 367, 1674–1681. [Google Scholar] [CrossRef]
- Verrier, R.L.; Klingenheben, T.; Malik, M.; El-Sherif, N.; Exner, D.V.; Hohnloser, S.H.; Ikeda, T.; Martinez, J.P.; Narayan, S.M.; Nieminen, T.; et al. Microvolt T-wave alternans: Physiological basis, methods of measurement, and clinical utility consensus guideline by International Society for Holter and Noninvasive Electrocardiology. J. Am. Coll. Cardiol. 2011, 58, 1309–1324. [Google Scholar] [CrossRef]
- Hnatkova, K.; Šišáková, M.; Smetana, P.; Toman, O.; Huster, K.M.; Novotný, T.; Schmidt, G.; Malik, M. Sex differences in heart rate responses to postural provocations. Int. J. Cardiol. 2019, 297, 126–134. [Google Scholar] [CrossRef]
- Linde, C.; Bongiorni, M.G.; Birgersdotter-Green, U.; Curtis, A.B.; Deisenhofer, I.; Furokawa, T.; Gillis, A.M.; Haugaa, K.H.; Lip, G.Y.H.; Van Gelder, I.; et al. ESC Scientific Document Group. Sex differences in cardiac arrhythmia: A consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace 2018, 20, 1565–1565ao. [Google Scholar] [CrossRef]
- Garnett, C.E.; Zhu, H.; Malik, M.; Fossa, A.A.; Zhang, J.; Badilini, F.; Li, J.; Darpö, B.; Sager, P.; Rodriguez, I. Methodologies to characterize the QT/corrected QT interval in the presence of drug-induced heart rate changes or other autonomic effects. Am. Heart J. 2012, 163, 912–930. [Google Scholar] [CrossRef]
- Malik, M.; Hnatkova, K.; Batchvarov, V.; Gang, Y.; Smetana, P.; Camm, A.J. Sample size, power calculations, and their implications for the cost of thorough studies of drug induced QT interval prolongation. Pacing Clin. Electrophysiol. 2004, 27, 1659–1669. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andršová, I.; Hnatkova, K.; Šišáková, M.; Toman, O.; Smetana, P.; Huster, K.M.; Barthel, P.; Novotný, T.; Schmidt, G.; Malik, M. Heart Rate Influence on the QT Variability Risk Factors. Diagnostics 2020, 10, 1096. https://doi.org/10.3390/diagnostics10121096
Andršová I, Hnatkova K, Šišáková M, Toman O, Smetana P, Huster KM, Barthel P, Novotný T, Schmidt G, Malik M. Heart Rate Influence on the QT Variability Risk Factors. Diagnostics. 2020; 10(12):1096. https://doi.org/10.3390/diagnostics10121096
Chicago/Turabian StyleAndršová, Irena, Katerina Hnatkova, Martina Šišáková, Ondřej Toman, Peter Smetana, Katharina M. Huster, Petra Barthel, Tomáš Novotný, Georg Schmidt, and Marek Malik. 2020. "Heart Rate Influence on the QT Variability Risk Factors" Diagnostics 10, no. 12: 1096. https://doi.org/10.3390/diagnostics10121096
APA StyleAndršová, I., Hnatkova, K., Šišáková, M., Toman, O., Smetana, P., Huster, K. M., Barthel, P., Novotný, T., Schmidt, G., & Malik, M. (2020). Heart Rate Influence on the QT Variability Risk Factors. Diagnostics, 10(12), 1096. https://doi.org/10.3390/diagnostics10121096




