Next Generation Sequencing in Non-Small Cell Lung Cancer: Pitfalls and Opportunities
Abstract
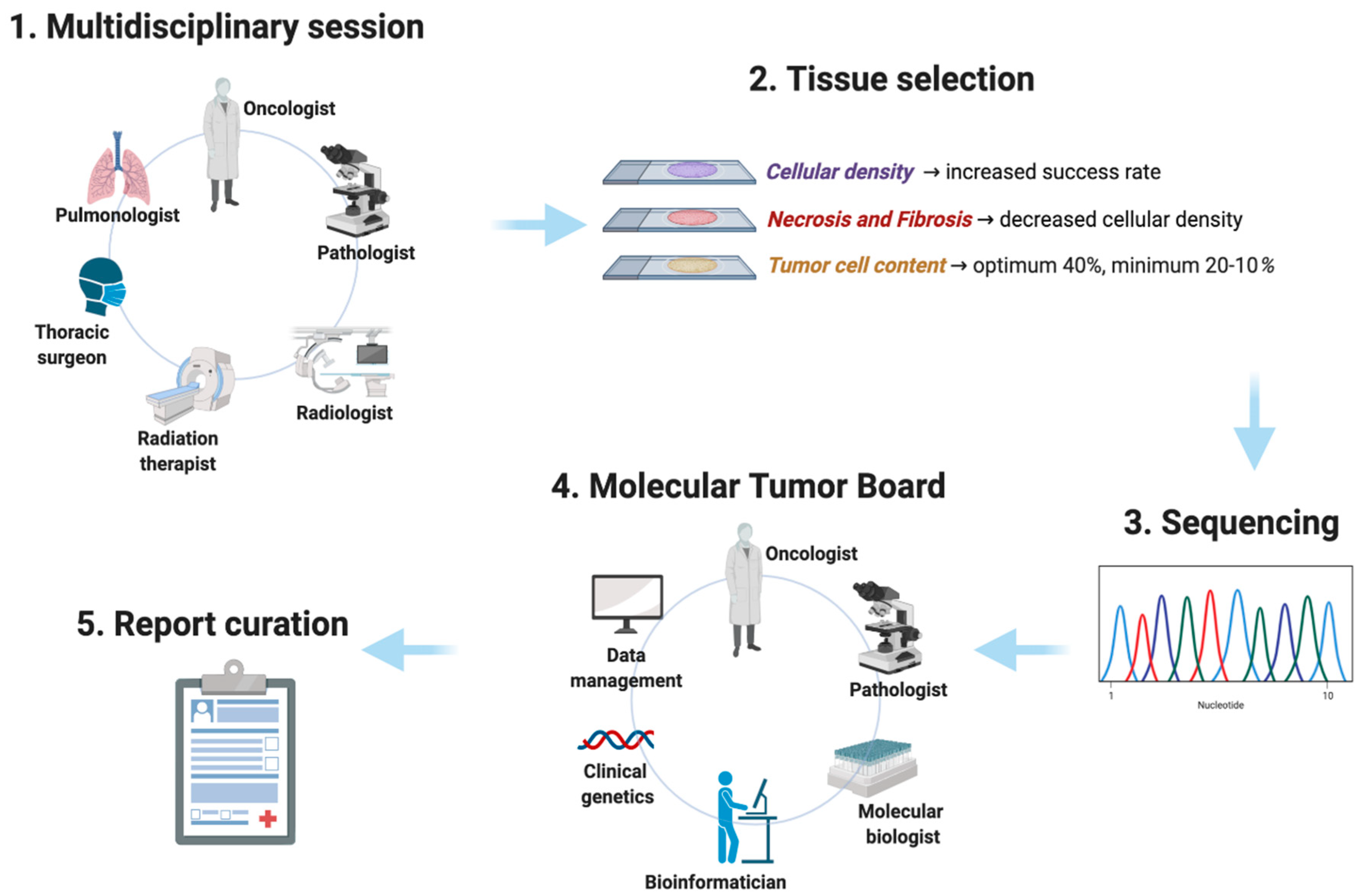
:1. Introduction
2. Next-Generation Sequencing: Tissue Requirements
3. Next-Generation Sequencing: Bioinformatic Issues
- Tier 1 (variants with strong clinical significance);
- Tier 2 (variants with potential clinical significance);
- Tier 3 (variants of unknown clinical significance)
- Tier 4 (variants deemed benign or likely benign).
- Tier I (targets ready for implementation in routine clinical decisions);
- Tier II (investigational targets that likely define a patient population that benefits from a targeted drug, but additional data are needed);
- Tier III (clinical benefit previously demonstrated in other tumor types or for similar molecular targets);
- Tier IV (preclinical evidence of actionability)
- Tier V (evidence supporting co-targeting approaches; and tier X, lack of evidence for actionability).
4. Previous and Ongoing Experiences with NGS in Patients with NSCLC
5. Comments and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomarkers Prev. 2015, 25, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosell, R.; Karachaliou, N. Large-scale screening for somatic mutations in lung cancer. Lancet 2016, 387, 1354–1356. [Google Scholar] [CrossRef]
- Kris, M.G.; Johnson, B.E.; Berry, L.D.; Kwiatkowski, D.J.; Iafrate, A.J.; Wistuba, I.I.; Varella-Garcia, M.; Franklin, W.A.; Aronson, S.L.; Su, P.-F.; et al. Using Multiplexed Assays of Oncogenic Drivers in Lung Cancers to Select Targeted Drugs. JAMA 2014, 311, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Barlesi, F.; Mazieres, J.; Merlio, J.P.; Debieuvre, D.; Mosser, J.; Lena, H.; Ouafik, H.; Besse, B.; Rouquette, I.; Westeel, V.; et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: Results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016, 387, 1415–1426. [Google Scholar] [CrossRef]
- Gagan, J.; Van Allen, E.M. Next-generation sequencing to guide cancer therapy. Genome Med. 2015, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Skoulidis, F.; Byers, L.A.; Diao, L.; Papadimitrakopoulou, V.A.; Tong, P.; Izzo, J.; Behrens, C.; Kadara, H.; Parra, E.R.; Canales, J.R.; et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015, 5, 860–877. [Google Scholar] [CrossRef] [Green Version]
- Helena, A.Y.; Suzawa, K.; Jordan, E.; Zehir, A.; Ni, A.; Kim, R.; Kris, M.G.; Hellmann, M.D.; Li, B.T.; Somwar, R.; et al. Concurrent Alterations in EGFR-Mutant Lung Cancers Associated with Resistance to EGFR Kinase Inhibitors and Characterization of MTOR as a Mediator of Resistance. Clin Cancer Res. 2018, 24, 3108–3118. [Google Scholar]
- Offin, M.; Rizvi, H.; Tenet, M.; Ni, A.; Sanchez-Vega, F.; Li, B.; Drilon, A.; Kris, M.G.; Rudin, C.M.; Schultz, N.; et al. Tumor Mutation Burden and Efficacy of EGFR-Tyrosine Kinase Inhibitors in Patients with EGFR-Mutant Lung Cancers. Clin. Cancer Res. 2019, 25, 1063–1069. [Google Scholar] [CrossRef] [Green Version]
- Sundaresan, T.K.; Sequist, L.V.; Heymach, J.V.; Riely, G.J.; Jänne, P.A.; Koch, W.H.; Sullivan, J.P.; Fox, D.B.; Maher, R.C.; Muzikansky, A.; et al. Detection of T790M, the Acquired Resistance EGFR Mutation, by Tumor Biopsy versus Noninvasive Blood-Based Analyses. Clin. Cancer Res. 2016, 22, 1103–1110. [Google Scholar] [CrossRef] [Green Version]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Qiu, T.; Ling, Y.; Gao, S.; Ying, J. Subjecting appropriate lung adenocarcinoma samples to next-generation sequencing-based molecular testing: Challenges and possible solutions. Mol. Oncol. 2018, 12, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzarella, L. Are we ready for routine precision medicine? Highlights from the Milan Summit on Precision Medicine, Milan, Italy, 8–9 February 2018. Ecancermedicalscience 2018, 12, 817. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Gao, J.; Phillips, S.M.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis. Oncol. 2017, 2017, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.C.D.; Dienstmann, R.; Jezdic, S. A framework to rank genomic alterations as targets for cancer precision medicine: The ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann. Oncol. 2018, 29, 1859–1902. [Google Scholar] [CrossRef] [PubMed]
- Fuh, K. Faculty Opinions recommendation of Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015, 16, 1324–1334. [Google Scholar]
- Massard, C.; Michiels, S.; Ferté, C.; Le Deley, M.C.; Lacroix, L.; Hollebecque, A.; Verlingue, L.; Ileana, E.; Rosellini, S.; Ammari, S.; et al. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov. 2017, 7, 586–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadimitrakopoulou, V.; Lee, J.J.; Wistuba, I.I.; Tsao, A.S.; Fossella, F.V.; Kalhor, N.; Gupta, S.; Byers, L.A.; Izzo, J.G.; Gettinger, S.N.; et al. The BATTLE-2 Study: A Biomarker-Integrated Targeted Therapy Study in Previously Treated Patients with Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 3638–3647. [Google Scholar] [CrossRef]
- Mangat, P.K.; Halabi, S.; Bruinooge, S.S.; Garrett-Mayer, E.; Alva, A.; Janeway, K.A.; Stella, P.J.; Voest, E.; Yost, K.J.; Perlmutter, J.; et al. Rationale and Design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. JCO Precis Oncol. 2018, 2018. [Google Scholar]
- Ahn, E.R.; Mangat, P.K.; Garrett-Mayer, E.; Halabi, S.; Dib, E.G.; Haggstrom, D.E.; Alguire, K.B.; Calfa, C.J.; Cannon, T.L.; Crilley, P.A.; et al. Palbociclib in Patients with Non-Small Cell Lung Cancer (NSCLC) with CDKN2A Alterations: Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. JCO Precis Oncol. 2019, 4, 757–766. [Google Scholar] [CrossRef]
- Fisher, J.G.; Tait, D.; Garrett-Mayer, E.; Halabi, S.; Mangat, P.K.; Schink, J.C.; Alvarez, R.H.; Veljovich, D.; Cannon, T.L.; Crilley, P.A.; et al. Cetuximab in Patients with Breast Cancer, Non-Small Cell Lung Cancer, and Ovarian Cancer Without KRAS, NRAS, or BRAF Mutations: Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. Target. Oncol. 2020, 15, 733–741. [Google Scholar] [CrossRef]
- Herbst, R.S.; Gandara, D.R.; Hirsch, F.R.; Redman, M.W.; Leblanc, M.; Mack, P.C.; Schwartz, L.H.; Vokes, E.; Ramalingam, S.S.; Bradley, J.D.; et al. Lung Master Protocol (Lung-MAP)—A Biomarker-Driven Protocol for Accelerating Development of Therapies for Squamous Cell Lung Cancer: SWOG S1400. Clin. Cancer Res. 2015, 21, 1514–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, C.; Redman, M.W.; Lara, P.N., Jr.; Borghaei, H.; Hoffman, P.; Bradley, J.D.; Newman, A.J., III; Feldman, M.J.; Minichiello, K.; Miao, J.; et al. SWOG S1400D (NCT02965378), a Phase II Study of the Fibroblast Growth Factor Receptor Inhibitor AZD4547 in Previously Treated Patients with Fibroblast Growth Factor Pathway-Activated Stage IV Squamous Cell Lung Cancer (Lung-MAP Substudy). J. Thorac. Oncol. 2019, 14, 1847–1852. [Google Scholar] [CrossRef] [PubMed]
- Edelman, M.J.; Redman, M.W.; Albain, K.S.; McGary, E.C.; Rafique, N.M.; Petro, D.; Waqar, S.N.; Minichiello, K.; Miao, J.; Papadimitrakopoulou, V.A.; et al. Brief Report: SWOG S1400C (NCT02154490)-A Phase II Study of Palbociclib for Previously Treated Cell Cycle Gene Alteration Positive Patients with Stage IV Squamous Cell Lung Cancer (Lung-MAP Sub-study). J. Thorac. Oncol. 2019, 14, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Langer, C.J.; Redman, M.W.; Wade, J.L., III; Aggarwal, C.; Bradley, J.D.; Crawford, J.; Stella, P.J.; Knapp, M.H.; Miao, J.; Minichiello, K.; et al. Brief Report: SWOG S1400B (NCT02785913), A Phase II Study of GDC-0032 (Taselisib) for Previously Treated PI3K-Positive Patients with Stage IV Squamous Cell Lung Cancer (Lung-MAP Sub-Study). J. Thorac. Oncol. 2019, 14, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Middleton, G.; Crack, L.R.; Popat, S.; Swanton, C.; Hollingsworth, S.J.; Buller, R.E.; Walker, I.; Carr, T.H.; Wherton, D.; Billingham, L.J. The National Lung Matrix Trial: Translating the biology of stratification in advanced non-small-cell lung cancer. Ann. Oncol. 2015, 26, 2464–2469. [Google Scholar] [CrossRef]
- Middleton, G.; Fletcher, P.; Popat, S.; Savage, J.; Summers, Y.; Greystoke, A.; Gilligan, D.; Cave, J.; O’Rourke, N.; Brewster, A.; et al. The National Lung Matrix Trial of personalized therapy in lung cancer. Nature 2020, 583, 807–812. [Google Scholar] [CrossRef]
- Van Allen, E.M.; Wagle, N.; Stojanov, P.; Perrin, D.L.; Cibulskis, K.; Marlow, S.; Jane-Valbuena, J.; Friedrich, D.C.; Kryukov, G.; Carter, S.L.; et al. Whole-exome sequencing and clinical interpretation of formalin-fixed, paraffin-embedded tumor samples to guide precision cancer medicine. Nat. Med. 2014, 20, 682–688. [Google Scholar] [CrossRef]
- Dienstmann, R.; Jang, I.S.; Bot, B.; Friend, S.; Guinney, J. Database of genomic biomarkers for cancer drugs and clinical targetability in solid tumors. Cancer Discov. 2015, 5, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Perez, C.; Tamborero, D.; Schroeder, M.P.; Antolín, A.A.; Deu-Pons, J.; Perez-Llamas, C.; Mestres, J.; Gonzalez-Perez, A.; Lopez-Bigas, N. In silico prescription of anticancer drugs to cohorts of 28 tumor types reveals targeting opportunities. Cancer Cell 2015, 27, 382–396. [Google Scholar] [CrossRef] [Green Version]
- Graig, L.A.; Phillips, J.K.; Moses, H.L. (Eds.) Biomarker Tests for Molecularly Targeted Therapies: Key to Unlocking Precision Medicine; The National Academies Collection: Reports funded by National Institutes of Health: Washington, DC, USA, 2016. [Google Scholar]
- Dees, N.D.; Zhang, Q.; Kandoth, C.; Wendl, M.C.; Schierding, W.; Koboldt, D.C.; Mooney, T.B.; Callaway, M.B.; Dooling, D.; Mardis, E.R.; et al. MuSiC: Identifying mutational significance in cancer genomes. Genome Res. 2012, 22, 1589–1598. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Bigas, N.; Lopez-Bigas, N. Functional impact bias reveals cancer drivers. Nucleic Acids Res. 2012, 40, e169. [Google Scholar]
- Davoli, T.; Xu, A.W.; Mengwasser, K.E.; Sack, L.M.; Yoon, J.C.; Park, P.J.; Elledge, S.J. Cumulative Haploinsufficiency and Triplosensitivity Drive Aneuploidy Patterns and Shape the Cancer Genome. Cell 2013, 155, 948–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| TIER 1 Variants of Strong Clinical Significance | TIER 2 Variants of Potential Clinical Significance | TIER 3 Variants of Unknown Clinical Significance | TIER 4 Benign or Likely Benign Variants |
|---|---|---|---|
| Therapeutic, Prognostic, and Diagnostic Value | Therapeutic, Prognostic, and Diagnostic Value | ||
| Level A evidence FDA approved therapy included in guidelines | Level C evidence FDA-approved therapies for different tumor types or investigational therapies Multiple small, published studies with consensus | Not observed at a significant allele frequency in the general or specific subpopulation databases or pan-cancer or tumor-specific variant databases No convincing published evidence or cancer association | Observed at significance allele frequency in the general or specific subpopulation databases No existing published evidence of cancer association |
| Level B evidence Well-powered studies with consensus from experts in the field | Level D evidence Preclinical trials or case reports without consensus |
| Trial (Reference) | Patients | Biomarkers | Enrollment Drugs |
|---|---|---|---|
| BATTLE 2 [17] | 200 patients with NSCLC, EGFR WT, EML4-ALK-, progressing to standard therapy 154 KRAS mutated | mRNA GeneChip Human Gene 1.0 ST Array from Affymetrix + NGS Foundation Medicine | enrollment closed Erlotinib Erlotinib + MK-2206 Selumetinib + MK-2206 Sorafenib |
| MOSCATO 01 [16] | 1035 patients with solid tumors progressing to at least one line 170 with lung tumor | NGS | enrollment closed ALK AR Cell cycle DNA damage EGFR 2 ERBB2 FGFR IDH IGF1R KIT MAPK MDM2 MET NOTCH PI3K–AKT–mTOR |
| TAPUR [18,19,20] | 3123 patients with advanced solid tumors, non-Hodgkin lymphoma, multiple myeloma progressing to standard therapy | molecular testing in a laboratory under the Clinical Laboratory Improvement Amendments and accreditation by the College of American Pathologists | enollment ongoing Crizotinib Palbociclib Sunitinib Temsirolimus Trastuzumab and Pertuzumab Vemurafenib and Cobimetinib Cetuximab Dasatinib Regorafenib Olaparib Pembrolizumab Nivolumab and Ipilimumab |
| S1400 Lung-MAP [21,22,23,24] (NCT02154490) | 10,000 patients with squamous NSCLC, progressing to first line therapy | NGS (200 genes) | enrollment ongoing Docetaxel Durvalumab Erlotinib AZD4547 Ipilimumab Nivolumab Palbociclib Rilotumumab Talazoparib Taselisib Tremelimumab |
| SAFIR02_Lung (NCT02117167) | 650 patients with NSCLC, EGFR WT, EML4-ALK-, with stable disease or partial response following 4 cycles of platinum based chemotherapy | DNA microarrays and NGS (50 geni, ampliSeq, ion torrent) | enrollment ongoing AZD2014 AZD4547 AZD5363 AZD8931 Selumetinib Vandetanib Pemetrexed Durvalumab savolitinib Olaparib |
| National Lung Matrix (NCT02664935) [25] | 5467 patients with NSCLC progressing to standard therapy | NGS (143 genes) | enrollment ongoing AZD4547 Vistusertib Palbociclib Crizotinib Selumetinib Docetaxel AZD5363 Osimertinib Durvalumab Sitravatinib |
| NCI-MATCH (NCT02465060) | 6452 advanced solid tumors, lymphomas, or multiple myeloma progressing to standard treatment | NGS (143 genes) | enrollment ongoing Adavosertib Afatinib Binimetinib Capivasertib Copanlisib Crizotinib Dabrafenib Dasatinib Defactinib Erdafitinib AZD4547 Ipatasertib Larotrectinib Nivolumab Osimertinib Palbociclib Pertuzumab GSK2636771 Sapanisertib Sunitinib Malate Taselisib Trametinib Trastuzumab Trastuzumab Emtansine Ulixertinib Vismodegib |
| ACC Lung | 1000 patients with naive advanced NSCLC | NGS (182 genes) | enrollment ongoing Afatinib Erlotinib Gefitinib Osimertinib Alectinib Crizotinib Dabrafenib + Trametinib Pembrolizumab Atezolizumab Nivolumab Cisplatin + Pemetrexed + Pembrolizumab Carboplatin + Pemetrexed + Pembrolizumab Cisplatin + gemcitabine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzari, C.; Bulotta, A.; Cangi, M.G.; Bucci, G.; Pecciarini, L.; Bonfiglio, S.; Lorusso, V.; Ippati, S.; Arrigoni, G.; Grassini, G.; et al. Next Generation Sequencing in Non-Small Cell Lung Cancer: Pitfalls and Opportunities. Diagnostics 2020, 10, 1092. https://doi.org/10.3390/diagnostics10121092
Lazzari C, Bulotta A, Cangi MG, Bucci G, Pecciarini L, Bonfiglio S, Lorusso V, Ippati S, Arrigoni G, Grassini G, et al. Next Generation Sequencing in Non-Small Cell Lung Cancer: Pitfalls and Opportunities. Diagnostics. 2020; 10(12):1092. https://doi.org/10.3390/diagnostics10121092
Chicago/Turabian StyleLazzari, Chiara, Alessandra Bulotta, Maria Giulia Cangi, Gabriele Bucci, Lorenza Pecciarini, Silvia Bonfiglio, Vincenza Lorusso, Stefania Ippati, Gianluigi Arrigoni, Greta Grassini, and et al. 2020. "Next Generation Sequencing in Non-Small Cell Lung Cancer: Pitfalls and Opportunities" Diagnostics 10, no. 12: 1092. https://doi.org/10.3390/diagnostics10121092
APA StyleLazzari, C., Bulotta, A., Cangi, M. G., Bucci, G., Pecciarini, L., Bonfiglio, S., Lorusso, V., Ippati, S., Arrigoni, G., Grassini, G., Doglioni, C., & Gregorc, V. (2020). Next Generation Sequencing in Non-Small Cell Lung Cancer: Pitfalls and Opportunities. Diagnostics, 10(12), 1092. https://doi.org/10.3390/diagnostics10121092






