Endoscopic Ultrasonography Diagnosis of Early Pancreatic Cancer †
Abstract
1. Introduction
2. Material and Methods
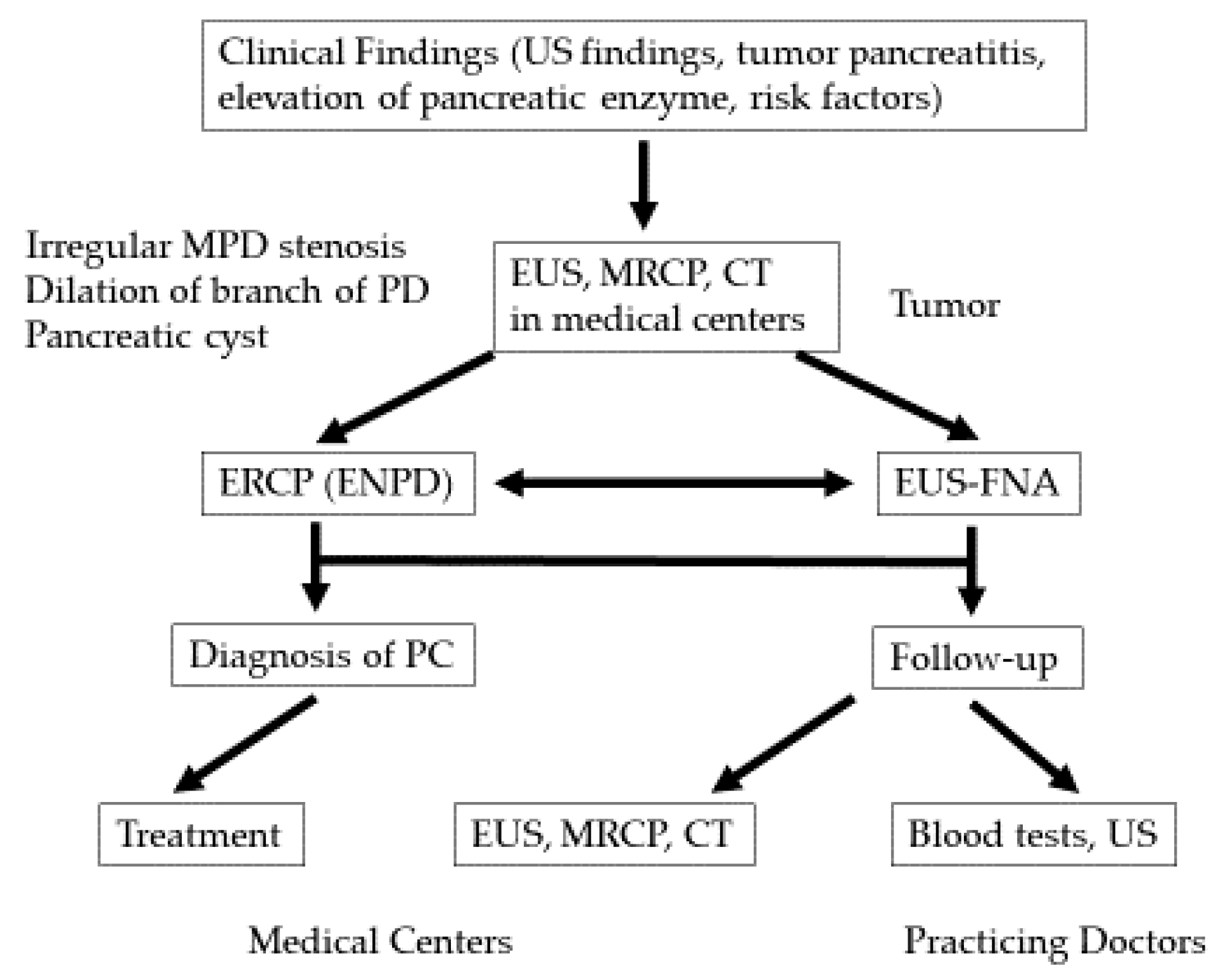
2.1. Early Diagnosis Initiative for PC (the Onomichi Project)
2.2. Patient Investigations
2.3. Risk Factors for PC
3. Results
3.1. The Clinical Characteristics and Imaging Findings of Stage 0 and Stage I PC
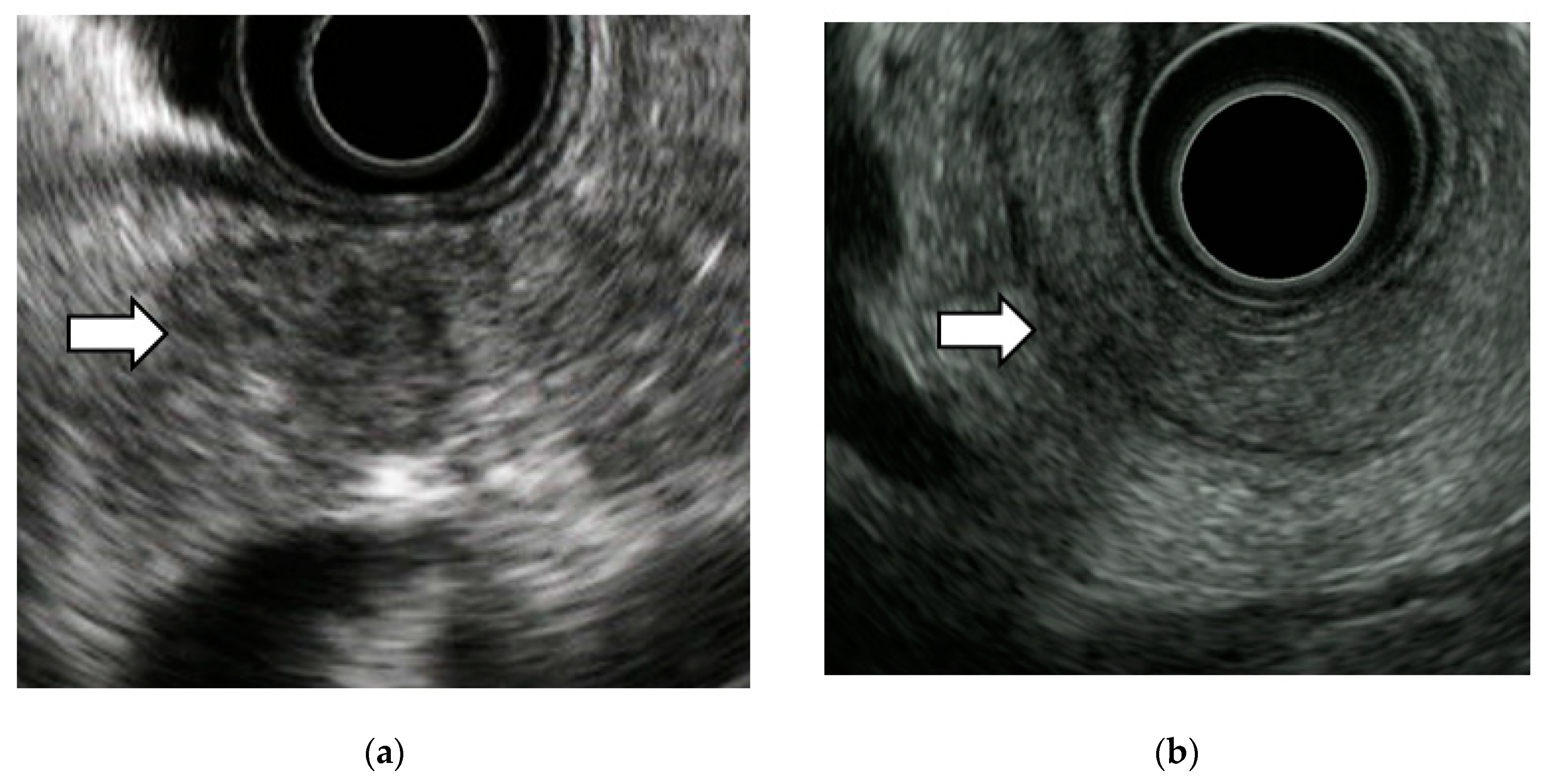
3.2. EUS Imaging Findings of Stage 0 PC
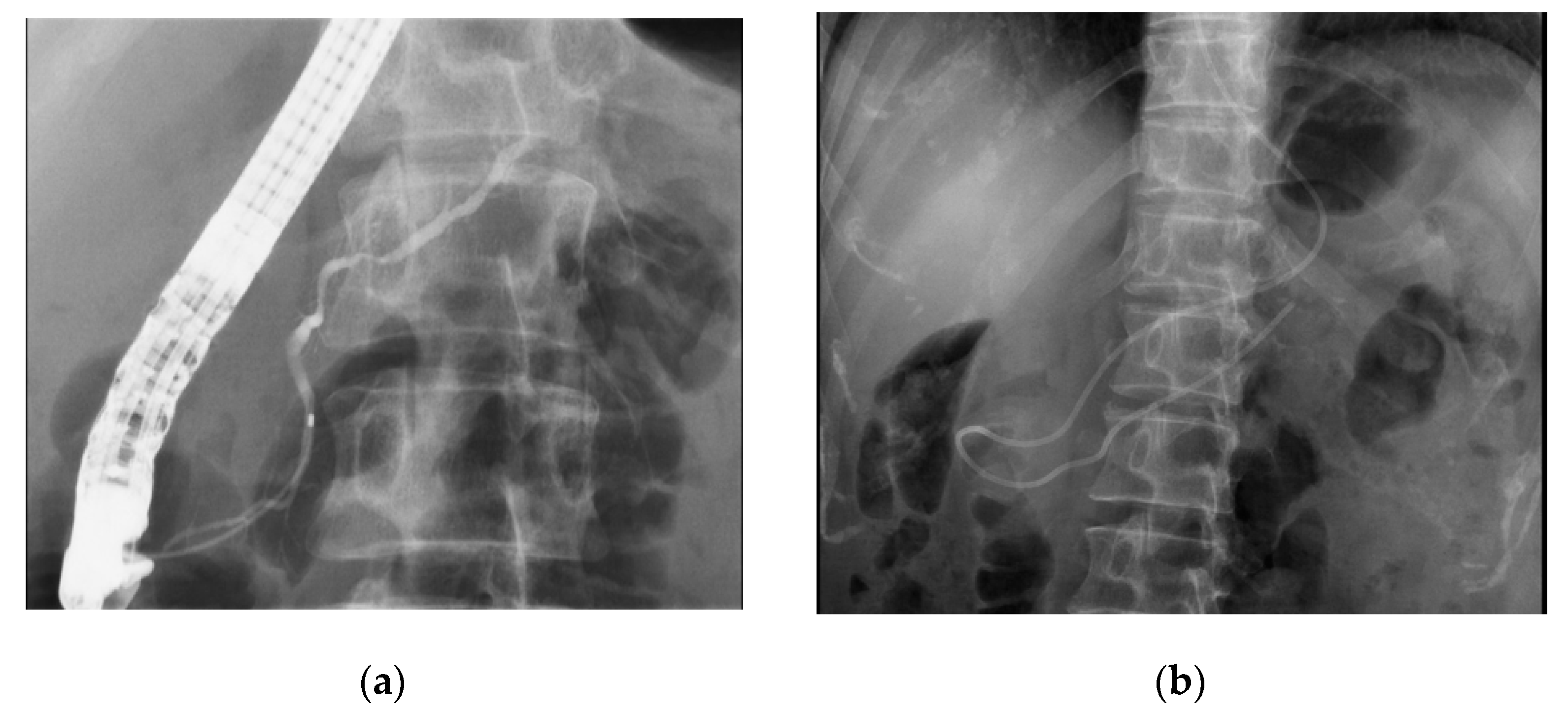
3.3. Diagnosis of Early-Stage PC
4. Discussion
4.1. The Utility of EUS in the Detection of Early-Stage PC
4.2. Diagnosis of Early-Stage PC
4.3. EUS Imaging Findings and Pathological Features of Stage 0 PC
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Vital Statistics Japan Reported by Ministry of Health, Labour and Welfare. Available online: https://ganjoho.jp/reg_stat/statistics/dl/ (accessed on 11 September 2020).
- Warshaw, A.L.; Castilo, C.F. Pancreatic carcinoma. N. Engl. J. Med. 1992, 326, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Rosewicz, S.; Wiedenmann, B. Pancreatic carcinoma. Lancet 1997, 349, 485–489. [Google Scholar] [CrossRef]
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. Japan pancreatic cancer registry; 30th year anniversary: Japan Pancreas Society. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Okazaki, A.; Hirano, N.; Izumi, Y.; Teraoka, Y.; Ikemoto, J.; Kanemitsu, K.; Hino, F.; Fukuda, T.; Yonehara, S. Diagnostic strategies for early pancreatic cancer. J. Gastroenterol. 2015, 50, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Okusaka, T.; Shimizu, K.; Furuse, J.; Ito, Y.; Hanada, K.; Shimosegawa, T.; Yamaguchi, K.; Okusaka, T.; Shimizu, K.; et al. EBM-based clinical guidelines for pancreatic cancer (2013) issued by the Japan Pancreatic Society: A synopsis. Jpn. J. Clin. Oncol. 2014, 10, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Kudo, M.; Maekawa, K.; Suetomi, Y.; Sakamoto, H.; Fukuta, N.; Nakaoka, R.; Kawasaki, T. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut 2004, 53, 854–859. [Google Scholar] [CrossRef]
- Okusaka, T.; Nakamura, M.; Yoshida, M.; Kitano, M.; Uesaka, K.; Ito, Y.; Furuse, J.; Hanada, K.; Okazaki, K. Clinical practice guidelines for pancreatic cancer 2019 from the Japan Pancreas Society. Pancreas 2020, 49, 326–335. [Google Scholar] [CrossRef]
- Kitano, M.; Yoshida, T.; Itonaga, M.; Tamura, T.; Hatamaru, K.; Yamashita, Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J. Gastroenterol. 2019, 54, 19–32. [Google Scholar] [CrossRef]
- Irisawa, A.; Sato, A.; Sato, M.; Ikeda, T.; Suzuki, R.; Ohira, H. Early diagnosis of small pancreatic cancer: Role of endoscopic ultrasonography. Dig. Endosc. 2009, 21, 92–96. [Google Scholar] [CrossRef]
- Müller, M.F.; Meyenberger, C.; Bertschinger, P.; Schaer, R.; Marincek, B. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology 1994, 190, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Maguchi, H. The roles of endoscopic ultrasonography in the diagnosis of pancreatic tumors. J. Hepatobiliary Pancreat Surg. 2004, 11, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Kitano, M.; Suetomi, Y.; Maekawa, K.; Takeyama, Y.; Kudo, M. Utility of Contrast-Enhanced Endoscopic Ultrasonography for Diagnosis of Small Pancreatic Carcinomas. Ultrasound Med. Biol. 2008, 34, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, B.; Abu-Hamda, E.; Molke, K.L.; Correa, A.M.; Ho, L. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am. J. Gastroenterol. 2004, 99, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Kudo, M.; Yamao, K.; Takagi, T.; Sakamoto, H.; Komaki, T.; Kamata, K.; Imai, H.; Chiba, Y.; Okada, M.; et al. Characterization of small solid tumors in the pancreas: The value of contrast-enhanced harmonic endoscopic ultrasonography. Am. J. Gastroenterol. 2012, 107, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology 2018, 18, 61–67. [Google Scholar] [CrossRef]
- Hewitt, M.J.; McPhail, M.J.; Possamai, L.; Dhar, A.; Vlavianos, P.; Monahan, K.J. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc. 2012, 75, 319–331. [Google Scholar] [CrossRef]
- Chen, J.; Yang, R.; Lu, Y.; Xia, Y.; Zhou, H. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: A systematic review. Cancer Res. Clin. Oncol. 2012, 138, 1433–1441. [Google Scholar] [CrossRef]
- Puli, S.R.; Kalva, N.; Bechtold, M.L.; Pamulaparthy, S.R.; Cashman, M.D.; Estes, N.C.; Pearl, R.H.; Volmar, F.H.; Dillon, S.; Shekleton, M.F.; et al. Diagnostic accuracy of endoscopic ultrasound in pancreatic neuroendocrine tumors: A systematic review and meta-analysis. World J. Gastroenterol. 2013, 19, 3678–3684. [Google Scholar] [CrossRef]
- Banafea, O.; Mghanga, F.P.; Zhao, J.; Zhao, R.; Zhu, L. Endoscopic ultrasonography with fine needle aspiration for histological diagnosis of solid pancreatic masses: A meta-analysis of diagnostic accuracy studies. BMC Gastroenterol. 2016, 16, 108. [Google Scholar] [CrossRef]
- Sugiura, R.; Kuwatani, M.; Hirata, K.; Sano, I.; Kato, S.; Kawakubo, K.; Sakamoto, N. Effect of pancreatic mass size on clinical outcomes of endoscopic ultrasound-guided fine-needle aspiration. Dig Dis Sci. 2019, 64, 2006–2013. [Google Scholar] [CrossRef]
- Uehara, H.; Ikezawa, K.; Kawada, N.; Fukutake, N.; Katayama, K.; Takakura, R.; Takano, Y.; Ishikawa, O.; Takenaka, A. Diagnostic accuracy of endoscopic malignancy in relation to the size of lesions. J. Gastroenterol. Hepatol. 2011, 26, 1256–1261. [Google Scholar] [CrossRef]
- Takagi, T.; Irisawa, A.; Sawaki, A.; Mizuno, N.; Hara, K.; Nakamura, T. Effectiveness of EUS-FNA for the diagnosis of small pancreatic cancer less than 10 mm. Tan Sui. 2009, 30, 361–367. [Google Scholar]
- Iiboshi, T.; Hanada, K.; Fukuda, T.; Yonehara, S.; Sasaki, T.; Chayama, K. Value of cytodiagnosis using endoscopic nasopancreatic drainage for early diagnosis of pancreatic cancer. Pancreas 2011, 23, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Minami, T.; Shimizu, A.; Fukuhara, M.; Yano, S.; Sasaki, K.; Koda, M.; Sugiyama, K.; Yonehara, S.; Yanagisawa, A. Roles of ERCP in the Early Diagnosis of Pancreatic Cancer. Diagnostics 2019, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Kitano, M.; Ashida, R. Value of endoscopy for early diagnosis of pancreatic carcinoma. Dig Endosc. 2020, 32, 27–36. [Google Scholar] [CrossRef]
- Kikuyama, M.; Kamisawa, T.; Kuruma, S.; Chiba, K.; Kawaguchi, S.; Terada, S.; Satoh, T. Early Diagnosis to Improve the Poor Prognosis of Pancreatic Cancer. Cancers 2018, 10, 48. [Google Scholar] [CrossRef]
- Izumi, Y.; Hanada, K.; Okazaki, A.; Minami, T.; Hirano, N.; Ikemoto, J.; Kanemitsu, K.; Nakadoi, K.; Shishido, T.; Katamura, Y.; et al. Endoscopic ultrasound findings and pathological features of pancreatic carcinoma in situ. Endosc. Int. Open. 2019, 7, E585–E593. [Google Scholar] [CrossRef]
- Kikuyama, M.; Hanada, K.; Ueki, T. Pancreatic carcinoma in situ presenting prominent fatty change of the pancreatic body on CT: Experiences from 3 cases. Suizo 2015, 30, 626–632, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Miyata, T.; Takenaka, M.; Omoto, S.; Kamata, K.; Minaga, K.; Yamao, K.; Imai, H.; Kudo, M. A case of pancreatic carcinoma in situ diagnosed by repeated pancreatic juice cytology. Oncology 2017, 93, 98–101. [Google Scholar] [CrossRef]

| Family history |
| Pancreatic cancer |
| Hereditary pancreatic cancer syndrome |
| Accompanying diseases |
| Diabetes mellitus |
| Obesity |
| Chronic pancreatitis |
| Hereditary pancreatitis |
| Intraductal papillary mucinous neoplasm |
| Pancreatic cyst |
| Habits |
| Tobacco use |
| Heavy drinking |
| All Cases (n = 64) | Stage 0 (n = 32) | Stage I (n = 32) | |
|---|---|---|---|
| Sex (male/female) | 33/31 | 19/13 | 14/18 |
| Age, mean (range) | 71.3 (38–87) | 71.3 (52–87) | 71.8 (39–84) |
| Observation period (year), median (range) | 3.9 (0.5–12.7) | 4.2 (1.7–12.7) | 3.4 (0.5–10.7) |
| Location, head/body/tail, n | 25/32/7 | 11/16/5 | 14/16/2 |
| 1 year survival rate (%) | 100 | 96.4 | |
| 5 year survival rate (%) | 78.9 | 66.7 |
| All Cases, n (%) | Stage 0, n (%) | Stage I, n (%) | ||
|---|---|---|---|---|
| EUS | 44/64 (68.8) | 22/32 (68.8) | 22/32 (68.8) | |
| MPD stenosis | 33/44 (75) | 16/22 (72.7) | 17/22 (77.3) | |
| MPD dilation | 33/44 (75) | 17/22 (77.3) | 16/22 (72.7) | |
| Tumor lesion | 28/44 (63.6) | 10/22 (45.5) | 18/22 (81.8) | |
| CT | 58/64 (90.6) | 31/32 (96.9) | 27/32 (84.4) | |
| MPD stenosis | 29/58 (50) | 14/31 (45.2) | 15/27 (55.6) | |
| MPD dilation | 37/58 (63.8) | 19/31 (61.3) | 18/27 (66.7) | |
| Tumor lesion | 18/58 (31) | 3/31 (9.7) | 15/27 (63) | |
| MRI | 54/64 (84.4) | 31/32 (96.9) | 23/32 (71.9) | |
| MPD stenosis | 33/54 (61.1) | 18/31 (58.1) | 15/23 (68.2) | |
| MPD dilation | 38/54 (70.4) | 20/31 (64.5) | 18/23 (78.3) | |
| Tumor lesion | 12/54 (22.2) | 3/31 (9.7) | 9/23 (39.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurihara, K.; Hanada, K.; Shimizu, A. Endoscopic Ultrasonography Diagnosis of Early Pancreatic Cancer. Diagnostics 2020, 10, 1086. https://doi.org/10.3390/diagnostics10121086
Kurihara K, Hanada K, Shimizu A. Endoscopic Ultrasonography Diagnosis of Early Pancreatic Cancer. Diagnostics. 2020; 10(12):1086. https://doi.org/10.3390/diagnostics10121086
Chicago/Turabian StyleKurihara, Keisuke, Keiji Hanada, and Akinori Shimizu. 2020. "Endoscopic Ultrasonography Diagnosis of Early Pancreatic Cancer" Diagnostics 10, no. 12: 1086. https://doi.org/10.3390/diagnostics10121086
APA StyleKurihara, K., Hanada, K., & Shimizu, A. (2020). Endoscopic Ultrasonography Diagnosis of Early Pancreatic Cancer. Diagnostics, 10(12), 1086. https://doi.org/10.3390/diagnostics10121086





