Comparing Absorbable and Nonabsorbable Suture Materials for Repair of Achilles Tendon Rupture: A Magnetic Resonance Imaging-Based Study
Abstract
1. Introduction
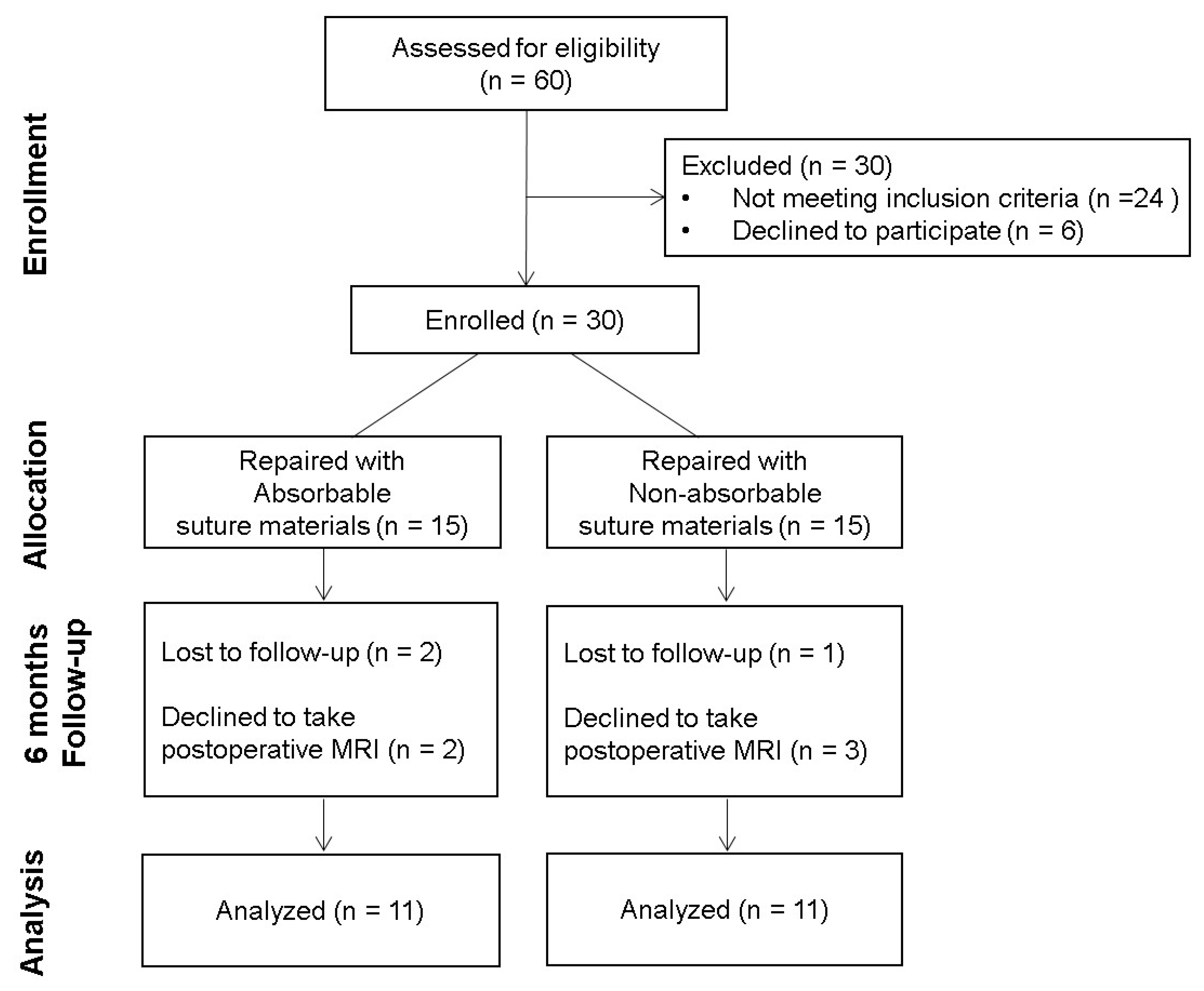
2. Materials and Methods
2.1. Patients
2.2. Operative Technique
2.3. Postoperative Management
2.4. Imaging Protocol
2.5. Assessment
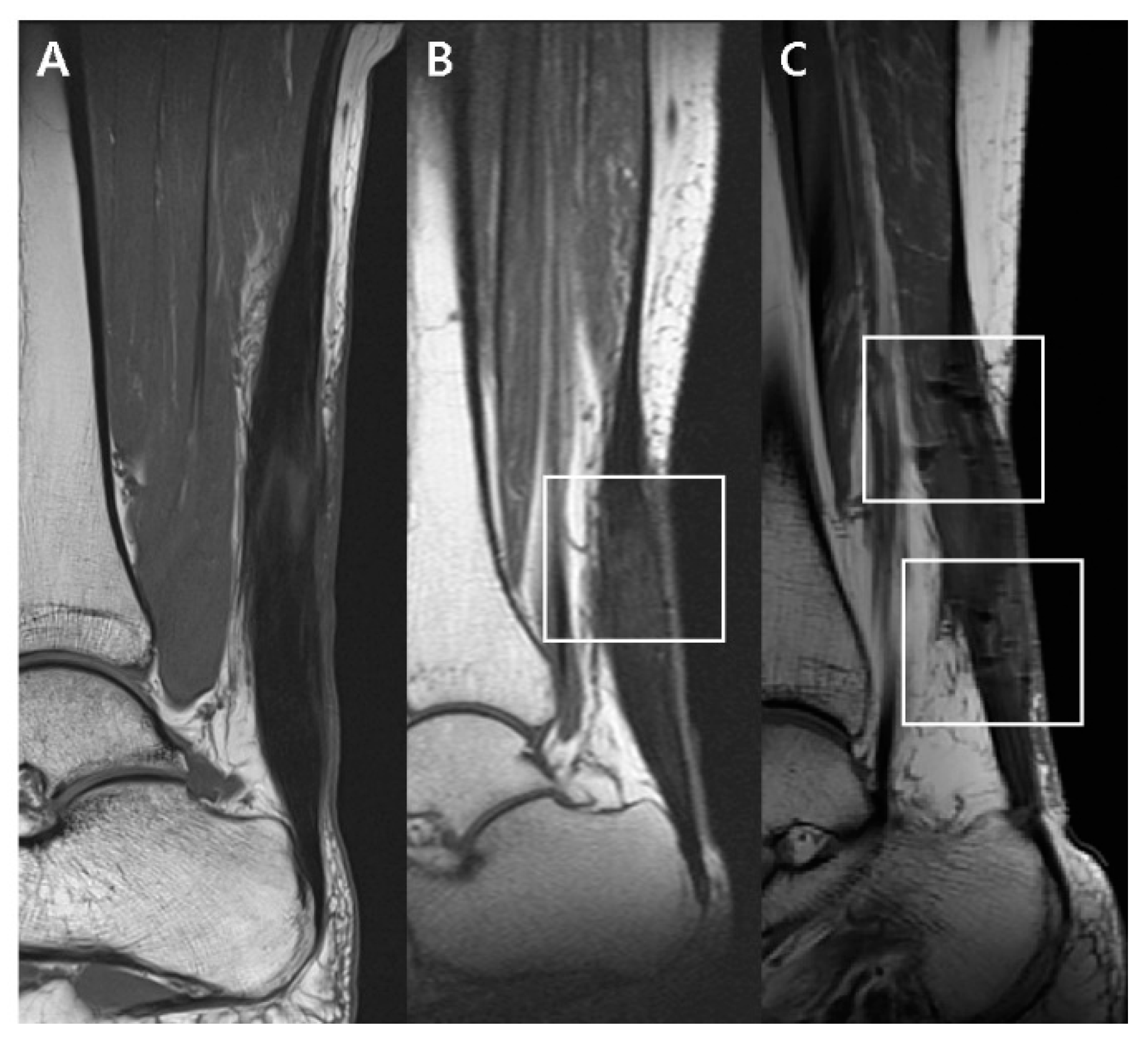
2.5.1. T1-Weighted Sagittal Image
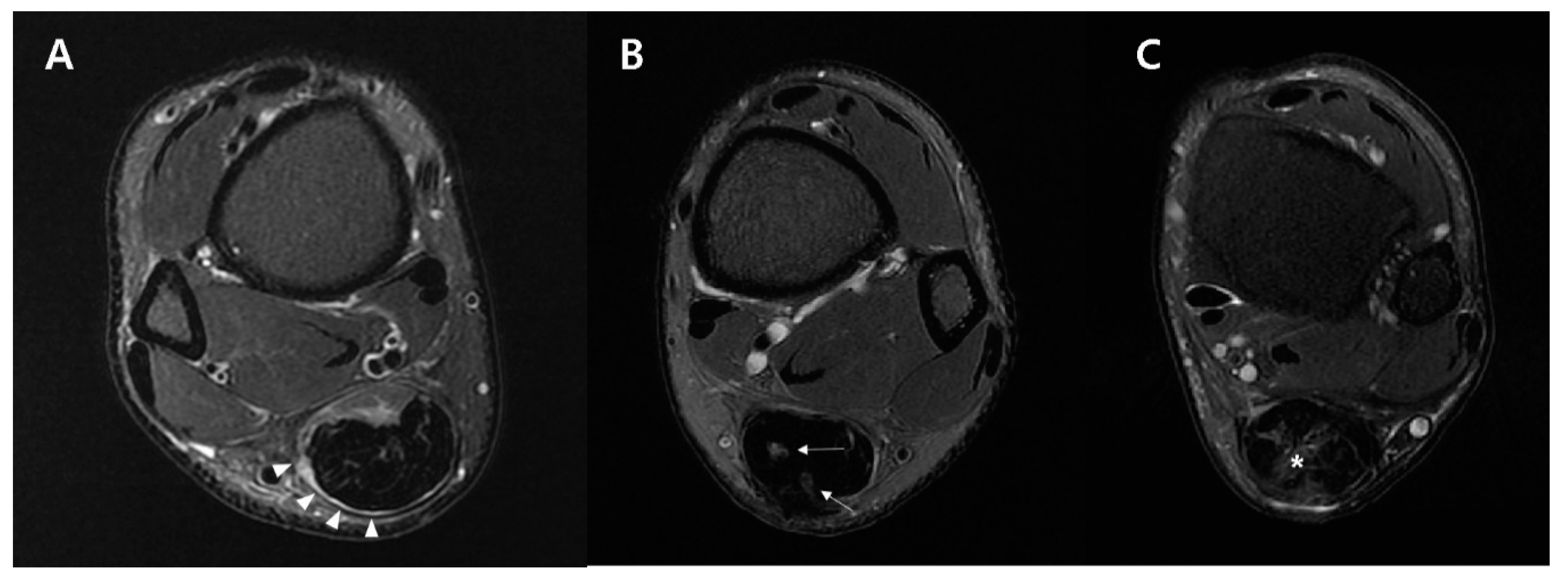
2.5.2. Fat-Saturated T2-Weighted Axial Image
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deng, S.; Sun, Z.; Zhang, C.; Chen, G.; Li, J. Surgical Treatment Versus Conservative Management for Acute Achilles Tendon Rupture: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Foot Ankle Surg. 2017, 56, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Movin, T.; Granhed, H.; Lind, K.; Faxén, E.; Karlsson, J. Acute rupture of tendon Achillis. A prospective randomised study of comparison between surgical and non-surgical treatment. J. Bone Joint Surg. 2001, 83, 843–848. [Google Scholar] [CrossRef]
- Barber, F.A.; Herbert, M.A.; Richards, D.P. Sutures and suture anchors: Update 2003. Arthroscopy 2003, 19, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.J.; Ochoa, L.; Rankin, D.; Owens, L.B.D. Biologic response to orthopedic sutures: A histologic study in a rabbit model. Orthopedics 2009, 32. [Google Scholar] [CrossRef]
- Baig, M.; Galbraith, J.G.; Yousaf, I.; Din, R. Absorbable Polydioxanone (PDS) Suture Provides Fewer Wound Complications than Polyester (Ethibond) Suture in Acute Tendo-Achilles Rupture Repair. Ir. Med. J. 2017. Available online: http://hdl.handle.net/10147/622537 (accessed on 4 September 2017).
- Kreszinger, M.; Kos, J.; Vuković, S.; Vnuk, D.; Matičić, D.; Pirkić, B.; Stejskal, M.; Pećin, M.; Smolec, O.; Kostešić, P. Influence of suture material on biomechanical and histological indicators of Achilles tendon heeling in rabbits. Veterinarski Arhiv 2011, 81, 223–233. [Google Scholar]
- Wichelhaus, D.A.; Beyersdoerfer, S.T.; Gierer, P.; Vollmar, B.; Mittlmeier, T. The effect of a collagen-elastin matrix on adhesion formation after flexor tendon repair in a rabbit model. Arch. Orthop. Trauma Surg. 2016, 136, 1021–1029. [Google Scholar] [CrossRef]
- Wada, A.; Kubota, H.; Akiyama, T.; Hatanaka, H.; Miura, H.; Iwamoto, Y. Effect of absorbable polydioxanone flexor tendon repair and restricted active mobilization in a canine model. J. Hand Surg. 2001, 26, 398–406. [Google Scholar] [CrossRef]
- Yildirim, Y.; Saygi, B.; Kara, H.; Cabukoğlu, C.; Esemenli, T. Tendon holding capacities of the suture materials used in repairing Achilles tendon rupture. Acta Orthop. Traumatol. Turc. 2006, 40, 164–168. [Google Scholar]
- Kocaoglu, B.; Ulku, T.K.; Gereli, A.; Karahan, M.; Turkmen, M. Evaluation of absorbable and nonabsorbable sutures for repair of achilles tendon rupture with a suture-guiding device. Foot Ankle Int. 2015, 36, 691–695. [Google Scholar] [CrossRef]
- Park, J.H.; Chun, D.-I.; Lee, S.H.; Cho, J.H. A Comparative Evaluation of Absorbable and Nonabsorbable Sutures for Open Repair of Achilles Tendon Rupture: A Pilot Study. Korean J. Phys. Anthropol. 2017, 30, 39–46. [Google Scholar] [CrossRef]
- Kitaoka, H.B.; Alexander, I.J.; Adelaar, R.S.; Nunley, J.A.; Myerson, M.S.; Sanders, M.; Lutter, L.D. Clinical Rating Systems for the Ankle-Hindfoot, Midfoot, Hallux, and Lesser Toes. Foot Ankle Int. 1997, 18, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, P.; Ahovuo, J.; Pihlajamäki, H.; Soila, K.; Aronen, H.J. Postoperative MR imaging and ultrasonography of surgically repaired Achilles tendon ruptures. Acta Radiol. 1996, 37, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, P.T.; Aronen, H.J.; Pihlajamäki, H.K.; Soila, K.; Paavonen, T.; Bostman, O.M. Magnetic resonance imaging during healing of surgically repaired Achilles tendon ruptures. Am. J. Sports Med. 1997, 25, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Thorpe, A.; Smith, F. Magnetic resonance imaging after operative repair of Achilles tendon rupture. Scand. J. Med. Sci. Sports 2001, 11, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Haramati, N.; Penrod, B.; Staron, R.B.; Barax, C.N. Surgical sutures: MR artifacts and sequence dependence. J. Magn. Reson. Imaging JMRI 1994, 4, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Park, J.S.; Ryu, K.N.; Rhee, Y.G.; Yoon, S.H.; Park, S.Y.; Jin, W. Repaired supraspinatus tendons in clinically improving patients: Early postoperative findings and interval changes on MRI. Korean J. Radiol. 2015, 16, 363–371. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Tendon injury and tendinopathy: Healing and repair. J. Bone Joint Surg. 2005, 87, 187–202. [Google Scholar]
- Manske, P.R.; Lesker, P.A. Biochemical evidence of flexor tendon participation in the repair process--an in vitro study. J. Hand Surg. (Edinb. Scotl.) 1984, 9, 117–120. [Google Scholar] [CrossRef]
- Gelberman, R.H.; Manske, P.R.; Vande Berg, J.S.; Lesker, P.A.; Akeson, W.H. Flexor tendon repair in vitro: A comparative histologic study of the rabbit, chicken, dog, and monkey. J. Orthop. Res. 1984, 2, 39–48. [Google Scholar] [CrossRef]
- Potenza, A.D. Tendon healing within the flexor digital sheath in the dog. J. Bone Joint Surg. 1962, 44-a, 49–64. [Google Scholar]
- Ackerman, J.E.; Studentsova, V.; Myers, M.; Buckley, M.R.; Richards, M.S.; Loiselle, A.E. Non-invasive ultrasound quantification of scar tissue volume identifies early functional changes during tendon healing. J. Orthop. Res. 2019, 37, 2476–2485. [Google Scholar] [CrossRef]
- Koob, T.J.; Summers, A.P. Tendon—Bridging the gap. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 905–909. [Google Scholar] [CrossRef]
- Koob, T.J. Biomimetic approaches to tendon repair. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 133, 1171–1192. [Google Scholar] [CrossRef]
- Whalen, W.P. Utilization of scar tissue in bridging tendon defects. Ann. Surg. 1951, 133, 567. [Google Scholar] [CrossRef] [PubMed]
- Tempfer, H.; Traweger, A. Tendon vasculature in health and disease. Front. Physiol. 2015, 6, 330. [Google Scholar] [CrossRef]
- Miyashita, H.; Ochi, M.; Ikuta, Y. Histological and biomechanical observations of the rabbit patellar tendon after removal of its central one-third. Arch. Orthop. Trauma Surg. 1997, 116, 454–462. [Google Scholar] [CrossRef]
- Fröberg, Å.; Cissé, A.-S.; Larsson, M.; Mårtensson, M.; Peolsson, M.; Movin, T.; Arndt, A. Altered patterns of displacement within the Achilles tendon following surgical repair. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1857–1865. [Google Scholar] [CrossRef]
- Nichols, A.E.C.; Best, K.T.; Loiselle, A.E. The cellular basis of fibrotic tendon healing: Challenges and opportunities. Transl. Res. J. Lab. Clin. Med. 2019, 209, 156–168. [Google Scholar] [CrossRef]
- Elliot, D.; Barbieri, C.H.; Evans, R.B.; Mass, D.; Tang, J.B. IFSSH Flexor Tendon Committee Report 2007. J. Hand Surg. 2007, 32, 346–356. [Google Scholar] [CrossRef]
- Brumann, M.; Baumbach, S.F.; Mutschler, W.; Polzer, H. Accelerated rehabilitation following Achilles tendon repair after acute rupture - Development of an evidence-based treatment protocol. Injury 2014, 45, 1782–1790. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Parks, W.C.; Rifkin, D.B.; Derwin, K.A. Mechanisms of tendon injury and repair. J. Orthop. Res. 2015, 33, 832–839. [Google Scholar] [CrossRef]
- Khan, K.M.; Tress, B.W.; Hare, W.S.C.; Wark, J.D. Treat the Patient, Not the X-ray: Advances in Diagnostic Imaging Do Not Replace the Need for Clinical Interpretation. Clin. J. Sport Med. 1998, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.; Aly, A. The Surgical Suture. Aesthet. Surg. J. 2019, 39, S67–S72. [Google Scholar] [CrossRef] [PubMed]
- Okoroha, K.; Ussef, N.; Jildeh, T.; Khalil, L.; Hasan, L.; Bench, C.; Zeni, F.; Eller, E.; Moutzouros, V. Comparison of Tendon Lengthening with Traditional vs. Accelerated Rehab Following Achilles Tendon Repair: A Prospective Randomized Controlled Trial. Am. J. Sports Med. 2020. [Google Scholar] [CrossRef]


| Sag T1 (1.5T) | Fat-Sat Sag T2 (1.5T) | Fat-Sat Ax T2 (1.5T) | Sag T1 (3T) | Fat-Sat Sag T2 (3T) | Fat-Sat Ax T2 (3T) | |
|---|---|---|---|---|---|---|
| Repetition time (msec) | 490 | 3000 | 4720 | 582 | 3489 | 5557 |
| Echo time (msec) | 12 | 81 | 97 | 7.30 | 78.4 | 82.76 |
| FOV (cm) | 220 | 220 | 160 | 200 | 200 | 140 |
| Matrix size | 384 × 176 | 384 × 204 | 320 × 163 | 416 × 288 | 416 × 288 | 384 × 256 |
| Slice thickness/interval (mm) | 3/0.3 | 3/0.3 | 5/1.5 | 3/0 | 3/0 | 4/0.8 |
| ETL | 1 | 9 | 11 | 4 | 14 | 12 |
| NEX | 2 | 2 | 3 | 2 | 3 | 3 |
| Absorbable (A) | Nonabsorbable (B) | |
|---|---|---|
| Number of cases | 11 | 11 |
| Age (years) | 41.73 ± 13.7 | 40.18 ± 10.26 |
| Gender | ||
| Male | 9 (81.82%) | 9 (81.82%) |
| Female | 2 (18.18%) | 2 (18.18%) |
| BMI (Kg/m2) | 26.11 ± 2.22 | 25.75 ± 3.16 |
| MRI Interval (Days) | 177 | 181 |
| Absorbable (A) | Nonabsorbable (B) | p-Value | |
|---|---|---|---|
| T1 sagittal image | |||
| Postop appearance | 0.0789 | ||
| Diffusely isointense thickened | 5 (45.45%) | 1 (9.1%) | |
| Diffusely elongated | 3 (27.27%) | 2 (18.2%) | |
| Focally fusiform | 3 (27.27%) | 8 (72.7%) | |
| Dark SI artifact | 0.0063 | ||
| None | 3 (27.27%) | 0 (0%) | |
| Mild | 8 (72.73%) | 5 (45.5%) | |
| Marked | 0 (0%) | 6 (54.5%) | |
| Fat-saturated T2 axial image | |||
| MTJ shape | 0.8204 | ||
| Convex | 4 (36.36%) | 6 (54.5%) | |
| Flat | 6 (54.55%) | 4 (36.4%) | |
| Concave | 1 (9.09%) | 1 (9.1%) | |
| Tendon signal changes | 0.7249 | ||
| Circumferential | 6 (54.55%) | 5 (45.4%) | |
| Focal | 3 (27.27%) | 3 (27.3%) | |
| Diffuse | 2 (18.18%) | 3 (27.3%) | |
| Postop circumferential area changes (times) | 2.43 ± 0.41 | 3.19 ± 0.51 | 0.0012 |
| Absorbable (A) | Nonabsorbable (B) | p-Value | |
|---|---|---|---|
| AOFAS ankle-hindfoot score | 92.27 ± 7.48 | 88.18 ± 8.58 | 0.2475 |
| Complications | |||
| Re-rupture, n | 1 | 1 | |
| Infection, n | 0 | 0 | |
| Foreign body reaction, n | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.; Kim, H.-J.; Lee, J.S.; Kim, J.; Won, S.H.; Yi, Y.; Chun, D.-I. Comparing Absorbable and Nonabsorbable Suture Materials for Repair of Achilles Tendon Rupture: A Magnetic Resonance Imaging-Based Study. Diagnostics 2020, 10, 1085. https://doi.org/10.3390/diagnostics10121085
Cho J, Kim H-J, Lee JS, Kim J, Won SH, Yi Y, Chun D-I. Comparing Absorbable and Nonabsorbable Suture Materials for Repair of Achilles Tendon Rupture: A Magnetic Resonance Imaging-Based Study. Diagnostics. 2020; 10(12):1085. https://doi.org/10.3390/diagnostics10121085
Chicago/Turabian StyleCho, Jaeho, Hyun-Joo Kim, Jeong Seok Lee, Jahyung Kim, Sung Hun Won, Young Yi, and Dong-Il Chun. 2020. "Comparing Absorbable and Nonabsorbable Suture Materials for Repair of Achilles Tendon Rupture: A Magnetic Resonance Imaging-Based Study" Diagnostics 10, no. 12: 1085. https://doi.org/10.3390/diagnostics10121085
APA StyleCho, J., Kim, H.-J., Lee, J. S., Kim, J., Won, S. H., Yi, Y., & Chun, D.-I. (2020). Comparing Absorbable and Nonabsorbable Suture Materials for Repair of Achilles Tendon Rupture: A Magnetic Resonance Imaging-Based Study. Diagnostics, 10(12), 1085. https://doi.org/10.3390/diagnostics10121085






