The Utility of Deep Learning in Breast Ultrasonic Imaging: A Review
Abstract
:1. Introduction
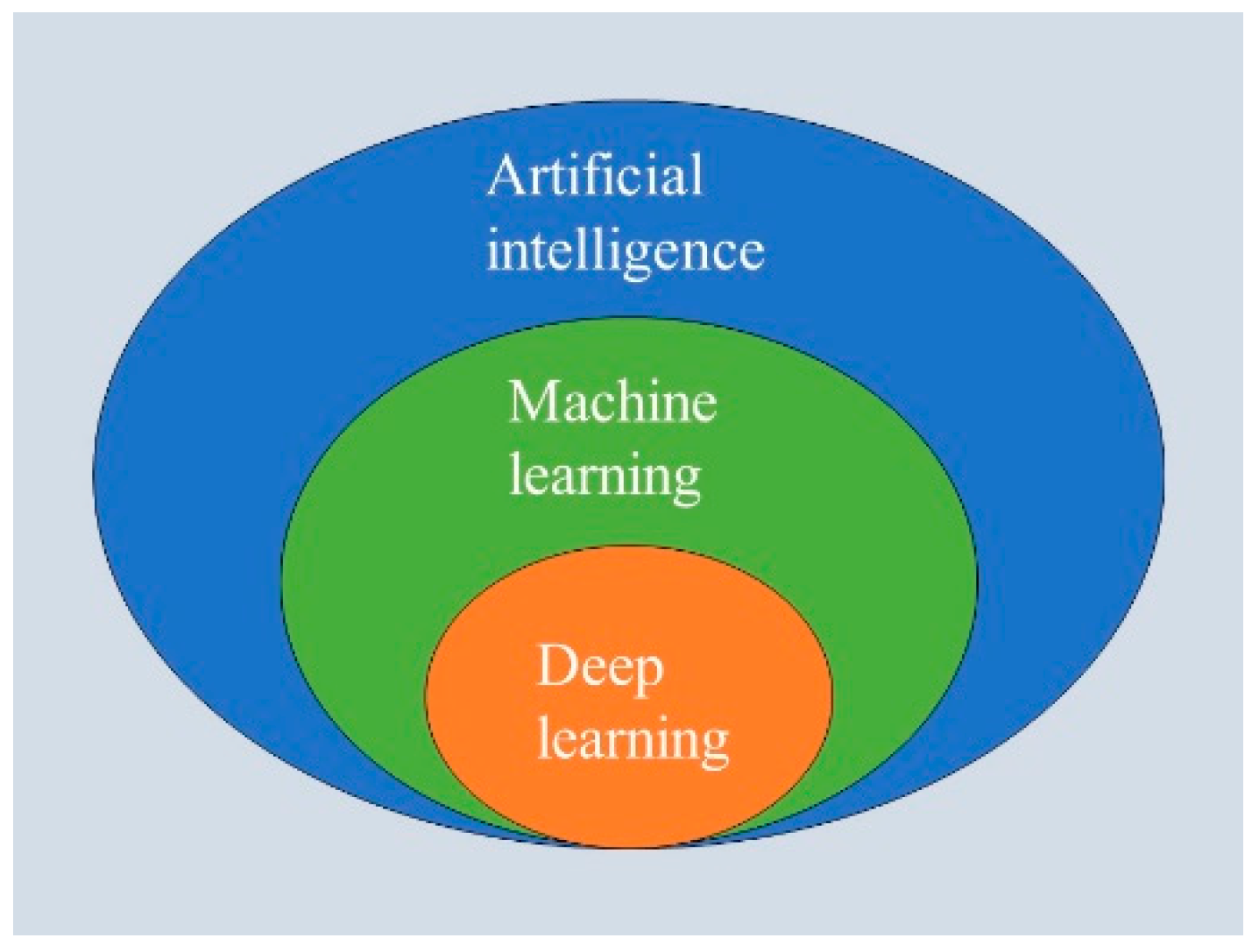
2. What Are AI, Machine Learning, and Deep Learning?
3. Development of AI Research on Breast Ultrasound
4. Image classification
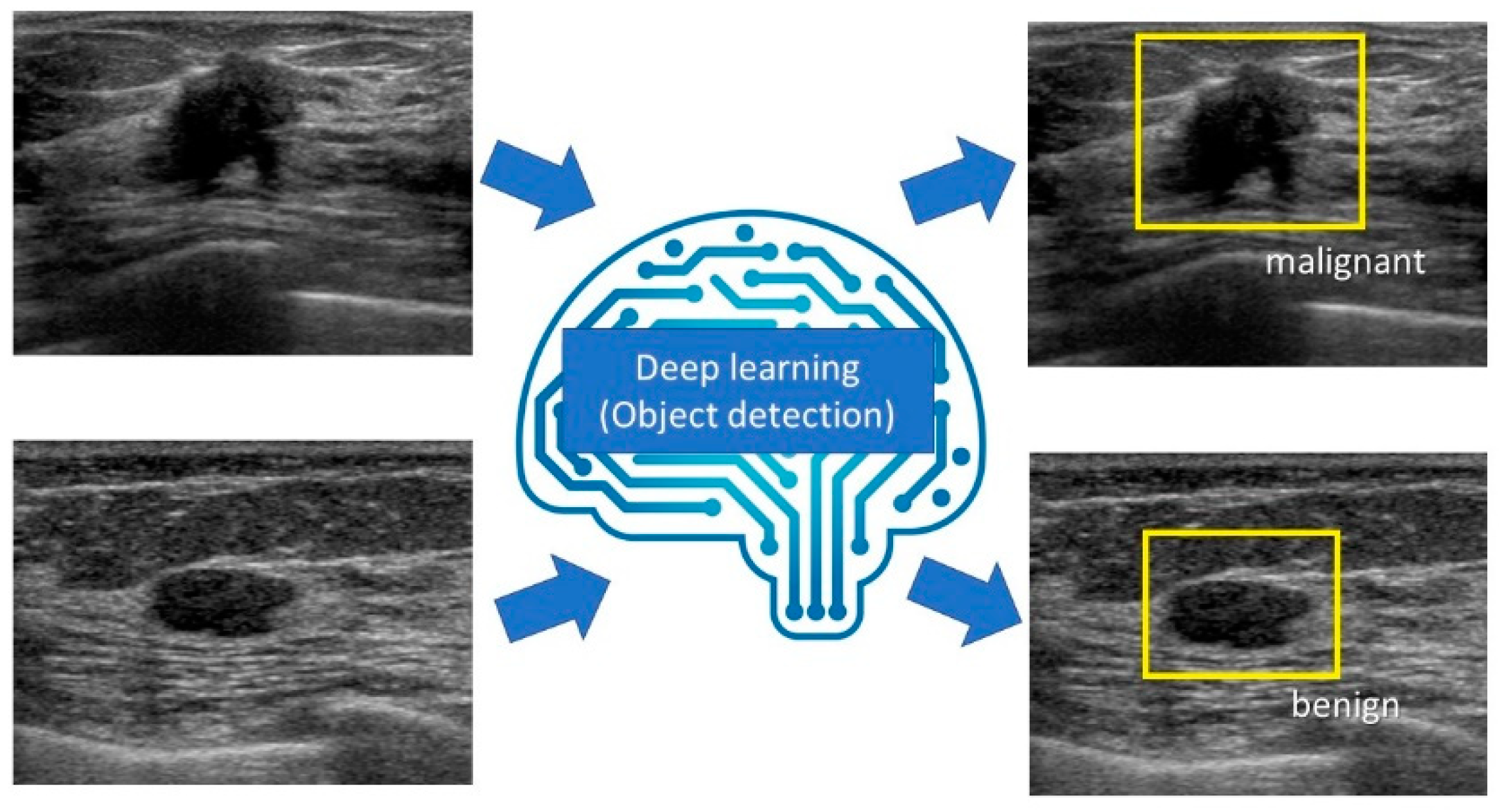
5. Object Detection
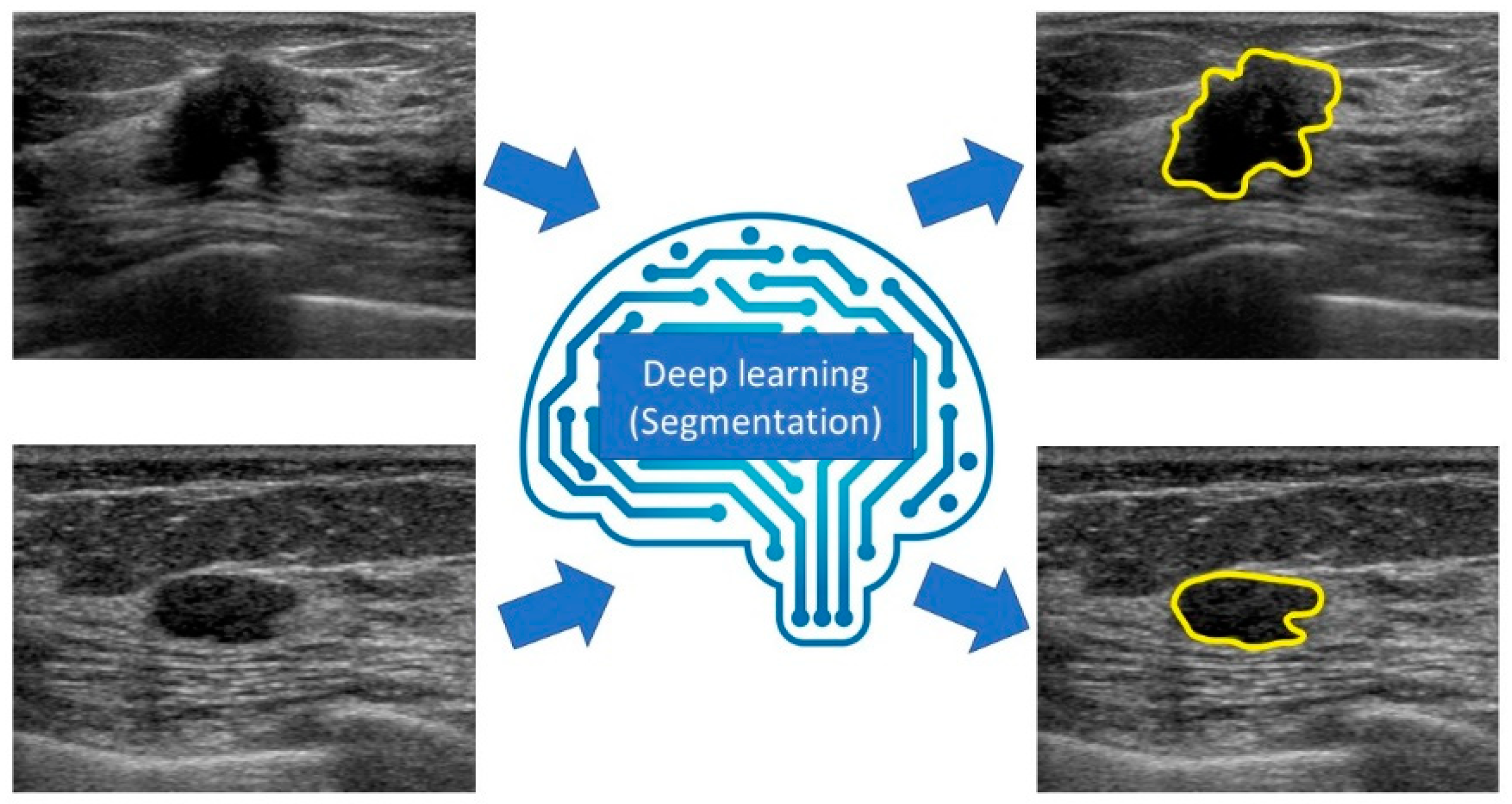
6. Segmentation
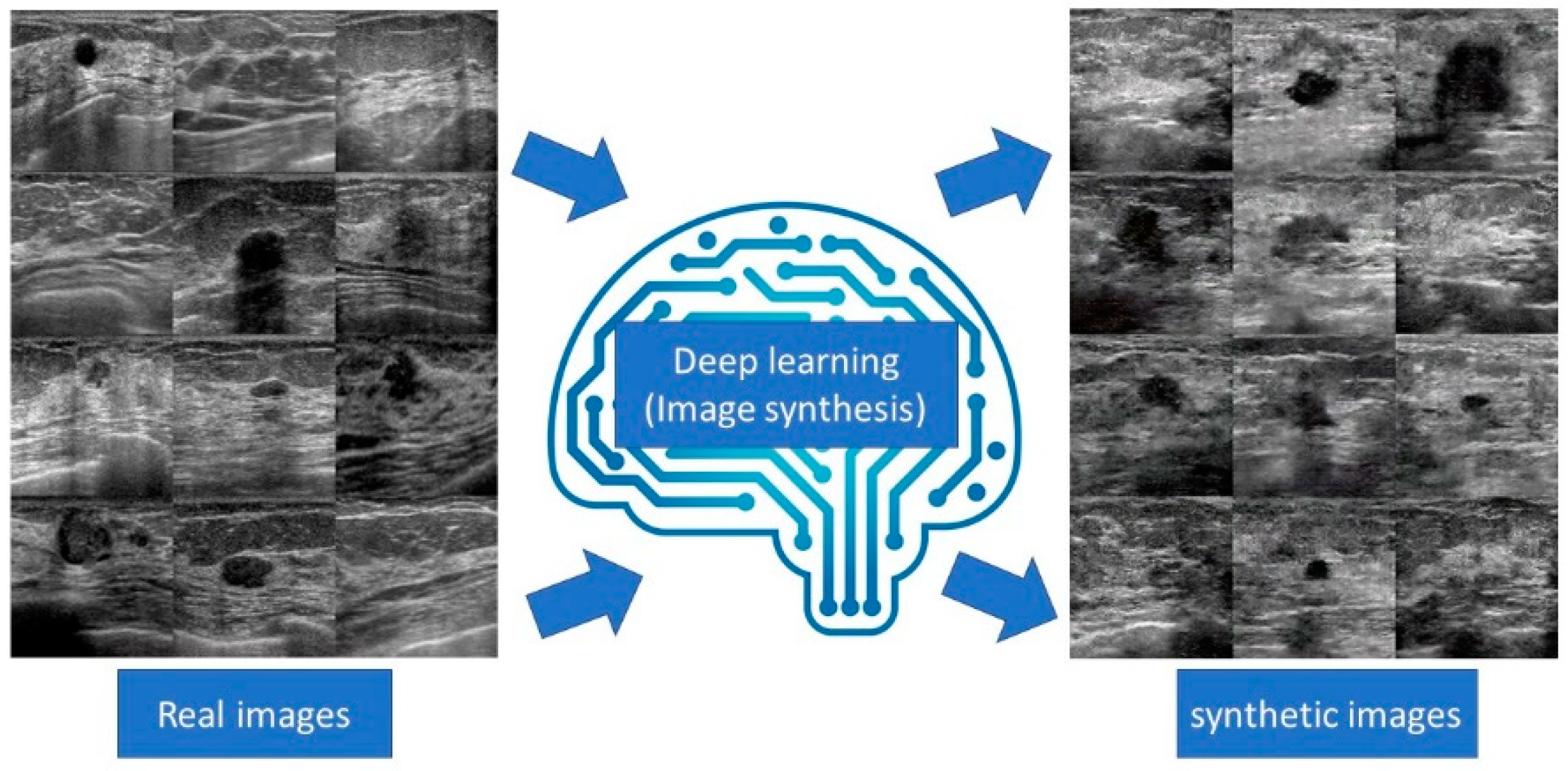
7. Image synthesis
8. Discussion
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.M.; Jemal, A. Cancer statistics, 2018. CA: A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Kornecki, A. Current Status of Breast Ultrasound. Can. Assoc. Radiol. J. 2011, 62, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohuchi, N.; Suzuki, A.; Sobue, T.; Kawai, M.; Yamamoto, S.; Zheng, Y.-F.; Shiono, Y.N.; Saito, H.; Kuriyama, S.; Tohno, E.; et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): A randomised controlled trial. Lancet 2016, 387, 341–348. [Google Scholar] [CrossRef]
- D’Orsi, C.; Sickles, E.; Mendelson, E.; Morris, E. ACR BIRADS® Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Mahmud, M.; Kaiser, M.; Hussain, A.; Vassanelli, S. Applications of deep learning and reinforcement learning to biological data. IEEE Trans. Neural Netw. Learn. Syst. 2018, 29, 2063–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, C.; Liu, F.; Tan, H.; Song, D.; Shu, W.; Li, W.; Zhou, Y.; Bo, X.; Xie, Z. Deep Learning and Its Applications in Biomedicine. Genom. Proteom. Bioinform. 2018, 16, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Ravi, D.; Wong, C.; Deligianni, F.; Berthelot, M.; Andreu-Perez, J.; Lo, B.; Yang, G.-Z. Deep Learning for Health Informatics. IEEE J. Biomed. Heal. Inform. 2017, 21, 4–21. [Google Scholar] [CrossRef] [Green Version]
- Zemouri, R.; Zerhouni, N.; Racoceanu, D. Deep learning in the biomedical applications: Recent and suture status. Appl. Sci. 2019, 9, 1526. [Google Scholar] [CrossRef] [Green Version]
- Lakhani, P.; Sundaram, B. Deep Learning at Chest Radiography: Automated Classification of Pulmonary Tuberculosis by Using Convolutional Neural Networks. Radiology 2017, 284, 574–582. [Google Scholar] [CrossRef]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.; van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [Green Version]
- Akkus, Z.; Galimzianova, A.; Hoogi, A.; Rubin, D.L.; Erickson, B.J. Deep Learning for Brain MRI Segmentation: State of the Art and Future Directions. J. Digit. Imaging 2017, 30, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Geras, K.J.; Mann, R.M.; Moy, L. Artificial Intelligence for Mammography and Digital Breast Tomosynthesis: Current Concepts and Future Perspectives. Radiology 2019, 293, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Le, E.; Wang, Y.; Huang, Y.; Hickman, S.; Gilbert, F. Artificial intelligence in breast imaging. Clin. Radiol. 2019, 74, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-G.; Zhou, L.-Q.; Xu, J.W.; Wang, J.-Y.; Wei, Q.; Deng, Y.-B.; Cui, X.-W.; Dietrich, C.F. Artificial intelligence in breast ultrasound. World J. Radiol. 2019, 11, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Sheth, D.; Giger, M.L. Artificial intelligence in the interpretation of breast cancer on MRI. J. Magn. Reson. Imaging 2020, 51, 1310–1324. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Fujioka, T.; Mori, M.; Kubota, K.; Kikuchi, Y.; Wu, X.T.; Oyama, J.; Kimura, K.; Oda, G.; Nakagawa, T.; et al. Detection and Diagnosis of Breast Cancer Using Artificial Intelligence Based Assessment of Maximum Intensity Projection Dynamic Contrast-Enhanced Magnetic Resonance Images. Diagnostics 2020, 10, 330. [Google Scholar] [CrossRef]
- Mori, M.; Fujioka, T.; Katsuta, L.; Kikuchi, Y.; Oda, G.; Nakagawa, T.; Kitazume, Y.; Kubota, K.; Tateishi, U. Feasibility of new fat suppression for breast MRI using pix2pix. Jpn. J. Radiol 2020, 10, 1075–1081. [Google Scholar] [CrossRef]
- Pouliakis, A.; Karakitsou, E.; Margari, N.; Bountris, P.; Haritou, M.; Panayiotides, J.; Koutsouris, D.; Karakitsos, P. Artificial Neural Networks as Decision Support Tools in Cytopathology: Past, Present, and Future. Biomed. Eng. Comput. Biol. 2016, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Janowczyk, A.; Madabhushi, A. Deep learning for digital pathology image analysis: A comprehensive tutorial with selected use cases. J. Pathol. Inform. 2016, 7, 29. [Google Scholar] [CrossRef]
- Baltres, A.; Al-Masry, Z.; R., R.M.Z.; Valmary-Degano, S.; Arnould, L.; Zerhouni, N.; Devalland, C. Prediction of Oncotype DX recurrence score using deep multi-layer perceptrons in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer 2020, 27, 1007–1016. [Google Scholar] [CrossRef]
- Zemouri, R.; Omri, N.; Morello, B.C.; Devalland, C.; Arnould, L.; Zerhouni, N.; Fnaiech, F. Constructive Deep Neural Network for Breast Cancer Diagnosis. IFAC-PapersOnLine 2018, 51, 98–103. [Google Scholar] [CrossRef]
- Chartrand, G.; Cheng, P.M.; Vorontsov, E.; Drozdzal, M.; Turcotte, S.; Pal, C.J.; Kadoury, S.; Tang, A. Deep Learning: A Primer for Radiologists. Radiographics 2017, 37, 2113–2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesapane, F.; Codari, M.; Sardanelli, F. Artificial intelligence in medical imaging: Threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur. Radiol. Exp. 2018, 2, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Yasaka, K.; Akai, H.; Kunimatsu, A.; Kiryu, S.; Abe, O. Deep learning with convolutional neural network in radiology. Jpn. J. Radiol. 2018, 36, 257–272. [Google Scholar] [CrossRef]
- Angermueller, C.; Pärnamaa, T.; Parts, L.; Stegle, O. Deep learning for computational biology. Mol. Syst. Biol. 2016, 12, 878. [Google Scholar] [CrossRef] [PubMed]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. Commun. ACM 2017, 60, 84–90. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Szegedy, C.; Liu, W.; Jia, Y.; Sermanet, P.; Reed, S.; Anguelov, D.; Erhan, D.; Vanhoucke, V.; Rabinovich, A. Going deeper with convolutions. In Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015; pp. 1–9. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Huang, G.; Liu, Z.; van der Maaten, L.; Weinberger, K.Q. Densely Connected Convolutional Networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; Volume 2017, pp. 2261–2269. [Google Scholar]
- Han, S.; Kang, H.-K.; Jeong, J.-Y.; Park, M.-H.; Kim, W.; Bang, W.-C.; Seong, Y.-K. A deep learning framework for supporting the classification of breast lesions in ultrasound images. Phys. Med. Biol. 2017, 62, 7714–7728. [Google Scholar] [CrossRef]
- Fujioka, T.; Kubota, K.; Mori, M.; Kikuchi, Y.; Katsuta, L.; Kasahara, M.; Oda, G.; Ishiba, T.; Nakagawa, T.; Tateishi, U. Distinction between benign and malignant breast masses at breast ultrasound using deep learning method with convolutional neural network. Jpn. J. Radiol. 2019, 37, 466–472. [Google Scholar] [CrossRef]
- Mango, V.L.; Sun, M.; Wynn, R.T.; Ha, R. Should We Ignore, Follow, or Biopsy? Impact of Artificial Intelligence Decision Support on Breast Ultrasound Lesion Assessment. Am. J. Roentgenol. 2020, 214, 1445–1452. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, Y.; Dai, W.; Suo, J.; Wang, C.; Shi, J.; Zheng, H. Deep learning based classification of breast tumors with shear-wave elastography. Ultrasonics 2016, 72, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Katsuta, L.; Kubota, K.; Mori, M.; Kikuchi, Y.; Kato, A.; Oda, G.; Nakagawa, T.; Kitazume, Y.; Tateishi, U. Classification of Breast Masses on Ultrasound Shear Wave Elastography using Convolutional Neural Networks. Ultrason Imaging 2020, 42, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Coronado-Gutiérrez, D.; Santamaría, G.; Ganau, S.; Bargalló, X.; Orlando, S.; Oliva-Brañas, M.E.; Perez-Moreno, A.; Burgos-Artizzu, X.P. Quantitative Ultrasound Image Analysis of Axillary Lymph Nodes to Diagnose Metastatic Involvement in Breast Cancer. Ultrasound Med. Biol. 2019, 45, 2932–2941. [Google Scholar] [CrossRef] [PubMed]
- Girshick, R.; Donahue, J.; Darrell, T.; Malik, J. Rich Feature Hierarchies for Accurate Object Detection and Semantic Segmentation. In Proceedings of the 2014 IEEE Conference on Computer Vision and Pattern Recognition, Columbus, OH, USA, 23–28 June 2014; pp. 580–587. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Spatial pyramid pooling in deep convolutional networks for visual recognition. IEEE Trans. Pattern Anal. Mach. Intell. 2015, 37, 1904–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girshick, R. Fast R-CNN. In Proceedings of the 2015 IEEE International Conference on Computer Vision (ICCV), Santiago, Chile, 7–13 December 2015; pp. 1440–1448. [Google Scholar]
- Ren, S.; He, K.; Girshick, R.; Sun, J. Faster r-cnn: Towards real-time object detection with region proposal networks. In Advances in Neural Information Processing Systems; Neural Information Processing Systems Foundation Inc.: San Diego, CA, USA, 2015; pp. 91–99. [Google Scholar]
- Redmon, J.; Divvala, S.; Girshick, R.; Farhadi, A. You only look once: Unified, real-time object detection. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 779–788. [Google Scholar]
- Liu, W.; Anguelov, D.; Erhan, D.; Szegedy, C.; Reed, S.; Fu, C.Y.; Berg, A.C. Ssd: Single shot multibox detector. In European Conference on Computer Vision; Springer: Cham, Switzerland, 2016; pp. 21–37. [Google Scholar]
- Lin, T.Y.; Dollár, P.; Girshick, R.; He, K.; Hariharan, B.; Belongie, S. Feature Pyramid Networks for Object Detection. arXiv 2016, arXiv:1612.03144. [Google Scholar]
- Lin, T.Y.; Goyal, P.; Girshick, R.; He, K.; Dollár, P. Focal loss for dense object detection. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017; pp. 2980–2988. [Google Scholar]
- Cao, Z.; Duan, L.; Yang, G.; Yue, T.; Chen, Q. An experimental study on breast lesion detection and classification from ultrasound images using deep learning architectures. BMC Med. Imaging 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Inciardi, M.F.; Edwards, A.V.; Papaioannou, J. Interpretation Time Using a Concurrent-Read Computer-Aided Detection System for Automated Breast Ultrasound in Breast Cancer Screening of Women with Dense Breast Tissue. Am. J. Roentgenol. 2018, 211, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gao, X.; Liu, L.; Shu, R.; Yan, J.; Zhang, G.; Xiao, Y.; Ju, Y.; Zhao, N.; Song, H. Performance and Reading Time of Automated Breast US with or without Computer-aided Detection. Radiology 2019, 292, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Bao, L.; Tan, Y.; Zhu, L.; Kong, F.; Wang, W. 1000-Case Reader Study of Radiologists’ Performance in Interpretation of Automated Breast Volume Scanner Images with a Computer-Aided Detection System. Ultrasound Med. Biol. 2018, 44, 1694–1702. [Google Scholar] [CrossRef]
- Long, J.; Shelhamer, E.; Darrell, T. Fully convolutional networks for semantic segmentation. In Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015. [Google Scholar]
- Badrinarayanan, V.; Kendall, A.; Cipolla, R. SegNet: A Deep Convolutional Encoder-Decoder Architecture for Image Segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 2481–2495. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI), Munich, Germany, 5–9 October 2015; Springer: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar]
- Zhuang, Z.; Li, N.; Raj, A.N.J.; Mahesh, V.G.V.; Qiu, S. An RDAU-NET model for lesion segmentation in breast ultrasound images. PLoS ONE 2019, 14, e0221535. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Guo, Y.; Wang, Y.; Yu, J.; Li, J.; Zhou, S.; Chang, C. Automatic tumor segmentation in breast ultrasound images using a dilated fully convolutional network combined with an active contour model. Med. Phys. 2019, 46, 215–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Webb, J.M.; Gregory, A.; Denis, M.; Meixner, D.D.; Bayat, M.; Whaley, D.H.; Fatemi, M.; Alizad, A. Automated and real-time segmentation of suspicious breast masses using convolutional neural network. PLoS ONE 2018, 13, e0195816. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, I.J.; Pouget-Abadie, J.; Mirza, M.; Xu, B.; Warde-Farley, D.; Ozair, S.; Courville, A.; Bengio, Y. Generative adversarial networks. arXiv 2014, arXiv:1406.2661. [Google Scholar] [CrossRef]
- Zemouri, R. Semi-Supervised Adversarial Variational Autoencoder. Mach. Learn. Knowl. Extr. 2020, 2, 361–378. [Google Scholar] [CrossRef]
- Yi, X.; Walia, E.; Babyn, P. Generative adversarial network in medical imaging: A review. Med. Image Anal. 2019, 58, 101552. [Google Scholar] [CrossRef] [Green Version]
- Fujioka, T.; Mori, M.; Kubota, K.; Kikuchi, Y.; Katsuta, L.; Adachi, M.; Oda, G.; Nakagawa, T.; Kitazume, Y.; Tateishi, U. Breast Ultrasound Image Synthesis using Deep Convolutional Generative Adversarial Networks. Diagnostics 2019, 9, 176. [Google Scholar] [CrossRef] [Green Version]
- Fujioka, T.; Kubota, K.; Mori, M.; Katsuta, L.; Kikuchi, Y.; Kimura, K.; Kimura, M.; Adachi, M.; Oda, G.; Nakagawa, T.; et al. Virtual Interpolation Images of Tumor Development and Growth on Breast Ultrasound Image Synthesis with Deep Convolutional Generative Adversarial Networks. J. Ultrasound Med. 2020. [Google Scholar] [CrossRef]
- Fujioka, T.; Kubota, K.; Mori, M.; Kikuchi, Y.; Katsuta, L.; Kimura, M.; Yamaga, E.; Adachi, M.; Oda, G.; Nakagawa, T.; et al. Efficient Anomaly Detection with Generative Adversarial Network for Breast Ultrasound Imaging. Diagnostics 2020, 10, 456. [Google Scholar] [CrossRef]
- Han, L.; Huang, Y.; Dou, H.; Wang, S.; Ahamad, S.; Luo, H.; Liu, Q.; Fan, J.; Zhang, J. Semi-supervised segmentation of lesion from breast ultrasound images with attentional generative adversarial network. Comput. Methods Programs Biomed. 2020, 189, 105275. [Google Scholar] [CrossRef]
- Benjamens, S.; Dhunnoo, P.; Meskó, B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: An online database. NPJ Digit. Med. 2020, 3. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Yashima, Y.; Oyama, J.; Mori, M.; Kubota, K.; Katsuta, L.; Kimura, K.; Yamaga, E.; Oda, G.; Nakagawa, T.; et al. Deep-learning approach with convolutional neural network for classification of maximum intensity projections of dynamic contrast-enhanced breast magnetic resonance imaging. Magn. Reson. Imaging 2021, 75. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-CAM: Visual Explanations from Deep Networks via Gradient-Based Localization. In Proceedings of the 2017 IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017; pp. 618–626. [Google Scholar]



| Purpose (Type of Image) | Model | Number of Training Set Images | Number of Test Set Images | Result | Study |
|---|---|---|---|---|---|
| Breast lesions (B-mode images) | GoogLeNet | 6579 | 829 | Sensitivity: 86% Specificity: 96% Accuracy: 90% AUC: 0.9 | [32] |
| Breast lesions (B-mode image) | GoogLeNet Inception v2 | 937 | 120 | Sensitivity: 95.8% Specificity: 87.5% Accuracy: 92.5% AUC: 0.913 | [33] |
| Breast lesions via CAD (B-mode image) | Koios DS | Over 400,000 | 900 | AUC without CAD: 0.83 AUC with CAD: 0.87 | [34] |
| Breast lesions (SWE image) | PGBM and RBM | 227 | Five-fold cross-validation | Sensitivity: 88.6% Specificity: 97.1% Accuracy: 93.4% AUC: 0.947 | [35] |
| Breast lesions (SWE image) | DenseNet 169 | 304 | 73 | Sensitivity: 85.7% Specificity: 78.9% AUC: 0.898 | [36] |
| Axillary lymph nodes (B-mode image) | VGG-M model | 118 | Five-fold cross-validation | Sensitivity: 84.9% Specificity: 87.7% Accuracy: 86.4% AUC: 0.937 | [37] |
| Purpose (Type of Image) | Model | Number of Training Set Images | Number of Test Set Images | Result | Study |
|---|---|---|---|---|---|
| Object detection of breast lesions (B-mode image) | SSD300 | 860 | 183 | Precision rate: 96.89% Recall rate: 67.23% F1 score: 79.38% | [46] |
| Object detection of breast lesions by CAD (ABUS image) | QVCAD | Over 20,000 | 185 | AUC without CAD: 0.828 AUC with CAD: 0.848 | [47] |
| Object detection of breast lesions by CAD (ABUS image) | QVCAD | Over 20,000 | 1485 | AUC without CAD: 0.88 AUC with CAD: 0.91 Sensitivity without CAD: 67% Sensitivity with CAD: 88% | [48] |
| Object detection of breast lesions by CAD (ABUS image) | QVCAD | Over 20,000 | 1000 | AUC without CAD: 0.747 AUC with CAD: 0.784 | [49] |
| Purpose (Type of image) | Model | Number of Training Set Images | Number of Test Set Images | Result | Study |
|---|---|---|---|---|---|
| Segmentation of breast lesions (B-mode image) | RDAU-NET | 857 | 205 | Precision rate: 88.58% Recall rate: 83.19% F1 score: 84.78 | [53] |
| Segmentation of breast lesions (B-mode image) | Combining DFCN with a PBAC model | 400 | 170 | Dice similarity coefficient: 88.97% Hausdorff distance: 35.54 pixels Mean absolute deviation: 7.67 pixels | [54] |
| Segmentation of breast lesions (B-mode image) | Multi U-net algorithm | 372 | 61 | Mean Dice coefficient: 0.82 True positive fraction: 0.84 False positive fraction: 0.01 | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujioka, T.; Mori, M.; Kubota, K.; Oyama, J.; Yamaga, E.; Yashima, Y.; Katsuta, L.; Nomura, K.; Nara, M.; Oda, G.; et al. The Utility of Deep Learning in Breast Ultrasonic Imaging: A Review. Diagnostics 2020, 10, 1055. https://doi.org/10.3390/diagnostics10121055
Fujioka T, Mori M, Kubota K, Oyama J, Yamaga E, Yashima Y, Katsuta L, Nomura K, Nara M, Oda G, et al. The Utility of Deep Learning in Breast Ultrasonic Imaging: A Review. Diagnostics. 2020; 10(12):1055. https://doi.org/10.3390/diagnostics10121055
Chicago/Turabian StyleFujioka, Tomoyuki, Mio Mori, Kazunori Kubota, Jun Oyama, Emi Yamaga, Yuka Yashima, Leona Katsuta, Kyoko Nomura, Miyako Nara, Goshi Oda, and et al. 2020. "The Utility of Deep Learning in Breast Ultrasonic Imaging: A Review" Diagnostics 10, no. 12: 1055. https://doi.org/10.3390/diagnostics10121055
APA StyleFujioka, T., Mori, M., Kubota, K., Oyama, J., Yamaga, E., Yashima, Y., Katsuta, L., Nomura, K., Nara, M., Oda, G., Nakagawa, T., Kitazume, Y., & Tateishi, U. (2020). The Utility of Deep Learning in Breast Ultrasonic Imaging: A Review. Diagnostics, 10(12), 1055. https://doi.org/10.3390/diagnostics10121055





