Clinical Impact of 18F-FDG PET/CT in the Diagnostic Workup of Pancreatic Ductal Adenocarcinoma: A Systematic Review
Abstract
1. Introduction
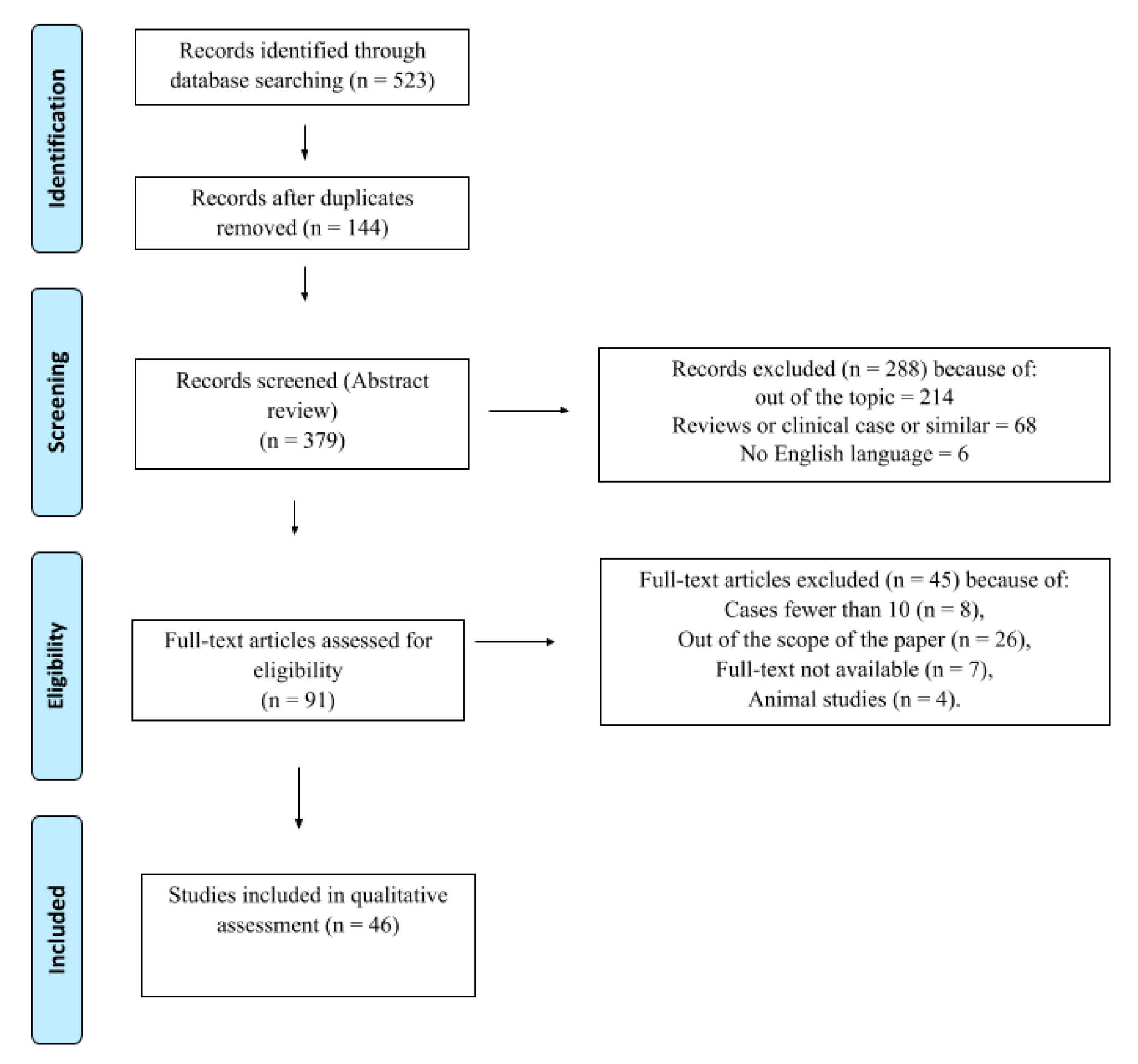
2. Materials and Methods
Quality Assessment
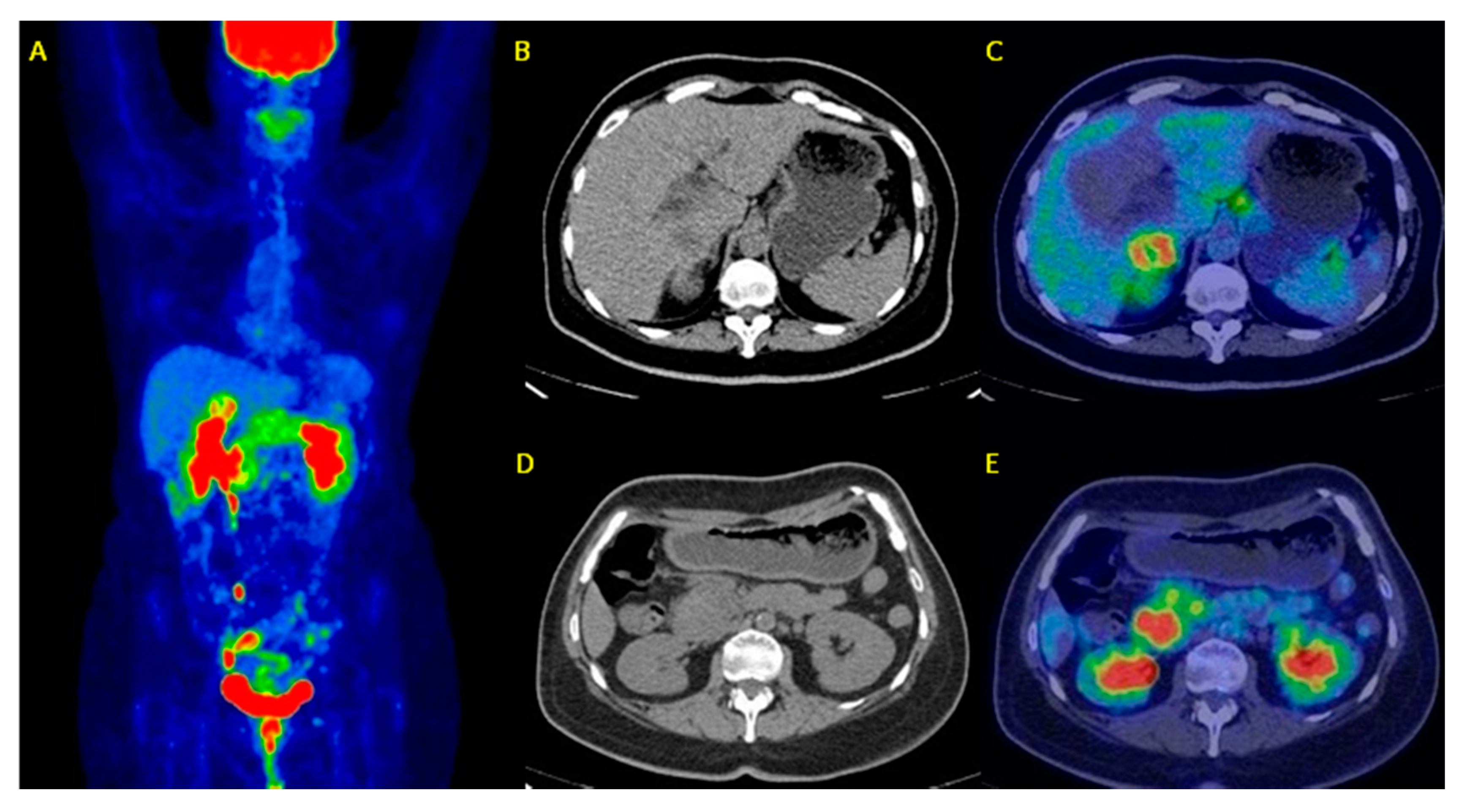
3. Results
3.1. Diagnosis
3.2. Preoperative Staging
3.3. Clinical Management
3.4. Tumor Recurrence
3.5. Treatment Response Assessment and Radiotherapy Planning
3.6. Prognosis
4. Discussion and Conclusions
Funding
Conflicts of Interest
References
- Simoes, P.K.; Olson, S.H.; Bs, A.S.; Kurtz, R.C. Epidemiology of pancreatic adenocarcinoma. Chin. Clin. Oncol. 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Addeo, P.; Velten, M.; Averous, G.; Faitot, F.; Nguimpi-Tambou, M.; Nappo, G.; Felli, E.; Fuchshuber, P.; Bachellier, P. Prognostic value of venous invasion in resected T3 pancreatic adenocarcinoma: Depth of invasion matters. Surgery 2017, 162, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Wray, C.J.; Ahmad, S.A.; Matthews, J.B.; Lowy, A.M. Surgery for Pancreatic Cancer: Recent Controversies and Current Practice. Gastroenterology 2005, 128, 1626–1641. [Google Scholar] [CrossRef]
- Katz, M.H.G.; Varadhachary, G.R.; Fleming, J.B.; Wolff, R.A.; Lee, J.E.; Pisters, P.W.T.; Vauthey, J.-N.; Abdalla, E.K.; Sun, C.C.; Wang, H.; et al. Serum CA 19-9 as a Marker of Resectability and Survival in Patients with Potentially Resectable Pancreatic Cancer Treated with Neoadjuvant Chemoradiation. Ann. Surg. Oncol. 2010, 17, 1794–1801. [Google Scholar] [CrossRef]
- Ferrone, C.R.; Finkelstein, D.M.; Thayer, S.P.; Muzikansky, A.; Fernandez-del Castillo, C.; Warshaw, A.L. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J. Clin. Oncol. 2006, 24, 2897–2902. [Google Scholar] [CrossRef]
- Seufferlein, T.; Bachet, J.; Van Cutsem, E.; Rougier, P. Pancreatic adenocarcinoma: ESMO–ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23, vii33–vii40. [Google Scholar] [CrossRef]
- Cascinu, S.; Falconi, M.; Valentini, V.; Jelic, S.; ESMO Guidelines Working Group. Pancreatic cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, v55–v58. [Google Scholar] [CrossRef]
- National Cancer Institute. Surveillance Epidemiology and End Results Program: Statistical Summaries. National Cancer Institute Website. Available online: Seer.cancer.gov/statistics (accessed on 9 November 2012).
- Di Sebastiano, K.M.; Yang, L.; Zbuk, K.; Wong, R.K.; Chow, T.; Koff, D.; Moran, G.R.; Mourtzakis, M. Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: The relationship with diabetes and anaemia. Br. J. Nutr. 2013, 109, 302–312. [Google Scholar] [CrossRef]
- Zaky, A.M.; Wolfgang, C.L.; Weiss, M.J.; Javed, A.A.; Fishman, E.K.; Zaheer, A. Tumor-Vessel Relationships in Pancreatic Ductal Adenocarcinoma at Multidetector CT: Different Classification Systems and Their Influence on Treatment Planning. Radiographics 2017, 37, 93–112. [Google Scholar] [CrossRef]
- Hidalgo, M. Pancreatic Cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Mangu, P.B.; Berlin, J.; Engebretson, A.; Hong, T.S.; Maitra, A.; Schulick, R.; Shapiro, M.; Urba, S.; Zeh, H.J.; et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 2324–2328. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Ergul, N.; Gundogan, C.; Tozlu, M.; Toprak, H.; Kadıoglu, H.; Aydin, M.; Çermik, T.F. Role of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in diagnosis and management of pancreatic cancer; comparison with Multidetector Row Computed Tomography, Magnetic Resonance Imaging and Endoscopic Ultrasonography. Rev. Esp. Med. Nucl. Imagen Mol. 2014, 33, 159–164. [Google Scholar]
- Zhang, Y.; Cheng, C.; Liu, Z.; Wang, L.; Pan, G.; Sun, G.; Chang, Y.; Zuo, C.; Yang, X. Radiomics analysis for the differentiation of autoimmune pancreatitis and pancreatic ductal adenocarcinoma in 18F-FDG PET/CT. Med. Phys. 2019, 46, 4520–4530. [Google Scholar] [CrossRef]
- Buchs, N.C.; Buhler, L.H.; Bucher, P.A.R.; Willi, J.-P.; Frossard, J.-L.; Roth, A.D.; Addeo, P.; Rosset, A.; Terraz, S.; Becker, C.; et al. Value of contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography/computed tomography in detection and presurgical assessment of pancreatic cancer: A prospective study. J. Gastroenterol. Hepatol. 2011, 26, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Nihashi, T.; Ikeda, M.; Abe, S.; Iwano, S.; Itoh, S.; Shimamoto, K.; Naganawa, S. Limited Efficacy of 18F-FDG PET/CT for Differentiation Between Metastasis-Free Pancreatic Cancer and Mass-Forming Pancreatitis. Clin. Nucl. Med. 2013, 38, 417–421. [Google Scholar] [CrossRef]
- Hu, S.; Yang, Z.; Zhou, Z.-R.; Yu, X.; Ping, B.; Zhang, Y.-J. Role of SUVmax obtained by 18F-FDG PET/CT in patients with a solitary pancreatic lesion. Nucl. Med. Commun. 2013, 34, 533–539. [Google Scholar] [CrossRef]
- Myssayev, A.; Myssayev, A.; Ideguchi, R.; Eguchi, S.; Adachi, T.; Sumida, Y.; Tobinaga, S.; Uetani, M.; Kudo, T. Usefulness of FDG PET/CT derived parameters in prediction of histopathological finding during the surgery in patients with pancreatic adenocarcinoma. PLoS ONE 2019, 14, e0210178. [Google Scholar] [CrossRef]
- Strobel, K.; Heinrich, S.; Bhure, U.; Soyka, J.; Veit-Haibach, P.; Pestalozzi, B.C.; Clavien, P.-A.; Hany, T.F. Contrast-Enhanced 18F-FDG PET/CT: 1-Stop-Shop Imaging for Assessing the Resectability of Pancreatic Cancer. J. Nucl. Med. 2008, 49, 1408–1413. [Google Scholar] [CrossRef]
- Asagi, A.; Ohta, K.; Nasu, J.; Tanada, M.; Nadano, S.; Nishimura, R.; Teramoto, N.; Yamamoto, K.; Inoue, T.; Iguchi, H. Utility of Contrast-Enhanced FDG-PET/CT in the Clinical Management of Pancreatic Cancer. Pancreas 2013, 42, 11–19. [Google Scholar] [CrossRef]
- Picchio, M.; Giovannini, E.; Passoni, P.; Busnardo, E.; Landoni, C.; Giovacchini, G.; Bettinardi, V.; Crivellaro, C.; Gianolli, L.; Di Muzio, N.G.; et al. Role of PET/CT in the clinical management of locally advanced pancreatic cancer. Tumori J. 2012, 9, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Casneuf, V.F.; Delrue, L.; Kelles, A.; Van Damme, N.; Van Huysse, J.; Berrevoet, F.; De Vos, M.; Duyck, P.; Peeters, M. Is combined 18F-fluorodeoxyglucose-positron emission tomography/computed tomography superior to positron emission tomography or computed tomography alone for diagnosis, staging and restaging of pancreatic lesions? Acta Gastro-Enterol. Belg. 2008, 70, 331–338. [Google Scholar] [PubMed]
- Lemke, A.-J.; Niehues, S.; Hosten, N.; Amthauer, H.; Boehmig, M.; Stroszczynski, C.; Rohlfing, T.; Rosewicz, S.; Felix, R. Retrospective digital image fusion of multidetector CT and 18F-FDG PET: Clinical value in pancreatic lesions--a prospective study with 104 patients. J. Nucl. Med. 2004, 45, 1279–1286. [Google Scholar] [PubMed]
- Yoneyama, T.; Tateishi, U.; Endo, I.; Inoue, T. Staging accuracy of pancreatic cancer: Comparison between non-contrast-enhanced and contrast-enhanced PET/CT. Eur. J. Radiol. 2014, 83, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, H.; Yang, F.; Teng, X.; Jiang, B. The value of 18F-FDG PET/CT and carbohydrate antigen 19-9 in predicting lymph node micrometastases of pancreatic cancer. Abdom. Radiol. 2019, 44, 4057–4062. [Google Scholar] [CrossRef]
- Kim, H.R.; Seo, M.; Nah, Y.W.; Park, H.W.; Park, S.H. Clinical impact of fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography in patients with resectable pancreatic cancer. Nucl. Med. Commun. 2018, 39, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Kaida, H.; Azuma, K.; Kawahara, A.; Yasunaga, M.; Kitasato, Y.; Hattori, S.; Taira, T.; Ureshino, H.; Kage, M.; Ishii, K.; et al. The correlation between FDG uptake and biological molecular markers in pancreatic cancer patients. Eur. J. Radiol. 2016, 85, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Ghaneh, P.; Hanson, R.; Titman, A.; Lancaster, G.; Plumpton, C.; Lloyd-Williams, H.; Yeo, S.T.; Edwards, R.T.; Johnson, C.; Abu Hilal, M.; et al. PET-PANC: Multicentre prospective diagnostic accuracy and health economic analysis study of the impact of combined modality 18fluorine-2-fluoro-2-deoxy-d-glucose positron emission tomography with computed tomography scanning in the diagnosis and management of pancreatic cancer. Health Technol. Assess. 2018, 22, 1–114. [Google Scholar]
- Nishiyama, Y.; Yamamoto, Y.; Yokoe, K.; Monden, T.; Sasakawa, Y.; Tsutsui, K.; Satoh, K.; Ohkawa, M. Contribution of whole body FDG-PET to the detection of distant metastasis in pancreatic cancer. Ann. Nucl. Med. 2005, 19, 491–497. [Google Scholar] [CrossRef]
- Albano, D.; Familiari, D.; Gentile, R.; Scalisi, S.; Midiri, F.; Messina, M.; Spada, M.; Fornito, M.C.; Galia, M.; Midiri, M.; et al. Clinical and prognostic value of 18F-FDG-PET/CT in restaging of pancreatic cancer. Nucl. Med. Commun. 2018, 39, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Burge, M.; O’Rourke, N.; Cavallucci, D.J.; Bryant, R.; Francesconi, A.; Houston, K.; Wyld, D.; Eastgate, M.; Finch, R.; Hopkins, G.; et al. A prospective study of the impact of fluorodeoxyglucose positron emission tomography with concurrent non-contrast CT scanning on the management of operable pancreatic and peri-ampullary cancers. HPB 2015, 17, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Im, H.-J.; Oo, S.; Jung, W.; Jang, J.-Y.; Kim, S.-W.; Cheon, G.J.; Kang, K.W.; Chung, J.-K.; Kim, E.E.; Lee, D.S. Prognostic Value of Metabolic and Volumetric Parameters of Preoperative FDG-PET/CT in Patients With Resectable Pancreatic Cancer. Medicine (Baltimore) 2016, 95, e3686. [Google Scholar] [CrossRef]
- Chang, J.S.; Choi, S.H.; Lee, Y.; Kim, K.H.; Park, J.Y.; Song, S.Y.; Cho, A.; Yun, M.; Lee, J.D.; Seong, J. Clinical Usefulness of 18F-Fluorodeoxyglucose-Positron Emission Tomography in Patients With Locally Advanced Pancreatic Cancer Planned to Undergo Concurrent Chemoradiation Therapy. Int. J. Radiat. Oncol. 2014, 90, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Kurahara, H.; Maemura, K.; Mataki, Y.; Sakoda, M.; Iino, S.; Kawasaki, Y.; Arigami, T.; Mori, S.; Kijima, Y.; Ueno, S.; et al. Significance of 18F-Fluorodeoxyglucose (FDG) Uptake in Response to Chemoradiotherapy for Pancreatic Cancer. Ann. Surg. Oncol. 2018, 26, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Shaban, E. Added value of 18-F-FDG-PET/CT in patients with pancreatic cancer: Initial observation. Egypt. J. Radiol. Nucl. Med. 2016, 47, 1275–1282. [Google Scholar] [CrossRef][Green Version]
- Korn, R.; Von Hoff, D.; Borad, M.J.; Renschler, M.F.; McGovern, D.; Bay, R.C.; Ramanathan, R.K. 18F-FDG PET/CT response in a phase 1/2 trial of nab-paclitaxel plus gemcitabine for advanced pancreatic cancer. Cancer Imaging 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Heilbrun, L.K.; Venkatramanamoorthy, R.; Lawhorn-Crews, J.M.; Zalupski, M.M.; Shields, A.F. Using 18F-Fluorodeoxyglucose Positron Emission Tomography to Monitor Clinical Outcomes in Patients Treated With Neoadjuvant Chemo-Radiotherapy for Locally Advanced Pancreatic Cancer. Am. J. Clin. Oncol. 2009, 33, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Eckel, F.; Lersch, C.; Lippl, F.; Schulte-Frohlinde, E.; Schusdziarra, V.; Helmberger, H.; Neverve, J.; Decker, M.; Frank, R.; Schwaiger, M.; et al. Monitoring of Tumour Glucose Metabolism by PET in a Phase I Study Evaluating Hormonal Therapy in Advanced Pancreatic Cancer. Scand. J. Gastroenterol. 2002, 37, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Sakahara, H.; Torizuka, T.; Nakamoto, Y.; Kanamori, S.; Hiraoka, M.; Imamura, M.; Nishimura, Y.; Tamaki, N.; Konishi, J. Evaluation of intraoperative radiation therapy for unresectable pancreatic cancer with FDG PET. J. Nucl. Med. 1999, 40, 1424–1433. [Google Scholar] [PubMed]
- Kishi, T.; Matsuo, Y.; Nakamura, A.; Nakamoto, Y.; Itasaka, S.; Mizowaki, T.; Togashi, K.; Hiraoka, M. Comparative evaluation of respiratory-gated and ungated FDG-PET for target volume definition in radiotherapy treatment planning for pancreatic cancer. Radiother. Oncol. 2016, 120, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Mukherjee, S.; Chu, K.-Y.; Brunner, T.B.; Partridge, M.; Hawkins, M.A. Challenges in using 18 F-fluorodeoxyglucose-PET-CT to define a biological radiotherapy boost volume in locally advanced pancreatic cancer. Radiat. Oncol. 2014, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Parlak, C.; Topkan, E.; Onal, C.; Reyhan, M.; Selek, U. Prognostic value of gross tumor volume delineated by FDG-PET-CT based radiotherapy treatment planning in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Radiat. Oncol. 2012, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kang, C.M.; Lee, W.J.; Song, S.Y.; Cho, A.; Yun, M.; Lee, J.D.; Kim, J.H.; Lee, J.-H. Prognostic Value of18F-Fluorodeoxyglucose Positron Emission Tomography in Patients with Resectable Pancreatic Cancer. Yonsei Med. J. 2013, 54, 1377–1383. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sugiura, T.; Mizuno, T.; Okamura, Y.; Aramaki, T.; Endo, M.; Uesaka, K. Preoperative FDG-PET Predicts Early Recurrence and a Poor Prognosis after Resection of Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2015, 22, 677–684. [Google Scholar] [CrossRef]
- Sperti, C.; Friziero, A.; Serafini, S.; Bissoli, S.; Ponzoni, A.; Grego, A.; Grego, E.; Moletta, L. Prognostic Implications of 18-FDG Positron Emission Tomography/Computed Tomography in Resectable Pancreatic Cancer. J. Clin. Med. 2020, 9, 2169. [Google Scholar] [CrossRef]
- Pergolini, I.; Crippa, S.; Salgarello, M.; Belfiori, G.; Partelli, S.; Ruffo, G.; Pucci, A.; Zamboni, G.; Falconi, M. SUVmax after (18)fluoro-deoxyglucose positron emission tomography/computed tomography: A tool to define treatment strategies in pancreatic cancer. Dig. Liver Dis. 2018, 50, 84–90. [Google Scholar] [CrossRef]
- Smeets, E.M.M.; Withaar, D.S.; Grootjans, W.; Hermans, J.J.; Van Laarhoven, C.J.H.M.; De Geus-Oei, L.-F.; Gotthardt, M.; Aarntzen, E.H. Optimal respiratory-gated [18F]FDG PET/CT significantly impacts the quantification of metabolic parameters and their correlation with overall survival in patients with pancreatic ductal adenocarcinoma. EJNMMI Res. 2019, 9, 24. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, J.W.; Kang, B.; Song, S.Y.; Lee, J.D.; Lee, J.-H. Prognostic Significance of Volume-Based FDG PET/CT Parameters in Patients with Locally Advanced Pancreatic Cancer Treated with Chemoradiation Therapy. Yonsei Med. J. 2014, 55, 1498–1506. [Google Scholar] [CrossRef]
- Su, W.; Ren, S.; Zhu, X.; Zhang, H.; Zuo, C. Standardized thresholds of volume-based PET/CT parameters predicting survival of patients with pancreatic head cancer treated with stereotactic body radiation therapy. Ann. Nucl. Med. 2020, 34, 379–387. [Google Scholar] [CrossRef]
- Ren, S.; Zhu, X.; Zhang, A.; Li, D.; Zuo, C.; Zhang, H. Prognostic value of 18F-FDG PET /CT metabolic parameters in patients with locally advanced pancreatic Cancer treated with stereotactic body radiation therapy. Cancer Imaging 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-X.; Chen, T.; Wang, W.-Q.; Wu, C.-T.; Liu, C.; Long, J.; Xu, J.; Zhang, Y.-J.; Chen, R.-H.; Liu, L.; et al. Metabolic tumour burden assessed by 18F-FDG PET/CT associated with serum CA19-9 predicts pancreatic cancer outcome after resection. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.; Needham, A.; Psarelli, E.; Carroll, M.; Vinjamuri, S.; Sanghera, B.; Wong, W.L.; Halloran, C.; Ghaneh, P. Prognostic value of 18FDG PET/CT volumetric parameters in the survival prediction of patients with pancreatic cancer. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kang, C.M.; Choi, H.J.; Lee, W.J.; Song, S.Y.; Lee, J.H.; Lee, J.D. Prognostic Value of Metabolic Tumor Volume and Total Lesion Glycolysis on Preoperative 18F-FDG PET/CT in Patients with Pancreatic Cancer. J. Nucl. Med. 2014, 55, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Paeng, J.C.; Cheon, G.J.; Lee, D.; Chung, J.-K.; Kang, K.W. Heterogeneity index evaluated by slope of linear regression on 18F-FDG PET/CT as a prognostic marker for predicting tumor recurrence in pancreatic ductal adenocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1995–2003. [Google Scholar] [CrossRef]
- Hyun, S.H.; Kim, H.S.; Choi, S.H.; Choi, D.W.; Lee, J.K.; Lee, K.H.; Park, J.O.; Lee, K.-H.; Kim, B.-T.; Choi, J.Y. Intratumoral heterogeneity of 18F-FDG uptake predicts survival in patients with pancreatic ductal adenocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1461–1468. [Google Scholar] [CrossRef]
- Toyama, Y.; Hotta, M.; Motoi, F.; Takanami, K.; Minamimoto, R.; Takase, K. Prognostic value of FDG-PET radiomics with machine learning in pancreatic cancer. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Lee, S.M.; Yeom, G.Y.; Lee, J.W.; Kim, S.-K.; Park, S.-J.; Han, S.-S. Improved Prognostic Value of Standardized Uptake Value Corrected for Blood Glucose Level in Pancreatic Cancer Using F-18 FDG PET. Clin. Nucl. Med. 2011, 36, 331–336. [Google Scholar] [CrossRef]
- Nakajo, M.; Kajiya, Y.; Tani, A.; Jinguji, M.; Nakajo, M.; Nihara, T.; Fukukura, Y.; Yoshiura, T. A pilot study of the diagnostic and prognostic values of FLT-PET/CT for pancreatic cancer: Comparison with FDG-PET/CT. Abdom. Radiol. 2016, 42, 1210–1221. [Google Scholar] [CrossRef]
- Murakami, K. FDG-PET for hepatobiliary and pancreatic cancer: Advances and current limitations. World J. Clin. Oncol. 2011, 2, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Berberat, P.; Friess, H.; Kashiwagi, M.; Beger, H.G.; Büchler, M.W. Diagnosis and staging of pancreatic cancer by positron emission tomography. World J. Surg. 1999, 23, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Stollfuss, J.C.; Glatting, G.; Friess, H.; Kocher, F.; Berger, H.G.; Reske, S.N. 2-(fluorine-18)-fluoro-2-deoxy-D-glucose PET in detection of pancreatic cancer: Value of quantitative image interpretation. Radiology 1995, 195, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Delbeke, D.; Meyerowitz, C.; Lapidus, R.L.; Maciunas, R.J.; Jennings, M.T.; Moots, P.L.; Kessler, R.M. Optimal cutoff levels of F-18 fluorodeoxyglucose uptake in the differentiation of low-grade from high-grade brain tumors with PET. Radiology 1995, 195, 47–52. [Google Scholar] [CrossRef]
- Chung, K.H.; Park, J.K.; Lee, S.H.; Hwang, D.W.; Cho, J.Y.; Yoon, Y.-S.; Han, H.S.; Hwang, J.-H. Lower maximum standardized uptake value of fluorine-18 fluorodeoxyglucose positron emission tomography coupled with computed tomography imaging in pancreatic ductal adenocarcinoma patients with diabetes. Am. J. Surg. 2015, 209, 709–716. [Google Scholar] [CrossRef]
- Heinrich, S.; Goerres, G.W.; Schäfer, M.; Sagmeister, M.; Bauerfeind, P.; Pestalozzi, B.C.; Hany, T.F.; Von Schulthess, G.K.; Clavien, P.-A. Positron Emission Tomography/Computed Tomography Influences on the Management of Resectable Pancreatic Cancer and Its Cost-Effectiveness. Ann. Surg. 2005, 242, 235. [Google Scholar] [CrossRef]
- Sperti, C.; Pasquali, C.; Bissoli, S.; Chierichetti, F.; Liessi, G.; Pedrazzoli, S. Tumor Relapse after Pancreatic Cancer Resection is Detected Earlier by 18-FDG PET than by CT. J. Gastrointest. Surg. 2010, 14, 131–140. [Google Scholar] [CrossRef]
- Yeh, R.; Dercle, L.; Garg, I.; Wang, Z.J.; Hough, D.M.; Goenka, A.H. The Role of 18F-FDG PET/CT and PET/MRI in Pancreatic Ductal Adenocarcinoma. Abdom. Radiol. 2017, 43, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Ruf, J.; Hänninen, E.L.; Oettle, H.; Plotkin, M.; Pelzer, U.; Stroszczynski, C.; Felix, R.; Amthauer, H. Detection of recurrent pancreatic cancer: Comparison of FDG-PET with CT/MRI. Pancreatology 2005, 5, 266–272. [Google Scholar] [CrossRef]
- Rayamajhi, S.; Balachandran, A.; Katz, M.; Reddy, A.; Rohren, E.; Bhosale, P. Utility of (18) F-FDG PET/CT and CECT in conjunction with serum CA 19-9 for detecting recurrent pancreatic adenocarcinoma. Abdom. Radiol. 2017, 43, 505–513. [Google Scholar] [CrossRef] [PubMed]
- El-Kholy, E.A.; Khaled, L. Diagnostic Accuracy of Dual-Time-Point Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography in Verification Local Recurrence in Pancreatic Cancer Patients. Indian J. Nucl. Med. 2019, 34, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Javery, O.; Shyn, P.; Mortele, K. FDG PET or PET/CT in patients with pancreatic cancer: When does it add to diagnostic CT or MRI? Clin. Imaging 2013, 37, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Dibble, E.H.; Karantanis, D.; Mercier, G.A.; Peller, P.J.; Kachnic, L.A.; Subramaniam, R. PET/CT of Cancer Patients: Part 1, Pancreatic Neoplasms. Am. J. Roentgenol. 2012, 199, 952. [Google Scholar] [CrossRef] [PubMed]




| N | Authors | Year | Study Design | Number of Patients and Age (Mean ± DS or Range) | Disease Phase and Eventual Treatment | Findings |
|---|---|---|---|---|---|---|
| Diagnosis | ||||||
| 1 | Ergul et al. [16] | 2013 | Retrospective study | 52 (63.4 ± 11.7) | Staging | Fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) seems to be useful, especially when applied with endoscopic ultrasound as first line diagnostic tools. |
| 2 | Zhang et al. [17] | 2019 | Retrospective study | 111 | Staging | FDG PET/CT and radiomics method help in non-invasive diagnosis of autoimmune pancreatitis, especially when biopsy is inconclusive. |
| 3 | Buchs et al. [18] | 2010 | Prospective study | 45 (69 (22–82)) | Staging | FDG PET/CT offers good sensitivity in the detection and assessment of pancreatic cancer, but at the price of a relatively low specificity |
| 4 | Kato et al. [19] | 2012 | Retrospective study | 47 pancretic lesions (33 PDAC) (66 ± 8.6) | Staging 20 patients then operated | Limit of the standardized uptake value (SUV) max values in distinguishing between chronic pancreatitis and pancreatic ductal adenocarcinoma (PDAC), except for extreme values. |
| 5 | Hu et al. [20] | 2013 | Retrospective study | 80 (57.3 ± 12.4) | Staging pancreatoduodenect-omy (30 patients), distal pancreatectomy (36 patients), total pancreatectomy (4 patients), proximal pancrea-tectomy (2 patients), and lesion resection (8 patients) | SUVmax of malignant tumors had a positive correlation with Ki-67, so helped in malignant lesions diagnosis. |
| Preoperative Staging | ||||||
| 6 | Myssayev et al. [21] | 2014 | Retrospective study | 48 (68.2) | Preoperative Staging biopsy-proven pancreatic adenocarcinoma | TLG (total lesion glycolysis) and MTV (metabolic tumor volume) are superior to SUV-based parameters for predicting tumor aggressiveness but are not directly related to vascular infiltration status and are not helpful alone for taking decision to perform surgery. |
| 7 | Strobel et al. [22] | 2015 | Retrospective study | 50 (64.3) | Preoperative staging biopsy-proven pancreatic adenocarcinoma | The one-stop-shop imaging approach is superior to unenhanced PET/CT in defining the resectability of PDAC, improving the detection of distant metastasis. |
| 8 | Asagi et al. [23] | 2013 | Prospective study | 108 | Preoperative staging in advanced disease | PET/contrast enhanced computed tomography (ceCT) imaging can provide useful information in the clinical management of pancreatic cancer. |
| 9 | Picchio et al. [24] | 2012 | Prospective study | 42 | Patients selection for helical tomotherapy with concurrent chemotherapy | PET/CT influenced the treatment strategy by detecting distant metastases not documented by CT, thus accurately selecting patients for hormonal-chemotherapy after induction chemotherapy |
| 10 | Casneuf et al. [25] | 2007 | Retrospective study | 46 | Diagnosis, staging, and restaging of pancreatic lesions | The accuracy rate of PET/CT (91.2%) for diagnosis of primary pancreatic lesions is higher compared to CT (88.2%) and PET alone (82.3%). Additionally, for locoregional staging, PET/CT has a higher accuracy rate (85.3%) compared to CT (83.8%) and PET (79.4%). |
| 11 | Lemke et al. [26] | 2004 | Retrospective study | 104 | Preoperative staging | The image fusion (PET/ceCT) permits a more accurate assessment of the resection criteria, also improving the correct anatomic localization of small lesions |
| 12 | Yoneyama et al. [27] | 2014 | Retrospective study | 95 (67 (36–83)) | Staging | The magnitude of diagnostic accuracy of PET/contrast enhanced CT in the detection of distant metastasis, lymph node metastasis, and peritoneal dissemination remains still unclear |
| 13 | Wang et al. [28] | 2019 | Retrospective study | 160 (66) | Preoperative staging surgical resection within 1 week after the 18F-FDG PET/CT scan | Either CT or PET are limited in evaluations of node metastasis. The best SUVmax and CA 19-9 cut-off values for predicting lymph node micrometastases is 7.05 and 240.55 U/mL, respectively. |
| 14 | Kim et al. [29] | 2018 | Retrospective study | 85 (69 (41–89)) | Preoperative staging | SUV of the lymph nodes (SUVLN) seems to be a more significant prognostic factor in pancreatic cancer than the primary tumor’s SUV |
| 15 | Kaida et al. [30] | 2016 | Retrospective study | 53 (68 (40–81)) | Preoperative staging | FDG uptake may predict the levels of endothelial growth factor receptor (EGFR) and p70S6 expressions, whilst mTOR did not correlate with FDG uptake |
| Tumor Recurrence | ||||||
| 16 | Ghaneh et al. [31] | 2018 | Prospective study | 589 | Whole management | FDG PET/CT, in addition to standard diagnostic work-up of PDAC, correctly changed the staging of PDAC in 10% of cases, influenced the planned management in 45%, avoided un-useful resection in 20% of patients scheduled for surgery, and got a limited role in chronic pancreatitis. |
| 17 | Nishiyama et al. [32] | 2005 | Retrospective study | 42 (65.8 (33–93)) | Restaging | FDG PET/CT adds information that was able to change the clinical initial staging in 11.9% of patients with a change of the therapy |
| 18 | Albano et al. [33] | 2018 | Retrospective study | 52 (59 (42–78)) | Restaging 28 surgery, 12 neoadjuvant chemotherapy+surgery+radiotherapy, s6 neoadjuvant chemotherapy+surgery, and 6 chemotherapy | PET/CT has a high diagnostic accuracy in the restaging process and significantly influences the therapeutic management in ∼30% of cases. |
| 19 | Burge et al. [34] | 2015 | Prospective study | 56 (64 (35–84)) | Staging and evaluation of impact of PET/CT on management | PET/CT was able to avoid potential risks and futile surgery in 16% of patients with advanced pancreatic cancer. However, it was not able to predict locally unresectable disease |
| 20 | Hyung-Jun et al. [35] | 2016 | Retrospective study | 51 | Staging Surgery and adjuvant treatment in all patients (concurrent chemo- radiotheraphy in 41, chemotheraphy in 9, radiotheraphy in 1) after scan | MTV and TLG are associated with the presence of lymphovascular invasion. |
| Therapy Assessment | ||||||
| 21 | Chang et al. [36] | 2014 | Retrospective study | 388 | Staging and post-Therapy assessment | PET/CT resulted in the ability to switch to systemic treatments, avoiding a futile surgical approach. Namely, the authors found that the presence of a low SUVmax in the primary tumor and the reduction of SUVmax > 60% after therapy was associated with a better overall survival (OS) and progression-free survival (PFS) |
| 22 | Kurahara et al. [37] | 2018 | Retrospective study | 125 | Pretreatment evaluation | FDG PET SUVmax was significantly associated with the therapeutic response to chemoradiotheraphy (CRT) in PDAC patients |
| 23 | Nasr Shaban et al. [38] | 2015 | Retrospective study | 20 (60.25 (57–74)) | Post-Therapy assessment | Combined FDG PET/CT significantly improves the sensitivity and specificity of isolated CT for depicting pancreatic tumors and distant metastases and can monitor response to treatment, distinguishing fibrosis from residual/recurrence |
| 24 | Korn et al. [39] | 2017 | Prospective study | 52 | Early PET imaging in patients with metastatic pancreatic adenocarcinoma (mPC) treated with nab-paclitaxel plus gemcitabine | PET effectively measured changes in tumor metabolic activity at 6 and 12 weeks. These results support the antitumor activity of nab-paclitaxel 125 mg/m2 plus gemcitabine 1000 mg/m2 for treating mPC and the utility of PET for measuring treatment response. Treatment response by PET analysis may be considered when evaluating investigational agents in mPC. |
| 25 | Choi et al. [40] | 2010 | Retrospective study | 20 | Early treatment response | FDG-PET-CT has an important role in defining the gross tumor volume (GTV) size in predicting outcomes of locally advanced pancreatic cancer (LAPC) |
| 26 | Eckel et al. [41] | 2002 | Prospective study | 19 (62 (43–76)) | PET at baseline and on days 14 and 28 in monitoring hormonal therapy using a highly selective, non-peptide cholecystokinin (CCK) receptor antagonist | No significant changes in FDG uptake by the primary tumors were observed. SR 27897B, when used alone at the limited doses employed, led neither to an impairment of tumor glucose metabolism nor to a reduction of tumor size in advanced pancreatic cancer. An unchanged FDG uptake cannot be used as a measure of disease stabilization. |
| 27 | Higashi et al. [42] | 1999 | Retrospective study | 14 | PET before (n = 12) and after IORT (0.7–11.9 mo, n = 14) | FDG PET was useful in monitoring patients after intraoperative radiotherapy (IORT), because the decrease of metabolism in pancreatic tumors could be detected earlier than the decrease in tumor size |
| 28 | Kishi et al. [43] | 2016 | Retrospective study | 14 | Four dimensional (4D)-PET in pancreatic cancer radiotherapy treatment planning | Tumor volume was significantly larger when delineated using 4D-PET than three dimensional (3D)-PET |
| 29 | Wilson et al. [44] | Retrospective study | 17 (65 (45–74)) | Staging | Low pre-CRT FDG-avidity related to less likely development of metastatic disease. | |
| 30 | Parlak et al. [45] | 2012 | Prospective study | 30 (57 (39–68)) | FDG-PET-CT based radiotherapy planning | Patients with lower gross tumor volume (GTV) assessed by FDG PET/CT have a significantly better OS than those with larger GTV during systemic therapies |
| Prognosis | ||||||
| 31 | Choi et al. [46] | 2013 | Retrospective study | 64 (63.5 (45–30)) | Restaging 34 (53.1%) underwent pylorus-preserving pancreatoduodenectomy, 18 (28.1%) distal pancreatectomy, 10 (15.6%) pancreatoduodenectomy, and two (3.1) total pancreatectomy. 40 patients had adjuvant treatment, 28 had chemotherapy, and 12 had chemoradiotherapy. | High SUVmax is an independent poor prognostic factor and may play an important role in risk stratification and treatment planning prior to undertaking surgical resection. |
| 32 | Yamamoto et al. [47] | 2014 | Retrospective study | 128 (67) | Staging pancreaticadenocarcinoma that preoperatively underwent FDG-PETexaminations | SUVmax cut off value 6.0 may be useful for selecting treatment strategies. |
| 33 | Sperti [48] | 2020 | Retrospective study | 144 (66.3) | Staging | The SUVmax calculated with 18-FDG-PET/CT is an important prognostic factor for patients with pancreatic cancer and may be useful in decisions concerning patients’ therapeutic management. |
| 34 | Pergolini et al. [49] | 2017 | Retrospective study | 46 (67) | Staging All pancreaticoduodenectomy | Preoperative SUVmax ≥ 6 is an independent predictor of poor disease-free survival (DFS) and disease specific survival (DSS) after surgery, identifying, in combination with other biomarkers of aggressiveness like CA 19.9, a subgroup that can benefit from a systemic approach with neoadjuvant treatment. |
| 35 | Smeets [50] | 2019 | Retrospective study | 69 (66 (40–82)) | Staging | Amplitude-based optimal respiratory gating (ORG) on quantification of PET-derived image features in PDAC has a significant impact on all measured metabolic parameters. |
| 36 | Choi et al. [51] | 2014 | Retrospective study | 60 (64.7) | Staging chemoradiation therapy after scan | The disease control rate (DCR) is significantly higher in patients with low SUVmax, MTV, or TLG, and has a strong correlation with longer survival times. |
| 37 | Su et al. [52] | 2020 | Prospective study | 35 (67.2 (45–84)) | Pre-SBRT Staging | MTV (40%) was the optimal prognosticator among the relative thresholds of SUVmax for tumour delineation on PET/CT for LAPHC patients receiving stereotactic body radiation therapy (SBRT). |
| 38 | Ren et al. [53] | 2020 | Retrospective study | 73 38 pts ≤ 68y 35 > 68y | Restaging | TLG was found to be the independent prognostic factor of OS, and PFS TLG was found to be the independent prognostic factor of OS and PFS |
| 39 | Huang-Xian et al. [54] | 2014 | Retrospective study | 122 (62 (35–84)) | Staging Radical pancreatectomy after scan | Preoperative MTV and TLG values are significantly associated with baseline serum CA19-9 level and tumor size. |
| 40 | Mohamed et al. [55] | 2020 | Retrospective study | 89 (69 (44–85)) | Staging | Tumor TLG offer an independent prognostic value in both potentially operable and metastatic disease settings |
| 41 | Lee et al. [56] | 2014 | Retrospective study | 87 (61 ± 10) | Staging | MTV and TLG measured on preoperative FDG PET/CT are independent and significant prognostic factors for predicting overall survival and recurrence free survival. |
| 42 | Yong-il Kim et al. [57] | 2017 | Retrospective study | 93 (64.2 ± 9.1) | Staging Surgery (+Adjuvantherapy in 76/93) after scan | Heterogeneity index could be a predictor of recurrence in surgically resected PDAC. Additionally, volumetric parameters, as well as venous invasion, are independent prognostic parameters. |
| 43 | Hyun et al. [58] | 2016 | Retrospective study | 137 (63 (36–87)) | Staging 80 Curative surgery with or without adjuvant therapy, 14 concurrent chemoradiotherapy, 17 Chemotherapy alone, and 26 Best supportive care | Tumoral heterogeneity of 18F-FDG PET/CT uptake by texture analysis is an independent predictor of survival along with tumor stage and serum Ca 19-9 level. |
| 44 | Toyama et al. [59] | 2020 | Retrospective study | 161 | Staging | Among the 42 features extracted, gray-level zone length matrix (GLZLM) gray-level non-uniformity (GLNU) was the only statistically significant PET parameter for predicting 1-year survival, followed by TLG |
| 45 | Lee et al. [60] | 2011 | Retrospective study | 43 (62 (31–79)) | Staging 23 pancreatoduodenectomy, 15 distal pancreatectomy, and 5 total pancreatectomy after scan Adjuvant radiotherapy in 29 patients within 1 month after the operation | Introduction of SUV correction for the blood glucose level calculated as SUVgluc (SUVmax x blood glucose level/100 mg/dL). Values are significantly higher in the recurrence group than in the non-recurrence group. |
| 46 | Nakajo et al. [61] | 2016 | Prospective study | 15 (69 ± 12) | Staging 4 pancreaticoduodenectomy (within 2 adjuvant chemotherapy) | Prognostic value of FLT-PET/CT is potentially equivalent to 18F- FDG PET/CT for detecting primary and metastatic PDAC, except liver metastasis. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnone, A.; Laudicella, R.; Caobelli, F.; Guglielmo, P.; Spallino, M.; Abenavoli, E.; Martini, A.L.; Filice, R.; Comis, A.D.; Cuzzocrea, M.; et al. Clinical Impact of 18F-FDG PET/CT in the Diagnostic Workup of Pancreatic Ductal Adenocarcinoma: A Systematic Review. Diagnostics 2020, 10, 1042. https://doi.org/10.3390/diagnostics10121042
Arnone A, Laudicella R, Caobelli F, Guglielmo P, Spallino M, Abenavoli E, Martini AL, Filice R, Comis AD, Cuzzocrea M, et al. Clinical Impact of 18F-FDG PET/CT in the Diagnostic Workup of Pancreatic Ductal Adenocarcinoma: A Systematic Review. Diagnostics. 2020; 10(12):1042. https://doi.org/10.3390/diagnostics10121042
Chicago/Turabian StyleArnone, Annachiara, Riccardo Laudicella, Federico Caobelli, Priscilla Guglielmo, Marianna Spallino, Elisabetta Abenavoli, Anna Lisa Martini, Rossella Filice, Alessio Danilo Comis, Marco Cuzzocrea, and et al. 2020. "Clinical Impact of 18F-FDG PET/CT in the Diagnostic Workup of Pancreatic Ductal Adenocarcinoma: A Systematic Review" Diagnostics 10, no. 12: 1042. https://doi.org/10.3390/diagnostics10121042
APA StyleArnone, A., Laudicella, R., Caobelli, F., Guglielmo, P., Spallino, M., Abenavoli, E., Martini, A. L., Filice, R., Comis, A. D., Cuzzocrea, M., Linguanti, F., Evangelista, L., & Alongi, P. (2020). Clinical Impact of 18F-FDG PET/CT in the Diagnostic Workup of Pancreatic Ductal Adenocarcinoma: A Systematic Review. Diagnostics, 10(12), 1042. https://doi.org/10.3390/diagnostics10121042









