Hormonal Environment and HER2 Status in Extra-Mammary Paget’s Disease (eMPD): A Systematic Literature Review and Meta-Analysis with Clinical Considerations
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Eligibility
2.3. Data Extraction
2.4. Risk of Bias across Studies
2.5. Data Analysis
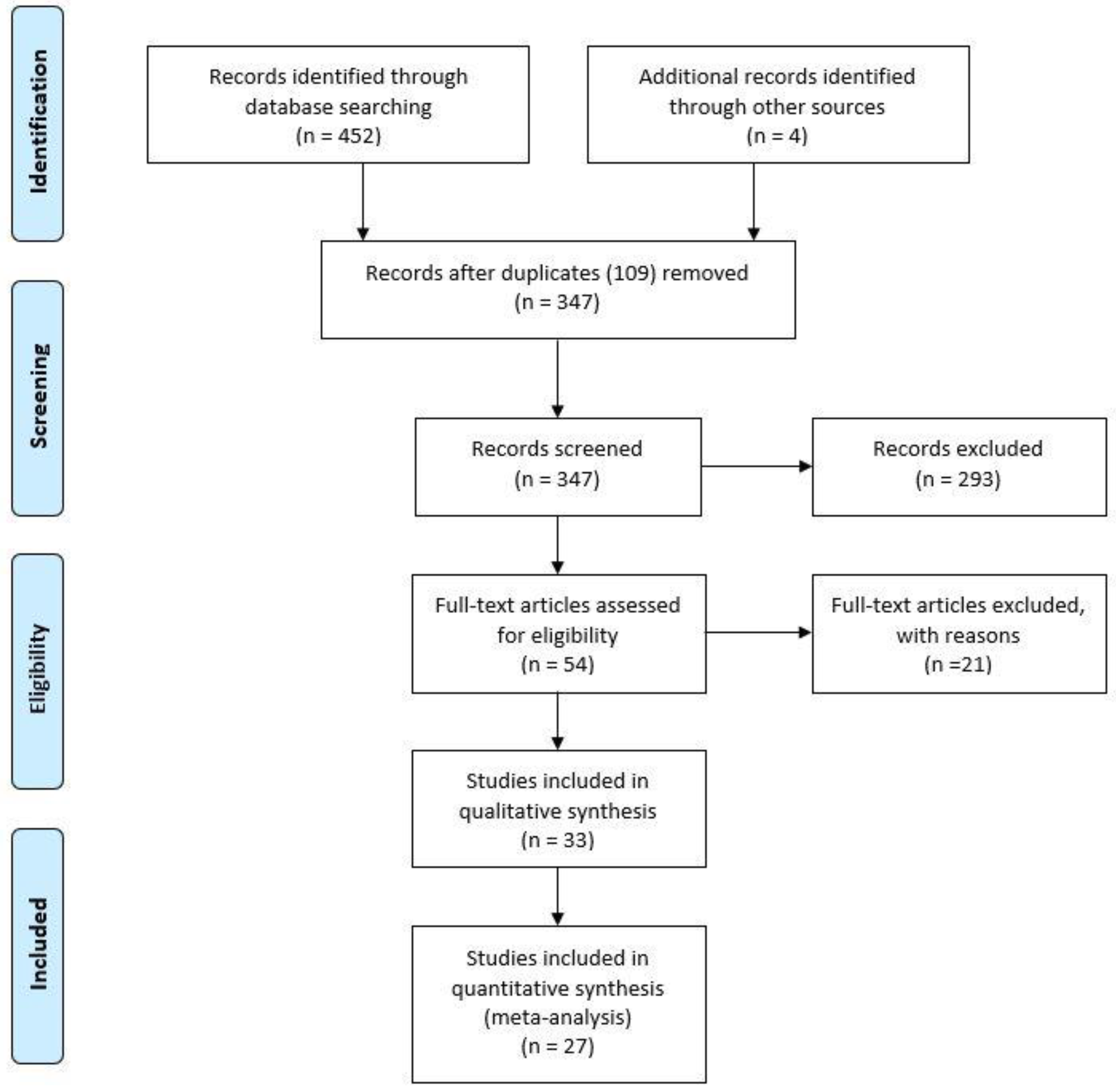
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crocker, H.R. Paget’s disease affecting the scrotum and penis. Trans. Pathol. Soc. Lond. 1889, 40, 187–191. [Google Scholar]
- Crawford, D.; Nimmo, M.; Clement, P.B.; Thomson, T.; Benedet, J.L.; Miller, D.; Gilks, C.B. Prognostic factors in Paget’s disease of the vulva: A study of 21 cases. Int. J. Gynecol. Pathol. 1999, 18, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wong, T.W.; Lee, J.Y. Depigmented genital extramammary Paget’s disease: A possible histogenetic link to Toker’s clear cells and clear cell papulosis. J. Cutan Pathol. 2001, 28, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Zollo, J.D.; Zeitouni, N.C. The Roswell Park Cancer Institute experience with extramammary Paget’s disease. Br. J. Dermatol. 2000, 142, 59–65. [Google Scholar] [CrossRef]
- Crum, C.P.; Herrington, C.S.; McCluggage, W.G.; Regauer, S.; Wilkinson, E.J. Tumours of the vulva. In WHO Classification of Tumours of Female Reproductive Organs, 4th ed.; Kurman, R.J., Carcangiu, M.L., Herrington, C.S., Young, R.H., Eds.; International Agency for Research on Cancer: Lyon, France, 2014; pp. 236–237. [Google Scholar]
- Mehta, N.J.; Torno, R.; Sorra, T. Extramammary Paget’s disease. South Med. J. 2000, 93, 713–715. [Google Scholar] [CrossRef]
- Willman, J.H.; Golitz, L.E.; Fitzpatrick, J.E. Vulvar clear cells of Toker: Precursors of extramammary Paget’s disease. Am. J. Dermatopathol. 2005, 27, 185–188. [Google Scholar] [CrossRef]
- Van der Putte, S.C. Mammary-like glands of the vulva and their disorders. Int. J. Gynecol. Pathol. 1994, 13, 150–160. [Google Scholar] [CrossRef]
- Wilkinson, E.J.; Brown, H.M. Vulvar Paget disease of urothelial origin: A report of three cases and a proposed classification of vulvar Paget disease. Hum. Pathol. 2002, 33, 549–554. [Google Scholar] [CrossRef]
- Van der Linden, M.; Meeuwis, K.A.; Bulten, J.; Bosse, T.; van Poelgeest, M.I.; de Hullu, J.A. Paget disease of the vulva. Crit. Rev. Oncol. Hematol. 2016, 101, 60–74. [Google Scholar] [CrossRef]
- Nowak, M.A.; Guerriere-Kovach, P.; Pathan, A.; Campbell, T.E.; Deppisch, L.M. Perianal Paget’s disease: Distinguishing primary and secondary lesions using immunohistochemical studies including gross cystic disease fluid protein-15 and cytokeratin 20 expression. Arch. Pathol. Lab. Med. 1998, 122, 1077–1081. [Google Scholar]
- Phyo, A.K.; Mun, K.S.; Kwan, K.C.; Ann, C.C.; Kuppusamy, S. Genitourinary extramammary Paget’s disease: Review and outcome in a multidisciplinary setting. Int. J. Clin. Exp. Pathol. 2020, 13, 2369–2376. [Google Scholar] [PubMed]
- Mantovani, G.; Fagotti, A.; Franchi, M.; Scambia, G.; Garganese, G. Reviewing vulvar Paget’s disease molecular bases. Looking forward to personalized target therapies: A matter of CHANGE. Int. J. Gynecol. Cancer 2019, 29. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, S.; Akiyama, M.; Shimizu, H. High expression of Ki-67 and cyclin D1 in invasive extramammary Paget’s disease. J. Dermatol. Sci. 2008, 50, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.K.; Vasef, M.A. HER-2 Gene Amplification in Paget Disease of the Nipple and Extramammary Site: A Chromogenic In Situ Hybridization Study. Diagn. Mol. Pathol. 2006, 15, 131–135. [Google Scholar] [CrossRef]
- Brummer, O.; Stegnerb, H.E.; Bfhmera, G.; Kqhnlea, H.; Petry, K.U. HER-2/neu expression in Paget disease of the vulva and the female breast. Ginecol. Oncol. 2004, 95, 336–340. [Google Scholar] [CrossRef]
- Diaz de Leon, E.; Carcangiu, M.L.; Prieto, V.G.; McCue, P.A.; Burchette, J.L.; To, G.; Norris, B.A.; Kovatich, A.J.; Sanchez, R.L.; Krigman, H.R.; et al. Extramammary Paget Disease Is Characterized by the Consistent Lack of Estrogen and Progesterone Receptors But Frequently Expresses Androgen Receptor. Am. J. Clin. Pathol. 2000, 113, 572–575. [Google Scholar] [CrossRef]
- Fujimoto, A.; Takata, M.; Hatta, N.; Takehara, K. Expression of Structurally Unaltered Androgen Receptor in Extramammary Paget’s Disease. Lab. Investig. 2000, 80, 1465–1471. [Google Scholar] [CrossRef][Green Version]
- Garganese, G.; Inzani, F.; Mantovani, G.; Santoro, A.; Valente, M.; Babini, G.; Petruzzellis, G.; Fragomeni, S.M.; Gentileschi, S.; Bove, S.; et al. The vulvar immunohistochemical panel (VIP) project: Molecular profiles of vulvar Paget’s disease. J. Cancer Res. Clin. Oncol. 2019, 145, 2211–2225. [Google Scholar] [CrossRef]
- Gatalica, Z.; Vranic, S.; Krušlin, B.; Poorman, K.; Stafford, P.; Kacerovska, D.; Senarathne, W.; Florento, E.; Contreras, E.; Leary, A.; et al. Comparison of the biomarkers for targeted therapies in primary extra-mammary and mammary Paget’s disease. Cancer Med. 2020, 9, 1441–1450. [Google Scholar] [CrossRef]
- Hanna, W.; Alowami, S.; Malik, A. The role of HER-2/neu oncogene and vimentin filaments in the production of the Paget’s phenotype. Breast J. 2003, 9, 485–490. [Google Scholar] [CrossRef]
- Hikita, T.; Ohtsuki, Y.; Maeda, T.; Furihata, M. Immunohistochemical and fluorescence in situ hybridization studies on noninvasive and invasive extramammary Paget’s disease. Int. J. Surg. Pathol. 2012, 20, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.C.; Purz, S.; Krumpe, C.; Bilek, K. COX-2 and Her-2/neu are overexpressed in Paget’s disease of the vulva and the breast: Results of a preliminary study. Arch. Gynecol. Obstet. 2008, 277, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Inoguchi, N.; Matsumura, Y.; Kanazawa, N.; Morita, K.; Tachibana, T.; Sakurai, T.; Utani, A.; Miyachi, Y. Expression of prostate-specific antigen and androgen receptor in extramammary Paget’s disease and carcinoma. Clin. Exp. Dermatol. 2007, 32, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Kasashima, S.; Ozaki, S.; Kawashima, A.; Zen, Y.; Moriya, T.; Inoue, M. Androgen receptor and 5alphareductase immunohistochemical profiles in extramammary Paget disease. Br. J. Dermatol. 2010, 162, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Liegl, B.; Horn, L.C.; Moinfar, F. Androgen receptors are frequently expressed in mammary and extramammary Paget’s disease. Mod. Pathol. 2005, 18, 1283–1288. [Google Scholar] [CrossRef]
- Liu, W.; Iqbal, J.; Khoury, T. Mammary Paget’s disease and extra-mammary Paget’s disease: Two morphologically similar but biologically different diseases. J. Cutan Pathol. 2010, 37, 1145–1149. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, P.; Zhu, Y.; Ye, D. Human epidermal growth factor receptor 2 amplification as a biomarker for treatment in patients with lymph node-metastatic penoscrotal extramammary Paget’s disease. Oncol. Lett. 2019, 17, 2677–2686. [Google Scholar] [CrossRef]
- Masuguchi, S.; Jinnin, M.; Fukushima, S.; Makino, T.; Sakai, K.; Inoue, Y.; Igata, T.; Ihn, H. The expression of HER-2 in extramammary Paget’s disease. BioScience Trends 2011, 5, 151–155. [Google Scholar] [CrossRef]
- Miyamoto, A.; Akasaka, K.; Oikawa, H.; Akasaka, T.; Masuda, T.; Maesawa, C. Immunohistochemical study of HER2 and TUBB3 proteins in extramammary Paget disease. Am. J. Dermatopathol. 2010, 32, 578–585. [Google Scholar] [CrossRef]
- Morbeck, D.; Tregnago, A.C.; Netto, G.B.; Sacomani, C.; Peresi, P.M.; Osório, C.T.; Schutz, L.; Bezerra, S.M.; de Brot, L.; Cunha, I.W. GATA3 expression in primary vulvar Paget disease: A potential pitfall leading to misdiagnosis of pagetoid urothelial intraepithelial neoplasia. Histopathology 2017, 70, 435–441. [Google Scholar] [CrossRef]
- Ogawa, T.; Nagashima, Y.; Wada, H.; Akimoto, K.; Chiba, Y.; Nagatani, T.; Inayama, Y.; Yao, M.; Aoki, I.; Ikezawa, Z. Extramammary Paget’s disease: Analysis of growth signal pathway from the human epidermal growth factor receptor 2 protein. Hum. Pathol. 2005, 36, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Plaza, J.A.; Torres-Cabala, C.; Ivan, D.; Prieto, V.G. HER-2/neu expression in extramammary Paget disease:a clinicopathologic and immunohistochemistry study of 47 cases with and without underlying malignancy. J. Cutan Pathol. 2009, 36, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Reich, O.; Liegl, B.; Tamussino, K.; Regauer, S. p185HER2 overexpression and HER2 oncogene amplification in recurrent vulvar Paget’s disease. Mod. Pathol. 2005, 18, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.E.; Hui, P.; Buza, N.; Silasi, D.A.; Azodi, M.; Santin, A.D.; Schwart, P.E.; Rutherford, T.J. HER-2/NEU overexpression in vulvar Paget disease: The Yale experience. J. Clin. Pathol. 2010, 63, 544–573. [Google Scholar] [CrossRef]
- Sekiguchi, N.; Kubota, S.; Noguchi, T.; Fukushima, T.; Kobayashi, T.; Kanda, S.; Koizumi, T.; Miyake, T.; Shirai, T.; Okuyama, R. Experiences of trastuzumab plus paclitaxel combination therapy in metastatic human epidermal growth factor receptor 2-positive extramammary Paget’s disease: Four cases and a review. J. Dermatol. 2020, 47, 1276–1279. [Google Scholar] [CrossRef]
- Tanaka, R.; Sasajima, Y.; Tsuda, H.; Namikawa, K.; Takahashi, A.; Tsutsumida, A.; Fujimoto, M.; Yamazaki, N. Concordance of the HER2 protein and gene status between primary and corresponding lymph node metastatic sites of extramammary Paget disease. Clin. Exp. Metastasis 2016, 33, 687–697. [Google Scholar] [CrossRef]
- Tanaka, R.; Sasajima, Y.; Tsuda, H.; Namikawa, K.; Tsutsumida, A.; Otsuka, F.; Yamazaki, N. Human epidermal growth factor receptor 2 protein overexpression and gene amplification in extramammary Paget disease. Br. J. Dermatol. 2013, 168, 1259–1266. [Google Scholar] [CrossRef]
- Tanskanen, M.; Jahkola, T.; Asko-Seljavaara, S.; Jalkanen, J.; Isola, J. HER2 oncogene amplification in extramammary Paget’s disease. Histopathology 2003, 42, 575–579. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, W.; Zhu, H.; Yu, H. Extramammary Paget’s Disease in Two Brothers. Indian J. Dermatol. 2015, 60, 423. [Google Scholar]
- Huedo-Medina, T.B.; Sanchez-Meca, J.; Marìn-Martìnez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, T.; Noguchi, T.; Nakayama, H.; Yoshida, Y.; Yamamoto, O.; Hayashi, N.; Ohara, K. Clinicopathological study of invasive extramammary Paget’s disease: Subgroup comparison according to invasion depth. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, Y.; Yoshino, K.; Kiyohara, Y.; Kadono, T.; Murata, Y.; Uhara, H.; Hatta, N.; Uchi, H.; Matsushita, S.; Takenouchi, T.; et al. The role of sentinel lymph node biopsy in the management of invasive extramammary Paget’s disease: Multi-center, retrospective study of 151 patients. J. Dermatol. Sci. 2015, 79, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Angelico, G.; Santoro, A.; Inzani, F.; Spadola, S.; Fiorentino, V.; Cianfrini, F.; Carbone, C.; Garganese, G.; Rossi, E.D.; Scambia, G.; et al. Ultrasound-guided FNA cytology of groin lymph nodes improves the management of squamous cell carcinoma of the vulva: Results from a comparative cytohistological study. Cancer Cytopathol. 2019, 127, 514–520. [Google Scholar] [CrossRef]
- Garganese, G.; Fragomeni, S.M.; Pasciuto, T.; Leombroni, M.; Moro, F.; Evangelista, M.T.; Bove, S.; Gentileschi, S.; Tagliaferri, L.; Paris, I.; et al. Ultrasound morphometric and cytologic preoperative assessment of inguinal lymph-node status in women with vulvar cancer: MorphoNode study. Ultrasound Obstet Gynecol. 2020, 55, 401–410. [Google Scholar] [CrossRef]
- Garganese, G.; Collarino, A.; Fragomeni, S.M.; Rufini, V.; Perotti, G.; Gentileschi, S.; Evangelista, M.T.; Ieria, F.P.; Zagaria, L.; Bove, S.; et al. Groin sentinel node biopsy and 18F-FDG PET/ CT-supported preoperative lymph node assessment in cN0 patients with vulvar cancer currently unfit for minimally invasive inguinal surgery: The GroSNaPET study. Eur. J. Surg. Oncol. 2017, 43, 1776–1783. [Google Scholar] [CrossRef]
- Collarino, A.; Fuoco, V.; Garganese, G.; Pereira Arias-Bouda, L.M.; Perotti, G.; Manca, G.; Vidal-Sicart, S.; Giammarile, F.; de Geus-Oei, L.F.; Scambia, G.; et al. Lymphoscintigraphy and sentinel lymph node biopsy in vulvar carcinoma: Update from a European expert panel. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1261–1274. [Google Scholar] [CrossRef]
- Inzani, F.; Angelico, G.; Santoro, A.; Musarra, T.; Valente, M.; Spadola, S.; Garganese, G.; Fragomeni, S.; Scambia, G.; Zannoni, G.F. A new entity in the pathological spectrum of vulvar neoplasms: The first report of a primary endometrioid adenocarcinoma. Int. J. Gynaecol. Obstet. 2019, 147, 270–272. [Google Scholar] [CrossRef]
- Yoneyama, K.; Kamada, N.; Kinoshita, K.; Kawashima, T.; Otani, M.; Endo, H.; Shinkai, H.; Utani, A. Androgen-deprivation regimen for multiple bone metastases of extramammary Paget disease. Br. J. Dermatol. 2005, 153, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Ieni, A.; Angelico, G.; Giuffrè, G.; Tuccari, G. Discordance Rate of HER2 Status in Primary Gastric Cancer and Synchronous Lymph Node Metastases: Its Impact on Therapeutic Decision and Clinical Management. Pathol. Oncol. Res. 2018, 24, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Buonavoglia, A.; Fasano, R.; Solimando, A.G.; De Re, V.; Cicco, S.; Vacca, A.; Racanelli, V. Insights into the Regulation of Tumor Angiogenesis by Micro-RNAs. J. Clin. Med. 2019, 8, 2030. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.E.; Lin, R.J. MicroRNA and HER2-overexpressing cancer. Microrna 2013, 2, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Goradel, N.H.; Mohammadi, N.; Haghi-Aminjan, H.; Farhood, B.; Negahdari, B.; Sahebkar, A. Regulation of tumor angiogenesis by microRNAs: State of the art. J. Cell Physiol. 2019, 234, 1099–1110. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Country | Age (Mean or Median) | Se X(% female) | Total Cases | Micro-Invasive/ Invasive Cases (%) | Positive Expression In Microinvasive/INVASIVE Cases (%) | Marker | Positive ihc Espression (%) | Her2 Amplificatin Status (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Aoyagi, et al. | 2008 | Japan | 70.6 | 34.7 | 23 | 6/23 (26) | 4/6 (66.6) | HER2 | F: 7/8 (87.5) M: 10/15 (66.6) | N/A |
| Bianco, et al. | 2006 | USA | 75 | 100 | 15 | N/A | N/A | HER2 | 6/15 (40) | 1/15 (7) |
| Brummer, et al. | 2004 | Germany | N/A | 100 | 10 | 2/10 (20) | 2/2 100 | HER2 | 8/10 (80) | N/A |
| Diaz de Leon, et al. | 2000 | USA | 64.5 | 82 | 28 | N/A | N/A | AR PR ER | F: 12/23 (52.2) M: 3/5 (60) 0/28 0/28 | |
| Fujimoto, et al. | 2000 | Japan | 67 | 26.6 | 30 | 13/30 (43.3) | 9/13 (26.6) | AR | F: 8/8 (100) M: 16/22 (72.7) | |
| Garganese, et al. | 2019 | Italy | 67 | 100 | 41 | 11/41 (26.8) | HER2 4/11 (36.3) AR 10/11 (90.9) PR 2/11 (18) ER 8/11 (72.7) | HER2 AR PR ER | 10/41 (24.4) 33/41 (80.5) 9/41 (22) 29/41 (70.7) | 10/41 (24.4) |
| Gatalica, et al. | 2020 | USA | 61 | 72.2 | 18 | 15/18 (83.3) | AR 9/15(60) ER (4)/15 (26.6) | AR ER | F: 9/13 (69.2) M: 3/5 (60) F: 2/13 (15.3) M: 2/5 (40) | |
| Hanna, et al. | 2003 | Canada | N/A | 100 | 20 | N/A | N/A | HER2 | 1/20 (5) | 0/19 |
| Hikita, et al. | 2012 | Japan | 70.47 | 64.70 | 17 | 23.5 | ER 0/2 PR 0/2 HER2 8/8 4/4(100) | HER2 | F: 9/11 (81.8) M: 3/6 (50) | 0/8 |
| Horn, et al. | 2008 | Germany | N/A | 100 | 8 | N/A | N/A | HER2 ER PR | 8/8 (100) 1/8 (12.5) 1/8 (12.5) | N/A |
| Inoguchi, et al. | 2006 | Japan | 71.7 | 17.6 | 34 | N/A | N/A | AR | F: 1/6 (16.6) M: 14/23 (60.8) | |
| Kasashima, et al. | 2010 | Japan | 71.5 | 44.8 | 58 | 16/58 (27.5) | 9/16 (56.2) | AR | F: 12/26 (46) M: 21/32 (65.6) | |
| Liegl, et al. | 2005 | Germany | N/A | 100 | 23 | N/A | N/A | HER2 AR PR ER | 12/23 (52) 18/23 (78) 0/23 (0) 1/23 (4) | N/A |
| Liu, et al. | 2009 | USA | 69 | 71.4 | 14 | N/A | N/A | HER2 | 5/14 (35.7) | N/A |
| Lu, et al. | 2018 | China | 63 | 0 | 11 | N/A | N/A | HER2 | 3/11 (27.2) | 2 (FISH+) + 1(genetic heterogeneity)/11 |
| Masuguchi, et al. | 2011 | Japan | N/A | 41.9 | 31 | 11/31 (35.4) | 10/11 (90.9) | HER-2 | F: 7/13 (53.8) M: 12/18 (66.6) | N/A |
| Miyamoto, et al. | 2010 | Japan | 74 | 43.7 | 32 | 19/32 (59.3) | 13/19 (68) | HER2 | F: 7/14 (50) M: 13/18 (72) | 2/5 (40) |
| Morbeck, et al. | 2016 | Brazil | 66.8 | 100 | 11 | 2/11 (18) | 2/2 (100) | HER2 | 6/11 (54.5) | 2/6 (33.3) |
| Ogawa, et al. | 2005 | Japan | 68.5 | 14.7 | 34 | 16/34 (47) 18/34 (52.9) | 5/18 (27.7) | HER2 | F: 1/5 (20) M: 6/29 (20.6) | 3/7 (42.8) |
| Plaza, et al. | 2009 | USA | 66 | 70.2 | 47 | 2/47 (4.2) | 0/2 (0) | HER2 | F: 14/33 (42.4) M: 1/14 (7) | N/A |
| Reich, et al. | 2005 | Austria | 63 | 100 | 6 | N/A | N/A | HER2 | 4/6 (66.6) | 4/6 (66.6) |
| Richter, et al. | 2010 | USA | 68.5 | 100 | 33/39 * | 7/33 (21) | 5/7 (71) | HER2 | 19/33 (57.5) | N/A |
| Sekiguchi, et al. | 2020 | Japan | 71 | 50 | 4 | N/A | N/A | HER2 | F: 2/2 (100) M: 2/2 (100) | 2 amplified 2 polysomic |
| Tanaka, et al. | 2016 | Japan | 72 | 15.3 | 26 | 26/26 (100) | 6/26 (23.07) | HER2 | F: 2/4 (50%) M: 4/22 (18) | 5/6 (83.3) |
| Tanaka, et al. | 2013 | Japan | 71.1 | 33.6 | 104 | 73/104 (36.5) | 10/73 (13.7) | HER2 | F: 5/35 (14.2) M: 7/69 (10) | 12/16 (75) |
| Tanskanen, et al. | 2003 | Finland | 65.47 | 60.8 | 23 | 3/23 (13.04) | 1/3 (33.3) | HER2 | F: 12/23 (52) M: 4/9 (44.44) | 10/23 (43.47) |
| Zhang, et al. | 2015 | China | 61.5 | 0 | 2 | 1/2 (50) | 1/2 (50) | HER2 | 1/2 (50) | N/A |
| Sex | K | N | Overall Rate of Expression (95% CI), % | Q | I2 | |
|---|---|---|---|---|---|---|
| Human epidermal growth factor Receptor 2 (HER 2/neu) | F M | 20 12 | 341 209 | 32 (27–38) 26 (18–36) | 23.74 26.04 | 19.98 57.76 |
| Estrogen Receptor (ER) | F M | 5 2 | 108 10 | 12 (3–36) 9 (0–68) | 17.30 3.57 | 76.88 72.02 |
| Androgen Receptor (AR) | F M | 7 5 | 140 87 | 40 (34–47) 40 (32–48) | 4.79 0.18 | 0.00 0.00 |
| Progesterone Receptor (PR) | F M | 4 1 | 95 5 | 9 (3–25) 2 (0–22) | 5.19 - | 42.15 - |
| Author | Marker | Positive IHC Expression (%) | Evaluation Criteria of IHC |
|---|---|---|---|
| Aoyagi, et al. | HER- 2/neu | F: 7/8 (87.5) M: 10/15 (66.6) | Expression of the antigen was assessed and compared with the reaction in known positive controls semi-quantitatively as follows: <5% of Paget cells positive (score 0); (+) 5–25% of Paget cells positive (score 1); (2+) 26–50% of Paget cells positive (score 2); (3+) >50% of Paget cells positive (score 3). |
| Bianco, et al. | HER-2/neu | 6/15 (40) | Intense staining of tumor cell membranes in the majority of tumor cells was graded as 3+, focal strong membrane staining as 2+, focal low intensity membrane staining as 1+, and granular cytoplasmic staining or no staining of tumor cells as 0. |
| Brummer, et al. | HER-2/neu | 8/10 (80) | In accordance with the Hercep Test kit guide, HER-2/neu overexpression was assessed as negative for scores of 0 and 1+ and positive for scores of 2+ and 3+. |
| Diaz de Leon, et al. | AR PR ER | F: 12/23 (52.2) M: 3/5 (60) 0/28 0/28 | Only nuclear staining with antibodies to steroid receptors was considered specific, and the percentage of cells stained was recorded. |
| Fujimoto, et al. | AR | F: 8/8 (100) M: 16/22 (72.7) | To roughly measure quantitatively androgen receptor content, a score corresponding to the sum of the percentage of tumor cells stained (0, no staining; 1, less than 25%; 2, 26–50%; 3, 51–75%; 4, 76–100%) and the staining intensity (0, absent; 1, weak; 2, moderate; 3, strong) was established |
| Garganese, et al. | HER-2/neu AR PR ER | 10/41 (24.4) 33/41 (80.5) 9/41 (22) 29/41 (70.7) | For HER2/neu expression, membrane staining was evaluated according to the ASCO-CAP guidelines described for breast cancer. A tumor showing ER, PR, or AR nuclear staining in a fraction of neoplastic cells ≥ 1% was considered positive. |
| Gatalica, et al. | AR ER | F: 9/13 (69.2) M: 3/5 (60) F: 2/13 (15.3) M: 2/5 (40) | AR, ER were analyzed using a ≥ 10% threshold for nuclear positivity. |
| Hanna, et al. | HER-2/neu | 1/20 (5) | Results for HER-2/neu status by immunohistochemistry were reported as follows: positive when at least 10% of the tumor showed moderate/strong complete membrane staining, negative when less than 10% of the tumor showed complete membrane staining or less than 30% showed weak or incomplete membrane staining, and equivocal when ≥ 30% of cells showed diffuse weak staining |
| Hikita, et al. | HER-2/neu | F: 9/11 (81.8) M: 3/6 (50) | For HER2/neu expression, membrane staining was evaluated according to the ASCO-CAP guidelines |
| Horn, et al. | HER-2/neu ER PR | 8/8 (100) 1/8 (12.5) 1/8 (12.5) | According to the recommendation for breast cancer, a tumor was counted as positive if a minimum of 10% of the cells showed positive intranuclear staining regardless of staining intensity. For HER-2/neu, only membranous staining results were scored using the system recommended in breast cancer |
| Inoguchi, et al. | AR | F: 1/6 (16.6) M: 14/23 (60.8) | The stained sections were evaluated microscopically as follows: − = no staining; + = focal deposition in the nest of tumor cells. |
| Kasashima, et al. | AR | F: 12/26 (46) M: 21/32 (65.6) | Immunopositive labelling of ≥10% among all cells was considered as a positive result |
| Liegl, et al. | HER-2 AR PR ER | 12/23 (52) 18/23 (78) 0/23 1/23 (4) | For HER2/neu expression, membrane staining was evaluated according to the ASCO-CAP guidelines described for breast cancer. A tumor showing ER, PR, or AR nuclear staining in a fraction of neoplastic cells ≥ 1% was considered positive. |
| Liu, et al. 2018 | HER-2/neu | 5/14 (35.7) | For HER2/neu expression, membrane staining was evaluated according to the ASCO-CAP guidelines |
| Lu, et al. | HER-2/neu | 3/11 (27.2) | For HER2/neu expression, membrane staining was evaluated according to the ASCO-CAP guidelines |
| Masuguchi, et al. | HER-2/neu | F: 7/13 (53.8) M: 4/18 (22.2) | Grading system: 1+ for slight staining, 3+ for strong staining, and 2+ for staining between 1+ and 3+. |
| Miyamoto, et al. | HER-2/neu | F: 7/14 (50) M: 13/18 (72) | Only membrane staining was evaluated using the 0 to 3+ scale illustrated in the HercepTest scoring guideline (0 for no staining or membrane staining in less than 30% of the cells; 1+ for partial, weak staining of the cell membrane in 30% of the cells; 2+ for moderate staining of the complete cell membrane in 30% of the cells; 3+ for intense staining of the complete membrane in.30% of the cells |
| Morbeck, et al. | HER-2/neu | 6/11 (54.5) | For HER2/neu expression, membrane staining was evaluated according to the ASCO-CAP guidelines |
| Ogawa, et al. | HER-2/neu | F: 1/5 (20) M: 6/29 (20.6) | 3+: more than 10% of tumor cells show strong complete membrane staining; 2+: more than 10% of tumor cells show weak or moderate and complete membrane staining; 1+: positively stained cells are less than 10% or show only faint staining, although more than 10% of cells are positive; 0, no staining. According to the recommendation of the US Food and Drug Administration, 3+ and 2+ were recorded as overexpressed and 1+ and 0 as non-overexpressed. |
| Plaza, et al. | HER-2/neu | F: 14/33 (42.4) M: 1/14 (7) | no staining or membrane staining in less than 10% of the cells; 1+ for partial, weak staining of the cell membrane in. 10% of the cells; 2+ for moderate staining of the complete cell membrane in 10% of the cells; 3+ for intense staining of the complete membrane in 10% of the cells). Overexpression was assessed as positive for scores 2 and 3+ and negative for scores 0 and 1+. |
| Reich, et al. | HER-2/neu | 4/6 (100) | For HER2/neu expression, membrane staining was evaluated according to the ASCO-CAP guidelines |
| Richter, et al. | HER-2/neu | 19/33 (57.5) | Staining was considered to be 0 for no staining, 1+ with less than 10% positively stained cells or cells that showed only faint staining, although more than 10% of cells were positive, 2+ when they showed more than 10% weak or moderate and complete membrane staining, and 3+ when more than 10% of tumor cells showed strong complete membrane staining.21 According to the standard guideline used for breast cancer, 2+ and 3+ tumors were recorded as ‘overexpressed’, 0 and 1+ tumors as ‘non-overexpressed’ |
| Sekiguchi, et al. | HER-2/neu | F: 2/2 (100) M: 2/2 (100) | For HER2/neu expression, membrane staining was evaluated according to the ASCO-CAP guidelines |
| Tanaka, et al. | HER-2/neu | F: 2/4 (50) M: 4/22 (18) | 3+, strong, complete membrane staining in more than 30% of the malignant cells; 2+, weak to moderate complete membrane staining in more than 10% of the malignant cells or strong, complete membrane staining in more than 10–30% of the malignant cells; 1+, weak to moderate incomplete membrane staining; 0, fewer than 10% of membrane staining or no membrane staining. Those cases scored 0 and 1+ were defined as negative. Cases scored 2+ and 3+ were defined as equivocal and overexpression, respectively |
| Tanaka, et al. | HER-2/neu | F: 5/35 (14.2) M: 7/69 (10) | 3+, strong, complete membrane staining in more than 30% of the malignant cells; 2+, weak to moderate complete membrane staining in more than 10% of the malignant cells or strong, complete membrane staining in more than 10–30% of the malignant cells; 1+, weak to moderate incomplete membrane staining; 0, fewer than 10% of membrane staining or no membrane staining. Those cases scored 0 and 1+ were defined as negative. Cases scored 2+ and 3+ were defined as equivocal and overexpression, respectively |
| Tanskanen, et al. | HER-2/neu | F: 12/23 (52) M: 4/9 (44.44) | For HER2/neu expression, membrane staining was evaluated according to the ASCO-CAP guidelines |
| Zhang, et al. | HER-2/neu | 1/2 (50) | For HER2/neu expression, membrane staining was evaluated according to the ASCO-CAP guidelines |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelico, G.; Santoro, A.; Inzani, F.; Straccia, P.; Arciuolo, D.; Mulè, A.; Valente, M.; Spadola, S.; D’Alessandris, N.; Garganese, G.; et al. Hormonal Environment and HER2 Status in Extra-Mammary Paget’s Disease (eMPD): A Systematic Literature Review and Meta-Analysis with Clinical Considerations. Diagnostics 2020, 10, 1040. https://doi.org/10.3390/diagnostics10121040
Angelico G, Santoro A, Inzani F, Straccia P, Arciuolo D, Mulè A, Valente M, Spadola S, D’Alessandris N, Garganese G, et al. Hormonal Environment and HER2 Status in Extra-Mammary Paget’s Disease (eMPD): A Systematic Literature Review and Meta-Analysis with Clinical Considerations. Diagnostics. 2020; 10(12):1040. https://doi.org/10.3390/diagnostics10121040
Chicago/Turabian StyleAngelico, Giuseppe, Angela Santoro, Frediano Inzani, Patrizia Straccia, Damiano Arciuolo, Antonino Mulè, Michele Valente, Saveria Spadola, Nicoletta D’Alessandris, Giorgia Garganese, and et al. 2020. "Hormonal Environment and HER2 Status in Extra-Mammary Paget’s Disease (eMPD): A Systematic Literature Review and Meta-Analysis with Clinical Considerations" Diagnostics 10, no. 12: 1040. https://doi.org/10.3390/diagnostics10121040
APA StyleAngelico, G., Santoro, A., Inzani, F., Straccia, P., Arciuolo, D., Mulè, A., Valente, M., Spadola, S., D’Alessandris, N., Garganese, G., Cianfrini, F., Piermattei, A., Scambia, G., & Zannoni, G. F. (2020). Hormonal Environment and HER2 Status in Extra-Mammary Paget’s Disease (eMPD): A Systematic Literature Review and Meta-Analysis with Clinical Considerations. Diagnostics, 10(12), 1040. https://doi.org/10.3390/diagnostics10121040








