Abstract
Background. Extra-mammary Paget’s disease (EMPD) is a rare neoplasm of epithelial origin, whose precise incidence is not clear. Starting from what is already known, we performed a systematic review and meta-analysis to investigate in male and female patients the immunohistochemical expression of biological markers that could serve as potential prognostic/therapeutic factors, including only human epidermal growth factor receptor 2 (HER2/neu), Estrogen Receptor (ER), Progesterone Receptor (PR), and Androgen Receptor (AR). Methods. A literature search was performed of the PubMed, Scopus, and Web of Science databases for English-language studies published from January 2000 to June 2020. Results. A total of 27 studies with 713 patients assessed the role of HER2/neu, AR, ER, and PR expression in male and female with EMPD. The overall rate of HER2/neu expression was 30%, the expression’s rate for ER and AR was 13% and 40%, respectively, and the overall rate for PR was 8%. The subgroup analysis revealed that there is a different expression of molecular markers between male and female patients. Conclusions. This study revealed that AR status and HER2/neu overexpression/amplification have been shown as two fundamental pathogenetic pathways in both female and male patients affected by EMPD.
1. Introduction
Extramammary Paget disease (EMPD) was first described by Crocker in 1889 in a man affected from bladder carcinoma and presented with an eczematous lesion involving the penoscrotal region, that was diagnosed as Paget disease in an extramammary site [1]. Subsequently EMPD has been reported involving more frequently the external female genitalia and less commonly, the perianal/perineal region, groin, axilla, umbilicus, eyelids, and also external ear canal [2,3,4].
EMPD has been defined by World Health Organization (WHO) as an intraepithelial neoplasm of epithelial origin expressing apocrine or eccrine glandular-like features and characterized by distinctive large cells with prominent cytoplasm, referred to as Paget cells’ [5].
The pathogenesis of EMPD is not fully understood; the stem cell compartment of the epidermis and hair follicle as well as Toker cells and mammary-like glands have been reported as possible sites of origin of Paget cells [6,7,8].
Over time, different attempts to classify EMPD have been made and, in particular, at the vulvar site, a histopathological classification of VPD has been conceived, distinguishing primary/cutaneous VPD (type 1) from secondary/non-cutaneous VPD [9]. In detail, cutaneous VPD (type 1) is further subdivided according to the presence or absence of dermal invasion: type 1a (intraepithelial disease arising within the epidermis and extending into the epithelium of skin appendages and less commonly arising from the skin appendages and migrate to the overlying epidermis by epidermotropism); type 1b when focal invasion can be observed; type 1c when there is a cutaneous “pagetoid spread” from an underlying vulvar adenocarcinoma of the skin appendage or subcutaneous vulvar glands.
The 5-year survival is highly variable, depending on the entity of infiltration, being, respectively, 100% and 88% for intraepithelial and micro-invasive disease (<1 mm), and only 15% when neoplastic invasion exceeds 1 mm [10].
On the other hand, secondary VPD can originate by epidermotropic metastases or by direct extension from a malignancy of the gastrointestinal tract (type 2) or the uro-genital tract (type 3) [11,12].
More recently, the WHO Classification of Tumours of Female Reproductive Organs (4th edition) considers to use the subdivision of cutaneous and non-cutaneous EMPD in routinary diagnosis [5].
Given the rarity of EMPD, data on genetic alterations are largely unexplored. Findings regarding the hormonal status including Her2/Neu amplification are probably the most studied genetic alteration, likely because of their therapeutic potential but the clinical significance of these abnormalities still remains to be fully understood [13]. Being aware that at present the need of a tailored treatment for EMPD is a critical clinical goal, but its concrete availability is still too far to achieve, we reviewed the current literature in order to study the impact of IHC expression in VPD and EMPD in both genders of biological markers that could serve as potential prognostic/therapeutic factors, including human epidermal growth factor receptor 2 (HER2/neu), Estrogen Receptor (ER), Progesterone Receptor (PR), and Androgen Receptor (AR).
2. Materials and Methods
2.1. Search Strategy
A systematic literature search (from January 2000 up to June 2020) was performed to identify articles regarding the expression of biological markers in vulvar (VPD) and extra-mammary Paget’s disease (EMPD). Since most published papers before 2000 failed to demonstrate ER, PR, and AR expression, we decided to begin our literature search from 2000, in order to obtain more uniform results. Pubmed, Web of Science, and Scopus were used simultaneously, with the combination of terms (extramammary OR extra mammary OR vulvar) AND (paget OR pagets OR paget’s) AND (molecular OR biological OR marker OR protein OR target OR expression). All articles were initially reviewed by abstract and title browsing to select the relevant reports, which were subjected to further screening.
2.2. Study Eligibility
Data retrieved from the studies included the following: author, country, year of publication, sex (% female), total number of cases with vulvar Paget’s disease (VPD) and/or extramammary Paget’s disease (EMPD), mean age, percentage of invasive cases, organ site, and molecular markers expression in immunohistochemistry (IHC). In detail, we selected heterogeneous female and male cases from a series of VPD and EMPD-patients. Our primary aim was to investigate the immunohistochemical expression in both sexes (male and female) of biological markers that could serve as potential prognostic/therapeutic factors, including only human epidermal growth factor receptor 2 (HER2/neu), Estrogen Receptor (ER), Progesterone Receptor (PR), and Androgen Receptor (AR). The language was limited to English.
2.3. Data Extraction
Starting from 452 identified references, 109 duplicates were removed. The first step consisted in an accurate reading of titles and abstracts and the analysis of all the references denoted high intra-rate reliability (98.62% agreement; Cohen K: 0.97). A total of 54 references were then selected and a full-text assessment was performed. Finally, 27 references which met the eligibility criteria were included in the current work [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40].
The present meta-analysis was conducted according to Guidelines in Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and PICOS (Participants, Intervention, Comparison, Outcomes, Study Design) model. Data from each eligible study were extracted without modification of original data. “Population” of our study was represented by patients diagnosed with VPD/EMPD. “Intervention” (or risk factor) was defined as the VPD/EMPD group with HER2/neu, ER, AR and PR expression, assessed by immunohistochemical analysis. “Comparator” was represented by the VPD/EMPD group without HER2/neu, ER, AR, and PR immunohistochemical expression. Flow diagram of the study selection process is shown in Figure 1.
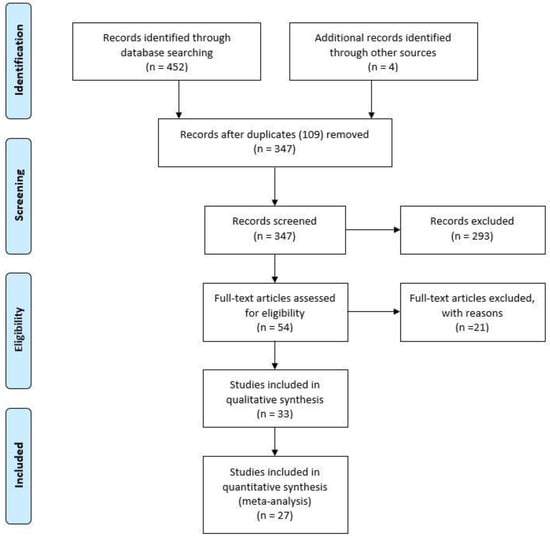
Figure 1.
Flow diagram of the study selection process.
2.4. Risk of Bias across Studies
Reporting bias across studies was evaluated by a graphic diagnostic tool named funnel plot (Figure 2).
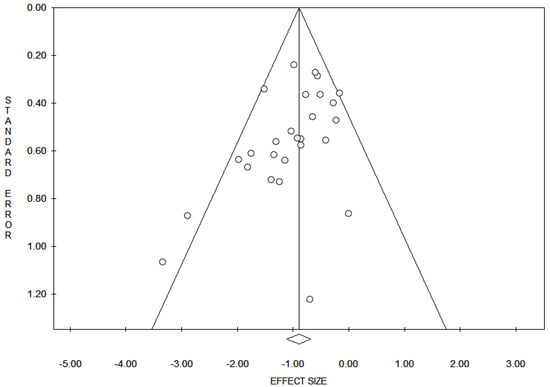
Figure 2.
Funnel plot for evaluation of bias across studies. The x-axis in the present analysis represents all the markers expression and the y-axis represents the standard error. In the absence of bias, a funnel plot should be a symmetrical inverted funnel. In the presence of bias, smaller studies with no expression would be missing, thus creating an asymmetrical funnel. Asymmetry in a funnel plot suggests that there is a systematic difference between larger and smaller studies and/or that there is publication bias.
2.5. Data Analysis
HER2/neu, ER, AR, and PR expression rates across all studies were aggregated using the meta-analytic software ProMeta 2.0 (Internovi, Cesena, Italy). The inverse-variance method was utilized to obtain an overall effect size of the pooled rates of malignancy across studies. Following this, a random effects model was used as a conservative approach to discriminate the different sources of variation among studies (i.e., within-study variance and between-studies variance) [41]. Q and I2 statistics were then conducted to evaluate heterogeneity across studies [42]. In detail, a significant Q value denotes the lack of homogeneity among studies; on the other hand, the proportion of observed variance, which indicates real differences in effect sizes was calculated with I2 statistics: values of 25%, 50%, and 75% were considered as low, moderate, and high, respectively [43]. Moreover, heterogeneity across study findings was determined using a moderator analysis. Sensitivity analyses were also performed to determine the stability of study results, computing how the overall rates would change by removing one study at a time. Finally, publication bias analyses were established with two tests: the regression method reported by Egger et al. and the Begg and Mazumdar rank correlation test [43,44]. The absence of publication bias is indicated in both tests by non-significant results.
3. Results
Based on our criteria, the articles that were published between 2000 and 2020 were analyzed and reported in Table 1. In detail, a total of 27 studies with 713 patients assessed the role of HER2/neu, AR, ER, and PR expression in male and female with VPD and EMPD. The median age was 68 years (range 61–75). The shapes of the funnel plots did not reveal evidence of obvious asymmetry (Figure 1). The results indicated that, in a highly heterogeneous set of 27 studies that compared VPD and EMPD, the overall rate of HER2/neu expression was 30% (95% CI = 0.25–0.36; Q = 34.47; I2 = 39.08), the expression’s rate for ER and AR was 13% (95% CI = 0.04–0.36; Q = 17.36; I2 = 77.48) and 40% (95% CI = 0.34–0.47; Q = 4.79; I2 = 0.00), respectively, and the overall rate for PR was 8% (95% CI = 0.02–0.24; Q = 5.98; I2 = 49.79) with p < 0.05. The result of publication bias analyses was: Egger test, −1.60; p = 0.014; Begg and Mazumdar test, −2.89; p = 0.04. Following this, we computed the rate of immuno-markers expression in male and female patients (Table 2). Table 3 illustrates the cut-off values for immunohistochemical markers in the selected studies.

Table 1.
Characteristics of Included Studies in the Meta-Analysis.

Table 2.
Summary of meta-analytic results.

Table 3.
Evaluation of immunohistochemical markers in the selected studies.
Human epidermal growth factor Receptor 2 (HER 2/neu)
The analyses indicated that the expression of HER2/neu in female and male patients was 32% (95% CI = 0.27–0.38) and 26% (95% CI=0.18-0.36), respectively, in a heterogeneous set of 22 studies involving a total of 550 patients.
Estrogen Receptor (ER)
The analyses indicated that the expression of ER was 12% (95% CI = 0.03–0.36) in female and 9% (95% CI= 0.00–0.68) in male patients, in a set of five studies involving a total of 118 patients.
Androgen Receptor (AR)
The analyses indicated that the expression of AR was 40% (95% CI = 0.34–0.47) in female and 40% (95% CI = 0.32–0.48) in male patients, in a set of seven studies involving a total of 227 patients.
Progesterone Receptor (PR)
The analyses indicated that the expression of PR was 9% (95% CI = 0.03–0.25) in female patients in a total set of four studies involving 95 patients. There was only one study that involved five male patients and the rate observed was 2%. Unfortunately, in these cases it was impossible to calculate the heterogeneity’s test.
4. Discussion
EMPD, also referred as in situ adenocarcinoma of the skin, is a rare malignant disorder of skin occurring on cutaneous sites with abundant apocrine sweat glands and hair follicles [1,2,3,4,5]. The most common sites of occurrence are represented by the vulvar region, perineal, perianal, scrotal, and penile skin. Axilla, buttocks, thighs, eyelids, and the external auditory canal represent other uncommon sites of occurrence [1,2,3,4,5]. Clinically, EMPD manifests as erythematous or persistent, eczema-like skin lesions [1,2,3,4,5].
The majority of primary EMPD, are confined to the epidermis, with a slow growth and exceptional metastases. However, cases with dermal invasion show an increased propensity for lymph node involvement and distant metastases [45]. In this subset of patients, imaging, ultrasound guided aspirative cytology, as well as sentinel lymph node biopsy have proven interesting results for the early detection of metastases and therapeutic management [46,47,48,49,50].
Before rendering the diagnosis of primary Paget disease, synchronous or metachronous secondary malignancies arising from the underlying dermis and adjacent or distant organs must be taken into consideration. In detail, sweat gland adenocarcinoma, colorectal carcinoma, prostatic carcinoma, endometrioid adenocarcinoma, and urothelial carcinoma represent possible etiologic factors of secondary EMPD [6,9,11,12,51].
In the present review and meta-analysis, we mainly focused on the hormonal environment and HER2 status in EMPD. Surprisingly, all papers before 2000 failed to demonstrate ER, PR, and AR expression, while, starting from 2000, we noted a hormonal background in EMPD mainly dominated by AR (Table 1). In detail, the observed expression rates for ER, PR, and AR were 13%, 9%, and 40%, respectively. Considering the patients’ sex, our results, in a total set of 4 studies involving 95 patients, have shown that the expression of ER was 12% (95% CI = 0.03–0.36) in female and 9% (95% CI= 0.00–0.68) in male patients and that the expression of PR was 9% (95% CI = 0.03–0.25) in female patients, and 2% in male patients. On the other hand, in a set of seven studies involving a total of 227 patients, higher expression rates of AR were detected both in female (40%; 95% CI = 0.34–0.47) and male (40%; 95% CI = 0.32–0.48) patients. According to these findings, anti-androgen target therapy seems promising tool in the management of EMPD [52].
Regarding ER and PR expression in EMPD, limited and conflicting results are still available. However, a recent study by Garganese et al., reported a remarkably high percentage of ER-positive EMPD (at least 70%), which may provide novel insights in the future hormonal treatment of this disease [19].
Regarding HER2 status, our results indicated that, in a highly heterogeneous set of 27 studies, the overall rate of HER2/neu expression was 30% (95% CI = 0.25–0.36; Q = 34.47; I2 = 39.08). Considering the patients’ sex, the performed analyses have also indicated that the expression of HER2/neu in female and male patients was 32% (95% CI = 0.27–0.38) and 26% (95% CI=0.18–0.36), respectively. Moreover, some authors highlighted a possible correlation between HER2 overexpression and disease recurrence, dermal invasion, and lymph-node metastases [33,34,35,36].
Few studies have also analyzed HER2 overexpression and gene amplification in metastatic patients. Ogawa et al. have found HER2 overexpression in 19.4% of the lesions, three of which with HER2 amplification by CISH [32]. Tanaka et al. reported that the ERBB2 gene was amplified in all cases with a HER2 score of 3+ [37]. Other authors detected by CISH HER2 gene amplification in 43% of the lesions. HER2 protein overexpression (score 3+ by IHC) was found in 12 tumors (52%), including all 10 tumors with gene amplification [39].
A good overall concordance between HER2 status in primary tumors and in the corresponding metastatic sites has also been described in EMPD [37]. This finding contrasts with the reported discordance rates of HER2 expression between primary and metastatic lesions reported for breast and gastric cancer [53].
According to these results, we can conclude that HER2/neu overexpression is found in at least one-third of EMPD lesions, probably characterized by poor outcome related to deep invasion, recurrence, and node metastases. However, therapies targeting HER2 may be useful in treating HER2 positive advanced and/or metastatic patients [28,36].
Moreover, several studies in the field of epigenetics have documented the pathogenic role of MicroRNAs (miRNAs) in different solid tumors, including HER2 positive breast cancer [54,55,56]. MiRNAs are small endogenous non-coding RNAs with a wide range of cellular functions. In breast cancer, both oncogenic and tumor suppressor properties have been related to specific miRNAs. In detail, miRNAs are involved in different stages of breast cancer progression, such as tumor growth, apoptosis, differentiation, angiogenesis, metastasis, and drug resistance [54,55,56]. Importantly, the tumor suppressor role of miRNAs has been recently highlighted also in HER2-overexpressing breast cancer where they mediate the downstream signaling of HER2, suppress the expression of HER2 and affect responses to anti-HER2 therapies [55].
In this regard, understanding the role of miRNAs in HER2-positive tumors is of great importance for the future development of novel and individualized target-therapies.
5. Conclusions
In conclusion, we believe that from the presented meta-analyses some relevant conclusions can be derived: AR status and HER2/neu overexpression/amplification have been shown as two fundamental pathogenetic pathways in both female and male patients affected by EMPD. Moreover, a possible relation between AR/HER2 and tumor invasion/recurrence/metastatic disease have been reported. These findings, anyway, need to be corroborate by further multicentric studies and confirmed by prospective clinical trials using appropriate standardized criteria for hormonal status assessment.
Author Contributions
Conceptualization, A.S., F.I., G.F.Z.; Methodology, P.S., D.A., A.M.; Software, P.S., D.A., A.P.; Validation, G.F.Z., G.S., G.G.; Formal Analysis, A.S., G.A., F.C.; Investigation, A.S., G.A., F.I.; Resources, G.G., M.V., S.S., N.D.; Data Curation, G.G., D.A.; Writing—Original Draft Preparation, A.S., G.A.; Writing—Review and Editing, A.S., G.A., F.I.; Supervision, G.F.Z., G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Antonino Mulè from Castelvetrano (Italy) for sharing his knowledge in the field of breast pathology and Paget disease.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Crocker, H.R. Paget’s disease affecting the scrotum and penis. Trans. Pathol. Soc. Lond. 1889, 40, 187–191. [Google Scholar]
- Crawford, D.; Nimmo, M.; Clement, P.B.; Thomson, T.; Benedet, J.L.; Miller, D.; Gilks, C.B. Prognostic factors in Paget’s disease of the vulva: A study of 21 cases. Int. J. Gynecol. Pathol. 1999, 18, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wong, T.W.; Lee, J.Y. Depigmented genital extramammary Paget’s disease: A possible histogenetic link to Toker’s clear cells and clear cell papulosis. J. Cutan Pathol. 2001, 28, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Zollo, J.D.; Zeitouni, N.C. The Roswell Park Cancer Institute experience with extramammary Paget’s disease. Br. J. Dermatol. 2000, 142, 59–65. [Google Scholar] [CrossRef]
- Crum, C.P.; Herrington, C.S.; McCluggage, W.G.; Regauer, S.; Wilkinson, E.J. Tumours of the vulva. In WHO Classification of Tumours of Female Reproductive Organs, 4th ed.; Kurman, R.J., Carcangiu, M.L., Herrington, C.S., Young, R.H., Eds.; International Agency for Research on Cancer: Lyon, France, 2014; pp. 236–237. [Google Scholar]
- Mehta, N.J.; Torno, R.; Sorra, T. Extramammary Paget’s disease. South Med. J. 2000, 93, 713–715. [Google Scholar] [CrossRef]
- Willman, J.H.; Golitz, L.E.; Fitzpatrick, J.E. Vulvar clear cells of Toker: Precursors of extramammary Paget’s disease. Am. J. Dermatopathol. 2005, 27, 185–188. [Google Scholar] [CrossRef]
- Van der Putte, S.C. Mammary-like glands of the vulva and their disorders. Int. J. Gynecol. Pathol. 1994, 13, 150–160. [Google Scholar] [CrossRef]
- Wilkinson, E.J.; Brown, H.M. Vulvar Paget disease of urothelial origin: A report of three cases and a proposed classification of vulvar Paget disease. Hum. Pathol. 2002, 33, 549–554. [Google Scholar] [CrossRef]
- Van der Linden, M.; Meeuwis, K.A.; Bulten, J.; Bosse, T.; van Poelgeest, M.I.; de Hullu, J.A. Paget disease of the vulva. Crit. Rev. Oncol. Hematol. 2016, 101, 60–74. [Google Scholar] [CrossRef]
- Nowak, M.A.; Guerriere-Kovach, P.; Pathan, A.; Campbell, T.E.; Deppisch, L.M. Perianal Paget’s disease: Distinguishing primary and secondary lesions using immunohistochemical studies including gross cystic disease fluid protein-15 and cytokeratin 20 expression. Arch. Pathol. Lab. Med. 1998, 122, 1077–1081. [Google Scholar]
- Phyo, A.K.; Mun, K.S.; Kwan, K.C.; Ann, C.C.; Kuppusamy, S. Genitourinary extramammary Paget’s disease: Review and outcome in a multidisciplinary setting. Int. J. Clin. Exp. Pathol. 2020, 13, 2369–2376. [Google Scholar] [PubMed]
- Mantovani, G.; Fagotti, A.; Franchi, M.; Scambia, G.; Garganese, G. Reviewing vulvar Paget’s disease molecular bases. Looking forward to personalized target therapies: A matter of CHANGE. Int. J. Gynecol. Cancer 2019, 29. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, S.; Akiyama, M.; Shimizu, H. High expression of Ki-67 and cyclin D1 in invasive extramammary Paget’s disease. J. Dermatol. Sci. 2008, 50, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.K.; Vasef, M.A. HER-2 Gene Amplification in Paget Disease of the Nipple and Extramammary Site: A Chromogenic In Situ Hybridization Study. Diagn. Mol. Pathol. 2006, 15, 131–135. [Google Scholar] [CrossRef]
- Brummer, O.; Stegnerb, H.E.; Bfhmera, G.; Kqhnlea, H.; Petry, K.U. HER-2/neu expression in Paget disease of the vulva and the female breast. Ginecol. Oncol. 2004, 95, 336–340. [Google Scholar] [CrossRef]
- Diaz de Leon, E.; Carcangiu, M.L.; Prieto, V.G.; McCue, P.A.; Burchette, J.L.; To, G.; Norris, B.A.; Kovatich, A.J.; Sanchez, R.L.; Krigman, H.R.; et al. Extramammary Paget Disease Is Characterized by the Consistent Lack of Estrogen and Progesterone Receptors But Frequently Expresses Androgen Receptor. Am. J. Clin. Pathol. 2000, 113, 572–575. [Google Scholar] [CrossRef]
- Fujimoto, A.; Takata, M.; Hatta, N.; Takehara, K. Expression of Structurally Unaltered Androgen Receptor in Extramammary Paget’s Disease. Lab. Investig. 2000, 80, 1465–1471. [Google Scholar] [CrossRef][Green Version]
- Garganese, G.; Inzani, F.; Mantovani, G.; Santoro, A.; Valente, M.; Babini, G.; Petruzzellis, G.; Fragomeni, S.M.; Gentileschi, S.; Bove, S.; et al. The vulvar immunohistochemical panel (VIP) project: Molecular profiles of vulvar Paget’s disease. J. Cancer Res. Clin. Oncol. 2019, 145, 2211–2225. [Google Scholar] [CrossRef]
- Gatalica, Z.; Vranic, S.; Krušlin, B.; Poorman, K.; Stafford, P.; Kacerovska, D.; Senarathne, W.; Florento, E.; Contreras, E.; Leary, A.; et al. Comparison of the biomarkers for targeted therapies in primary extra-mammary and mammary Paget’s disease. Cancer Med. 2020, 9, 1441–1450. [Google Scholar] [CrossRef]
- Hanna, W.; Alowami, S.; Malik, A. The role of HER-2/neu oncogene and vimentin filaments in the production of the Paget’s phenotype. Breast J. 2003, 9, 485–490. [Google Scholar] [CrossRef]
- Hikita, T.; Ohtsuki, Y.; Maeda, T.; Furihata, M. Immunohistochemical and fluorescence in situ hybridization studies on noninvasive and invasive extramammary Paget’s disease. Int. J. Surg. Pathol. 2012, 20, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.C.; Purz, S.; Krumpe, C.; Bilek, K. COX-2 and Her-2/neu are overexpressed in Paget’s disease of the vulva and the breast: Results of a preliminary study. Arch. Gynecol. Obstet. 2008, 277, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Inoguchi, N.; Matsumura, Y.; Kanazawa, N.; Morita, K.; Tachibana, T.; Sakurai, T.; Utani, A.; Miyachi, Y. Expression of prostate-specific antigen and androgen receptor in extramammary Paget’s disease and carcinoma. Clin. Exp. Dermatol. 2007, 32, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Kasashima, S.; Ozaki, S.; Kawashima, A.; Zen, Y.; Moriya, T.; Inoue, M. Androgen receptor and 5alphareductase immunohistochemical profiles in extramammary Paget disease. Br. J. Dermatol. 2010, 162, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Liegl, B.; Horn, L.C.; Moinfar, F. Androgen receptors are frequently expressed in mammary and extramammary Paget’s disease. Mod. Pathol. 2005, 18, 1283–1288. [Google Scholar] [CrossRef]
- Liu, W.; Iqbal, J.; Khoury, T. Mammary Paget’s disease and extra-mammary Paget’s disease: Two morphologically similar but biologically different diseases. J. Cutan Pathol. 2010, 37, 1145–1149. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, P.; Zhu, Y.; Ye, D. Human epidermal growth factor receptor 2 amplification as a biomarker for treatment in patients with lymph node-metastatic penoscrotal extramammary Paget’s disease. Oncol. Lett. 2019, 17, 2677–2686. [Google Scholar] [CrossRef]
- Masuguchi, S.; Jinnin, M.; Fukushima, S.; Makino, T.; Sakai, K.; Inoue, Y.; Igata, T.; Ihn, H. The expression of HER-2 in extramammary Paget’s disease. BioScience Trends 2011, 5, 151–155. [Google Scholar] [CrossRef]
- Miyamoto, A.; Akasaka, K.; Oikawa, H.; Akasaka, T.; Masuda, T.; Maesawa, C. Immunohistochemical study of HER2 and TUBB3 proteins in extramammary Paget disease. Am. J. Dermatopathol. 2010, 32, 578–585. [Google Scholar] [CrossRef]
- Morbeck, D.; Tregnago, A.C.; Netto, G.B.; Sacomani, C.; Peresi, P.M.; Osório, C.T.; Schutz, L.; Bezerra, S.M.; de Brot, L.; Cunha, I.W. GATA3 expression in primary vulvar Paget disease: A potential pitfall leading to misdiagnosis of pagetoid urothelial intraepithelial neoplasia. Histopathology 2017, 70, 435–441. [Google Scholar] [CrossRef]
- Ogawa, T.; Nagashima, Y.; Wada, H.; Akimoto, K.; Chiba, Y.; Nagatani, T.; Inayama, Y.; Yao, M.; Aoki, I.; Ikezawa, Z. Extramammary Paget’s disease: Analysis of growth signal pathway from the human epidermal growth factor receptor 2 protein. Hum. Pathol. 2005, 36, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Plaza, J.A.; Torres-Cabala, C.; Ivan, D.; Prieto, V.G. HER-2/neu expression in extramammary Paget disease:a clinicopathologic and immunohistochemistry study of 47 cases with and without underlying malignancy. J. Cutan Pathol. 2009, 36, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Reich, O.; Liegl, B.; Tamussino, K.; Regauer, S. p185HER2 overexpression and HER2 oncogene amplification in recurrent vulvar Paget’s disease. Mod. Pathol. 2005, 18, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.E.; Hui, P.; Buza, N.; Silasi, D.A.; Azodi, M.; Santin, A.D.; Schwart, P.E.; Rutherford, T.J. HER-2/NEU overexpression in vulvar Paget disease: The Yale experience. J. Clin. Pathol. 2010, 63, 544–573. [Google Scholar] [CrossRef]
- Sekiguchi, N.; Kubota, S.; Noguchi, T.; Fukushima, T.; Kobayashi, T.; Kanda, S.; Koizumi, T.; Miyake, T.; Shirai, T.; Okuyama, R. Experiences of trastuzumab plus paclitaxel combination therapy in metastatic human epidermal growth factor receptor 2-positive extramammary Paget’s disease: Four cases and a review. J. Dermatol. 2020, 47, 1276–1279. [Google Scholar] [CrossRef]
- Tanaka, R.; Sasajima, Y.; Tsuda, H.; Namikawa, K.; Takahashi, A.; Tsutsumida, A.; Fujimoto, M.; Yamazaki, N. Concordance of the HER2 protein and gene status between primary and corresponding lymph node metastatic sites of extramammary Paget disease. Clin. Exp. Metastasis 2016, 33, 687–697. [Google Scholar] [CrossRef]
- Tanaka, R.; Sasajima, Y.; Tsuda, H.; Namikawa, K.; Tsutsumida, A.; Otsuka, F.; Yamazaki, N. Human epidermal growth factor receptor 2 protein overexpression and gene amplification in extramammary Paget disease. Br. J. Dermatol. 2013, 168, 1259–1266. [Google Scholar] [CrossRef]
- Tanskanen, M.; Jahkola, T.; Asko-Seljavaara, S.; Jalkanen, J.; Isola, J. HER2 oncogene amplification in extramammary Paget’s disease. Histopathology 2003, 42, 575–579. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, W.; Zhu, H.; Yu, H. Extramammary Paget’s Disease in Two Brothers. Indian J. Dermatol. 2015, 60, 423. [Google Scholar]
- Huedo-Medina, T.B.; Sanchez-Meca, J.; Marìn-Martìnez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, T.; Noguchi, T.; Nakayama, H.; Yoshida, Y.; Yamamoto, O.; Hayashi, N.; Ohara, K. Clinicopathological study of invasive extramammary Paget’s disease: Subgroup comparison according to invasion depth. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, Y.; Yoshino, K.; Kiyohara, Y.; Kadono, T.; Murata, Y.; Uhara, H.; Hatta, N.; Uchi, H.; Matsushita, S.; Takenouchi, T.; et al. The role of sentinel lymph node biopsy in the management of invasive extramammary Paget’s disease: Multi-center, retrospective study of 151 patients. J. Dermatol. Sci. 2015, 79, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Angelico, G.; Santoro, A.; Inzani, F.; Spadola, S.; Fiorentino, V.; Cianfrini, F.; Carbone, C.; Garganese, G.; Rossi, E.D.; Scambia, G.; et al. Ultrasound-guided FNA cytology of groin lymph nodes improves the management of squamous cell carcinoma of the vulva: Results from a comparative cytohistological study. Cancer Cytopathol. 2019, 127, 514–520. [Google Scholar] [CrossRef]
- Garganese, G.; Fragomeni, S.M.; Pasciuto, T.; Leombroni, M.; Moro, F.; Evangelista, M.T.; Bove, S.; Gentileschi, S.; Tagliaferri, L.; Paris, I.; et al. Ultrasound morphometric and cytologic preoperative assessment of inguinal lymph-node status in women with vulvar cancer: MorphoNode study. Ultrasound Obstet Gynecol. 2020, 55, 401–410. [Google Scholar] [CrossRef]
- Garganese, G.; Collarino, A.; Fragomeni, S.M.; Rufini, V.; Perotti, G.; Gentileschi, S.; Evangelista, M.T.; Ieria, F.P.; Zagaria, L.; Bove, S.; et al. Groin sentinel node biopsy and 18F-FDG PET/ CT-supported preoperative lymph node assessment in cN0 patients with vulvar cancer currently unfit for minimally invasive inguinal surgery: The GroSNaPET study. Eur. J. Surg. Oncol. 2017, 43, 1776–1783. [Google Scholar] [CrossRef]
- Collarino, A.; Fuoco, V.; Garganese, G.; Pereira Arias-Bouda, L.M.; Perotti, G.; Manca, G.; Vidal-Sicart, S.; Giammarile, F.; de Geus-Oei, L.F.; Scambia, G.; et al. Lymphoscintigraphy and sentinel lymph node biopsy in vulvar carcinoma: Update from a European expert panel. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1261–1274. [Google Scholar] [CrossRef]
- Inzani, F.; Angelico, G.; Santoro, A.; Musarra, T.; Valente, M.; Spadola, S.; Garganese, G.; Fragomeni, S.; Scambia, G.; Zannoni, G.F. A new entity in the pathological spectrum of vulvar neoplasms: The first report of a primary endometrioid adenocarcinoma. Int. J. Gynaecol. Obstet. 2019, 147, 270–272. [Google Scholar] [CrossRef]
- Yoneyama, K.; Kamada, N.; Kinoshita, K.; Kawashima, T.; Otani, M.; Endo, H.; Shinkai, H.; Utani, A. Androgen-deprivation regimen for multiple bone metastases of extramammary Paget disease. Br. J. Dermatol. 2005, 153, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Ieni, A.; Angelico, G.; Giuffrè, G.; Tuccari, G. Discordance Rate of HER2 Status in Primary Gastric Cancer and Synchronous Lymph Node Metastases: Its Impact on Therapeutic Decision and Clinical Management. Pathol. Oncol. Res. 2018, 24, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Buonavoglia, A.; Fasano, R.; Solimando, A.G.; De Re, V.; Cicco, S.; Vacca, A.; Racanelli, V. Insights into the Regulation of Tumor Angiogenesis by Micro-RNAs. J. Clin. Med. 2019, 8, 2030. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.E.; Lin, R.J. MicroRNA and HER2-overexpressing cancer. Microrna 2013, 2, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Goradel, N.H.; Mohammadi, N.; Haghi-Aminjan, H.; Farhood, B.; Negahdari, B.; Sahebkar, A. Regulation of tumor angiogenesis by microRNAs: State of the art. J. Cell Physiol. 2019, 234, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).