Predictive Value of Carcinoembryonic Antigen in Symptomatic Patients without Colorectal Cancer: A Post-Hoc Analysis within the COLONPREDICT Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Measurements and Definitions
2.4. Follow-Up and Main Outcome
2.5. Statistical Analysis
3. Results
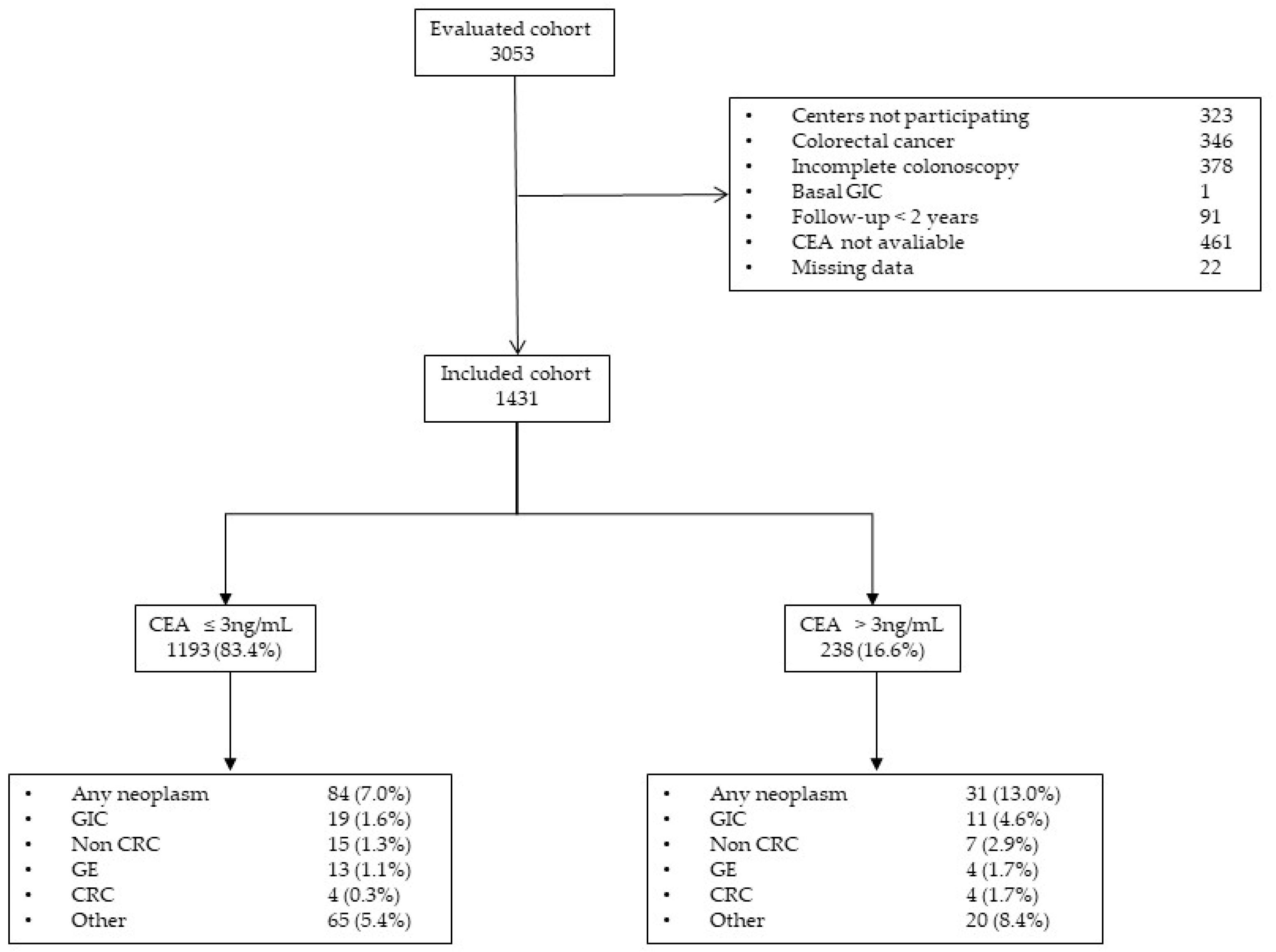
3.1. Participants
3.2. Cancer Diagnosis
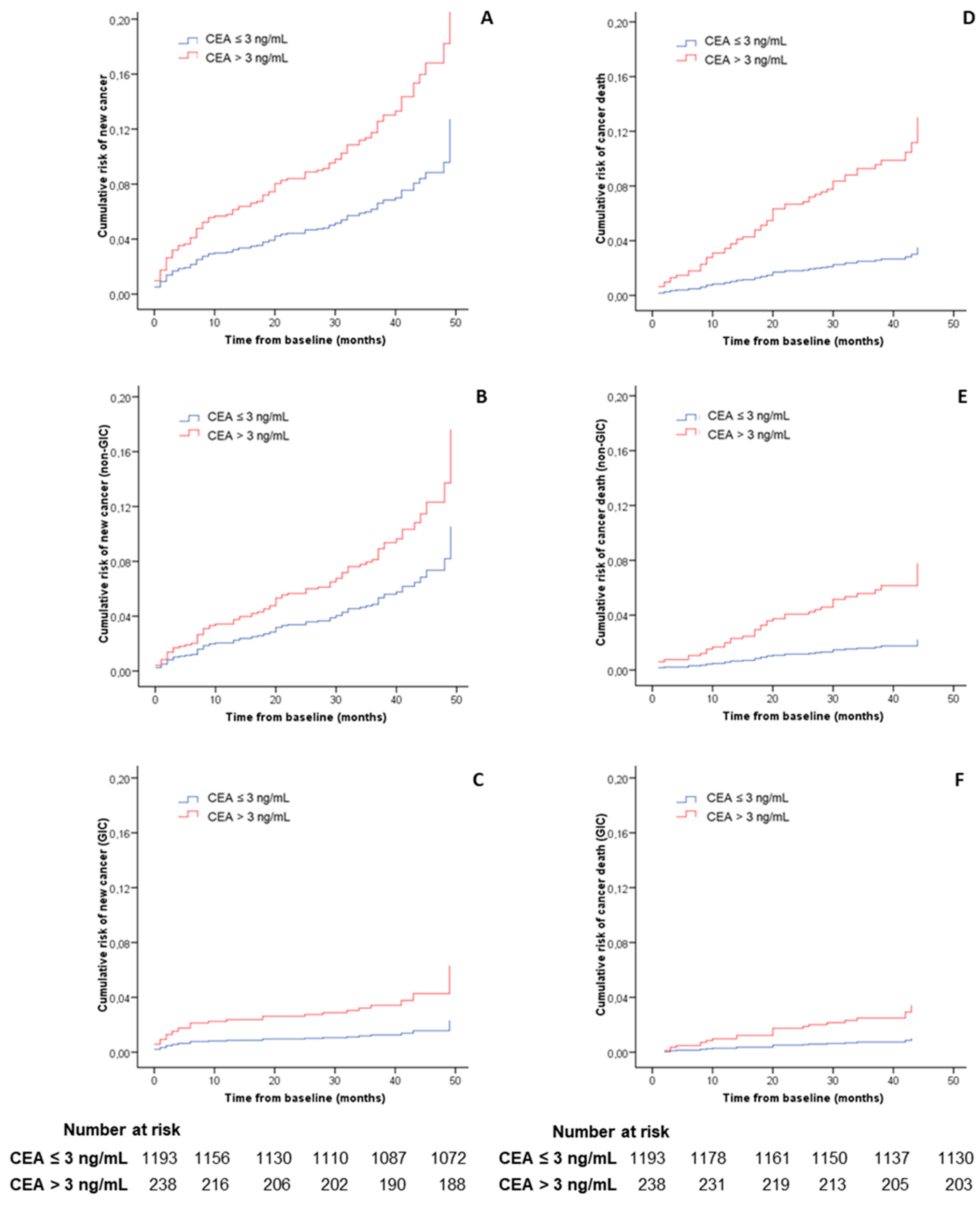
3.3. Main Outcome
3.4. Risk of Cancer Diagnosis during the First Year of Follow-Up
3.5. Diagnostic Accuracy of CEA for Cancer Diagnosis the First Year of Follow-Up
4. Discussion
4.1. Statement of Principal Findings
4.2. Strengths and Weaknesses of Our Study
4.3. Strengths and Weaknesses in Relation to Other Studies
4.4. Implications for Clinical Practice and Research
4.5. Unanswered Questions and Future Research
4.6. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koo, M.M.; Hamilton, W.; Walter, F.M.; Rubin, G.P.; Lyratzopoulos, G. Symptom Signatures and Diagnostic Timeliness in Cancer Patients: A Review of Current Evidence. Neoplasia 2018, 20, 165–174. [Google Scholar] [CrossRef]
- Koo, M.M.; Swann, R.; McPhail, S.; Abel, G.A.; Elliss-Brookes, L.; Rubin, G.P.; Lyratzopoulos, G. Presenting symptoms of cancer and stage at diagnosis: Evidence from a cross-sectional, population-based study. Lancet Oncol. 2020, 21, 73–79. [Google Scholar] [CrossRef]
- Lee, J.K.; Liles, E.G.; Bent, S.; Levin, T.R.; Corley, D.A. Accuracy of fecal immunochemical tests for colorectal cancer: Systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 171. [Google Scholar] [CrossRef]
- Pin-Vieito, N.; Zarraquiños, S.; Cubiella, J. High-risk symptoms and quantitative faecal immunochemical test accuracy: Systematic review and meta-analysis. World J. Gastroenterol. 2019, 25, 2383–2401. [Google Scholar] [CrossRef]
- NICE Diagnostics Guidance DG30. Quantitative Faecal Immunochemical Tests to Guide Referral for Colorectal Cancer in Primary Care. 2017. Available online: https://www.nice.org.uk/guidance/dg30 (accessed on 9 May 2019).
- Hall, C.; Clarke, L.; Pal, A.; Buchwald, P.; Eglinton, T.; Wakeman, C.; Frizelle, F. A Review of the Role of Carcinoembryonic Antigen in Clinical Practice. Ann. Coloproctol. 2019, 35, 294–305. [Google Scholar] [CrossRef]
- Holdenrieder, S.; Wehnl, B.; Hettwer, K.; Simon, K.; Uhlig, S.; Dayyani, F. Carcinoembryonic antigen and cytokeratin-19 fragments for assessment of therapy response in non-small cell lung cancer: A systematic review and meta-analysis. Br. J. Cancer 2017, 116, 1037–1045. [Google Scholar] [CrossRef]
- Li, X.; Dai, D.; Chen, B.; Tang, H.; Xie, X.; Wei, W. Clinicopathological and Prognostic Significance of Cancer Antigen 15-3 and Carcinoembryonic Antigen in Breast Cancer: A Meta-Analysis including 12,993 Patients. Dis. Markers 2018, 2018, 9863092. [Google Scholar] [CrossRef]
- Kim, J.R.; Jang, J.Y.; Kang, M.J.; Park, T.; Lee, S.Y.; Jung, W.; Chang, J.; Shin, Y.; Han, Y.; Kim, S.W. Clinical implication of serum carcinoembryonic antigen and carbohydrate antigen 19-9 for the prediction of malignancy in intraductal papillary mucinous neoplasm of pancreas. J. Hepatobiliary Pancreat. Sci. 2015, 22, 699–707. [Google Scholar] [CrossRef]
- Locker, G.Y.; Hamilton, S.; Harris, J.; Jessup, J.M.; Kemeny, N.; Macdonald, J.S.; Somerfield, M.R.; Hayes, D.F.; Bast, R.C. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol. 2006, 24, 5313–5327. [Google Scholar] [CrossRef]
- Wilson, A.P.M.; van Dalen, A.; Sibley, P.E.C.; Kasper, L.A.; Durham, A.P.; El Shami, A.S. Multicenter tumour marker reference range study. Anticancer Res. 1999, 19, 2749–2752. [Google Scholar]
- Lim, Y.K.; Kam, M.H.; Eu, K.W. Carcinoembryonic antigen screening: How far should we go? Singapore Med. J. 2009, 50, 862–865. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: +GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Pin-Vieito, N.; Iglesias, M.J.; Remedios, D.; Rodríguez-Alonso, L.; Rodriguez-Moranta, F.; Álvarez-Sánchez, V.; Fernández-Bañares, F.; Boadas, J.; Martínez-Bauer, E.; Campo, R.; et al. On Behalf of the Colonpredict Study Investigators. Risk of gastrointestinal cancer in a symptomatic cohort after a complete colonoscopy: Role of faecal immunochemical test. World J. Gastroenterol. 2020, 26, 70–85. [Google Scholar] [CrossRef]
- Cubiella, J.; Vega, P.; Salve, M.; Díaz-Ondina, M.; Alves, M.T.; Quintero, E.; Álvarez-Sánchez, V.; Fernández-Bañares, F.; Boadas, J.; Campo, R.; et al. COLONPREDICT study investigators. Development and external validation of a faecal immunochemical test-based prediction model for colorectal cancer detection in symptomatic patients. BMC Med. 2016, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef]
- Cubiella, J.; Castells, A.; Andreu, M.; Bujanda, L.; Carballo, F.; Jover, R.; Lanas, Á.; Morillas, J.D.; Salas, D.; Quintero, E. COLONPREV study investigators. Correlation between adenoma detection rate in colonoscopy- and fecal immunochemical testing-based colorectal cancer screening programs. United Eur. Gastroenterol. J. 2017, 5, 255–260. [Google Scholar] [CrossRef]
- Pin-Vieito, N.; García-Nimo, L.; Bujanda, L.; Román-Alonso, B.; Gutiérrez-Stampa, M.Á.; Aguilar-Gama, V.; Portillo, I.; Cubiella, J. Optimal diagnostic accuracy of quantitative faecal immunochemical test positivity thresholds for colorectal cancer detection in primary health care: A community-based cohort study. United Eur. Gastroenterol. J. 2020, 10. [Google Scholar] [CrossRef]
- Jover, R.; Herráiz, M.; Alarcón, O.; Brullet, E.; Bujanda, L.; Bustamante, M.; Campo, R.; Carreño, R.; Castells, A.; Cubiella, J.; et al. Spanish Society of Gastroenterology; Spanish Society of Gastrointestinal Endoscopy Working Group. Clinical practice guidelines: Quality of colonoscopy in colorectal cancer screening. Endoscopy 2012, 44, 444–451. [Google Scholar] [CrossRef]
- Kim, N.H.; Lee, M.Y.; Park, J.H.; Park, D.I.; Sohn, C.I.; Choi, K.; Jung, Y.S. Serum CEA and CA 19-9 Levels are Associated with the Presence and Severity of Colorectal Neoplasia. Yonsei Med. J. 2017, 58, 918–924. [Google Scholar] [CrossRef]
- Shinkins, B.; Nicholson, B.D.; James, T.; Pathiraja, I.; Pugh, S.; Perera, R.; Primrose, J.; Mant, D. What carcinoembryonic antigen level should trigger further investigation during colorectal cancer follow-up? A systematic review and secondary analysis of a randomised controlled trial. Health Technol. Assess. 2017, 21, 1–60. [Google Scholar] [CrossRef]
- Lahner, E.; Capasso, M.; Carabotti, M.; Annibale, B. Incidence of cancer (other than gastric cancer) in pernicious anaemia: A systematic review with meta-analysis. Dig. Liver Dis. 2018, 50, 780–786. [Google Scholar] [CrossRef]
- Mashlab, S.; Large, P.; Laing, W.; Ng, O.; D’Auria, M.; Thurston, D.; Thomson, S.; Acheson, A.G.; Humes, D.J.; Banerjea, A.; et al. Anaemia as a risk stratification tool for symptomatic patients referred via the two-week wait pathway for colorectal cancer. Ann. R. Coll. Surg. Engl. 2018, 100, 350–356. [Google Scholar] [CrossRef]
- Kok, V.C.; Sung, F.C.; Kao, C.H.; Lin, C.C.; Tseng, C.H. Cancer risk in East Asian patients associated with acquired haemolytic anaemia: A nationwide population-based cohort study. BMC Cancer. 2016, 16, 57. [Google Scholar] [CrossRef][Green Version]
- Hung, N.; Shen, C.C.; Hu, Y.W.; Hu, L.Y.; Yeh, C.M.; Teng, C.J.; Kuan, A.S.; Chen, S.C.; Chen, T.J.; Liu, C.J. Risk of cancer in patients with iron deficiency anemia: A nationwide population-based study. PLoS ONE 2015, 10, e0119647. [Google Scholar] [CrossRef]
- Morris, E.J.; Rutter, M.D.; Finan, P.J.; Thomas, J.D.; Valori, R. Post-colonoscopy colorectal cancer (PCCRC)rates vary considerably depending on the method used to calculate them: A retrospective observational population-based study of PCCRC in the English National Health Service. Gut 2015, 64, 1248–1256. [Google Scholar] [CrossRef]
- Allgar, V.L.; Neal, R.D. Delays in the diagnosis of six cancers: Analysis of data from the National Survey of NHS Patients: Cancer. Br. J. Cancer 2005, 92, 1959–1970. [Google Scholar] [CrossRef]
- Hamilton, W.; Walter, F.M.; Rubin, G.; Neal, R.D. Improving early diagnosis of symptomatic cancer. Nat. Rev. Clin. Oncol. 2016, 13, 740–749. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence Suspected Cancer: Recognition and Referral. NICE Guideline (NG12). 2015. Available online: https://www.nice.org.uk/guidance/ng12 (accessed on 5 September 2020).
- Koo, M.M.; von Wagner, C.; Abel, G.A.; McPhail, S.; Hamilton, W.; Rubin, G.P.; Lyratzopoulos, G. The nature and frequency of abdominal symptoms in cancer patients and their associations with time to help-seeking: Evidence from a national audit of cancer diagnosis. J. Public Health 2018, 40, e388–e395. [Google Scholar] [CrossRef]
| Characteristics | Overall (n = 1431) | CEA ≤ 3ng/mL (n = 1193) | CEA > 3 ng/mL (n = 238) | p |
|---|---|---|---|---|
| Demographic | ||||
| Age, (years) | 66.7 (20.0) | 66.2 (19.5) | 69.1 (22.0) | <0.01 |
| Female sex, no. (%) | 748 (52.3) | 634 (53.1) | 114 (47.9) | 0.16 |
| Primary healthcare referral, no. (%) | 390 (27.3) | 335 (28.1) | 55 (23.1) | 0.13 |
| Previous colonoscopy, no. (%) | 398 (27.8) | 326 (27.3) | 72 (30.3) | 0.38 |
| Daily using ASA, no. (%) | 280 (19.6) | 223 (18.7) | 57 (23.9) | 0.07 |
| f-Hb concentration | 33.0 (140.0) | 33.0 (139.5) | 31.0 (142.0) | 0.8 |
| Indications, no. (%) | ||||
| Rectal bleeding | 796 (55.6) | 675 (56.6) | 121 (50.8) | 0.12 |
| Change of bowel habit | 839 (58.6) | 705 (59.1) | 134 (56.3) | 0.43 |
| Anaemia 1 | 338 (34.1) | 279 (33.4) | 59 (38.1) | 0.27 |
| Abdominal pain 1 | 451 (45.6) | 378 (45.3) | 73 (47.1) | 0.73 |
| Weight loss 1 | 240 (24.2) | 197 (23.6) | 43 (27.7) | 0.26 |
| Basal colonoscopy findings, no. (%) | ||||
| Benign anorectal lesion | 612 (42.8) | 521 (43.7) | 91 (38.2) | 0.13 |
| Significant colonic lesions | 277 (19.4) | 233 (19.5) | 44 (18.5) | 0.79 |
| Advanced adenoma | 206 (14.4) | 176 (14.8) | 30 (12.6) | 0.42 |
| Follow-up, (months) | 38.0 (10.0) | 38.0 (10.0) | 37.0 (11.0) | 0.03 |
| EVENT | Risk | Overall (n = 1431) | CEA ≤ 3 ng/mL (n = 1193) | CEA > 3 ng/mL (n = 238) | RR/IR (95% CI) | p | HR (95% CI) | p |
|---|---|---|---|---|---|---|---|---|
| Gastrointestinal cancer | Cumulative 1 | 2.1 (1.5–3.0) | 1.6 (1.0–2.5) | 4.6 (2.6–8.1) | 2.9 (1.4–6.0) | <0.01 | 2.7 (1.3–5.7) | 0.01 |
| Density 2 | 6.9 (4.7–9.9) | 5.1 (2.9–8.0) | 16.1 (8.0–28.5) | 3.1 (1.5–6.5) | <0.01 | |||
| Cumulative death 1 | 1.5 (1.0–2.2) | 1.0 (0.6–1.7) | 3.8 (2.0–7.0) | 3.8 (1.6–8.8) | 0.03 | 3.4 (1.4–8.1) | 0.01 | |
| Death density 2 | 4.7 (2.9–7.3) | 3.3 (1.8–5.5) | 12.8 (5.8–24.4) | 4.0 (1.7–9.4) | <0.01 | |||
| Upper gastrointestinal cancer 3 | Cumulative 1 | 1.5 (1.0–2.3) | 1.3 (0.8–2.1) | 2.9 (1.4–5.9) | 2.3 (1.0–5.7) | 0.10 | 2.2 (0.9–5.4) | 0.09 |
| Density 2 | 5.1 (3.3–7.7) | 4.0 (2.2–6.6) | 10.2 (4.0–20.8) | 2.5 (1.0–6.1) | 0.04 | |||
| Cumulative death 1 | 1.0 (0.6–1.7) | 0.8 (0.5–1.5) | 2.1 (0.9–4.8) | 2.5 (0.9–7.3) | 0.16 | 2.3 (0.8–6.8) | 0.13 | |
| Death density 2 | 3.3 (1.8–5.5) | 2.6 (1.5–5.1) | 7.3 (2.2–16.8) | 2.6 (0.9–7.7) | 0.06 | |||
| Colorectal cancer | Cumulative 1 | 0.6 (0.3–1.1) | 0.3 (0.1–0.9) | 1.7 (0.7–4.2) | 5.0 (1.3–19.9) | 0.04 | 4.4 (1.1–17.7) | 0.04 |
| Density 2 | 1.8 (0.7–3.7) | 1.1 (0.4-2.9) | 5.8 (1.5–14.6) | 5.3 (1.3–21.3) | <0.01 | |||
| Cumulative death 1 | 0.4 (0.2–0.9) | 0.2 (0.0–0.6) | 1.7 (0.7–4.2) | 10.0 (1.8–54.4) | <0.01 | 8.8 (1.6–48.5) | <0.01 | |
| Death density 2 | 1.5 (0.4–2.9) | 0.4 (0.0–1.8) | 5.8 (1.5–14.6) | 10.6 (1.9–57.8) | <0.01 | |||
| Non gastrointestinal cancer | Cumulative 1 | 5.9 (4.8–7.3) | 5.4 (4.3–5.5) | 8.4 (5.5–12.6) | 1.5 (1.0–2.5) | 0.11 | 1.7 (1.0–2.8) | 0.04 |
| Density 2 | 19.7 (15.7–24.5) | 17.9 (13.9–22.6) | 29.6 (17.9–45.7) | 1.7 (1.0–2.7) | 0.05 | |||
| Cumulative death 1 | 2.7 (1.9–3.6) | 1.8 (1.2–2.8) | 6.7 (4.2–10.6) | 3.6 (1.9–6.8) | < 0.01 | 3.5 (1.8–6.7) | <0.01 | |
| Death density 2 | 8.8 (6.2–11.7) | 5.8 (3.7–9.1) | 22.6 (13.1–36.9) | 3.8 (2.0–7.3) | <0.01 | |||
| Cancer | Cumulative 1 | 8.0 (6.7–9.6) | 7.0 (5.7–8.6) | 13.0 (9.3–18.0) | 1.8 (1.3–2.7) | <0.01 | 2.0 (1.3–3.1) | <0.01 |
| Density 2 | 27.0 (22.3–32.1) | 23.4 (18.6–28.9) | 46.8 (31.8–66.5) | 2.0 (1.3–3.0) | <0.01 | |||
| Cumulative death 1 | 4.1 (3.2–5.3) | 2.9 (2.0–4.0) | 10.5 (7.2–15.0) | 3.7 (2.2–6.1) | <0.01 | 3.7 (2.2–6.2) | <0.01 | |
| Death density 2 | 13.5 (10.2–17.2) | 9.1 (6.2–12.8) | 35.8 (23.0–52.6) | 3.9 (2.3–6.5) | <0.01 | |||
| Death | Cumulative 1 | 7.0 (5.8–8.4) | 5.4 (4.2–6.8) | 15.1 (11.1–20.2) | 2.8 (1.9–4.1) | <0.01 | 2.6 (1.7–3.9) | <0.01 |
| Density 2 | 22.6 (18.6–27.4) | 17.2 (13.1–21.9) | 51.5 (35.8–71.2) | 3.0 (2.0–4.5) | <0.01 |
| New Cancer | Odds Ratio (95% CI) | Odds Ratio (95% CI) 1 | |
|---|---|---|---|
| Sex | |||
| Male (683) | 28 (4.0%) | 1 | |
| Female (748) | 23 (3.1%) | 0.8 (0.4–1.3) | |
| Age | |||
| ≤70 years (846) | 19(2.2%) | 1 | |
| >70 years (585) | 32(5.5%) | 2.4 (1.4–4.3) | |
| Primary healthcare referral | |||
| No (1041) | 35 (3.4%) | 1 | |
| Yes (390) | 16 (4.1%) | 1.2 (0.7–2.2) | |
| Rectal bleeding | |||
| No (635) | 37 (5.8%) | 1 | 1 |
| Yes (796) | 14 (1.8%) | 0.3 (0.2–0.6) | 0.3 (0.1–0.7) |
| Change of bowel habit | |||
| No (592) | 25 (4.2%) | 1 | |
| Yes (839) | 26 (3.1%) | 0.7 (0.4–1.3) | |
| Anaemia | |||
| No (652) | 12 (1.8%) | 1 | 1 |
| Yes (338) | 22 (6.5%) | 3.5 (1.8–7.1) | 2.8 (1.3–5.8) |
| Abdominal pain | |||
| No (539) | 21 (3.9%) | 1 | |
| Yes (451) | 13 (2.9%) | 0.7 (0.4–1.5) | |
| Weight loss | |||
| No (750) | 25 (3.3%) | 1 | |
| Yes (240) | 9 (3.4%) | 1.1 (0.5–2.4) | |
| Faecal immunochemical test | |||
| ≤10 µg/g (n = 948) | 30 (3.2%) | 1 | |
| >10 µg/g (n = 483) | 21 (4.3%) | 1.4 (0.8–2.4) | |
| Carcinoembryonic antigen value | |||
| ≤3 ng/mL (n = 1193) | 31 (2.3%) | 1 | 1 |
| >3 ng/mL (n = 238) | 20(8.4%) | 3.2 (1.9–5.6) | 3.4 (1.7–7.1) |
| Advanced adenoma | |||
| No (1225) | 43 (3.5%) | 1 | |
| Yes (206) | 8 (3.9%) | 1.1 (0.5–2.3) |
| Threshold | Type of Cancer | Anaemia | Prevalence | %AT | Sensitivity 1 | Specificity 1 | NPV ‡,1 | PPV 1 | AUC | LR+ | LR− | DOR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CEA > 3 ng/mL | GI cancer | No | 0.6 | 14.7 | 50.0 (15.0–85.0) | 85.5 (82.6–88.0) | 99.6 (98.7–99.9) | 2.1 (0.6–7.3) | 0.70 (0.41–0.99) | 3.4 | 0.6 | 5.82 |
| Yes | 3.0 | 17.5 | 30.0 (10.8–60.3) | 82.9 (78.5–86.6) | 97.5 (94.9–98.8) | 5.1 (1.7–13.9) | 0.57 (0.39–0.74) | 1.8 | 0.8 | 2.08 | ||
| Upper GI cancer 2 | No | 0.2 | 14.7 | 0.0 (0.0–79.3) | 85.3 (82.3–87.8) | 99.8 (99.0–99.96) | 0.0 (0.0–3.8) | 0.48 (0.44–0.53) | 0.0 | NA | NA | |
| Yes | 3.0 | 17.5 | 30.0 (10.8–60.3) | 82.9 (78.5–86.6) | 97.5 (94.9–98.8) | 5.1 (1.7–13.9) | 0.57 (0.39–0.74) | 1.8 | 0.8 | 2.08 | ||
| Colorectal cancer | No | 0.5 | 14.7 | 66.7 (20.8–93.9) | 85.5 (82.6–88.0) | 99.8 (99.0–99.96) | 2.1 (0.6–7.3) | 0.77 (0.42–1.0) | 4.6 | 0.4 | 11.80 | |
| Yes | 0.0 | 17.5 | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Non-GI cancer | No | 1.2 | 14.7 | 50.0 (21.5–78.5) | 85.7 (82.8–88.5) | 99.3 (98.2–99.7) | 4.2 (1.6–10.2) | 0.60 (0.32–0.87) | 3.5 | 0.6 | 6.00 | |
| Yes | 3.6 | 17.5 | 33.3 (13.8–60.9) | 83.1 (78.7–86.8) | 97.1 (94.4–98.5) | 6.8 (2.7–16.2) | 0.49 (0.28–0.70) | 2.0 | 0.8 | 2.46 | ||
| Cancer | No | 1.8 | 14.7 | 50.0 (25.4–74.6) | 85.9 (83.0–88.4) | 98.9 (97.7–99.5) | 6.3 (2.9–13.0) | 0.63 (0.42–0.84) | 3.6 | 0.6 | 6.11 | |
| Yes | 6.5 | 17.5 | 31.8 (16.4–52.7) | 83.5 (79.1–87.2) | 94.6 (91.3–96.7) | 11.9 (5.9–22.5) | 0.53 (0.38–0.67) | 1.9 | 0.8 | 2.37 | ||
| CEA >5 ng/mL | GI cancer | No | 0.6 | 5.1 | 50.0 (15.0–85.0) | 99.2 (93.3–96.6) | 99.7 (98.8–99.9) | 6.1 (1.7–19.6) | 0.70 (0.41–0.99) | 10.5 | 0.5 | 19.91 |
| Yes | 3.0 | 8.6 | 20.0 (5.7–51.0) | 91.8 (88.3–94.3) | 97.4 (95.0–98.7) | 6.9 (1.9–22.0) | 0.57 (0.39–0.74) | 2.4 | 0.9 | 2.79 | ||
| Upper GI cancer 2 | No | 0.2 | 5.1 | 0.0 (0.0–79.3) | 94.9 (93.0–96.4) | 99.8 (99.1–99.97) | 0.0 (0.0–10.4) | 0.48 (0.44–0.53) | 0.0 | NA | NA | |
| Yes | 3.0 | 8.6 | 20.0 (5.7–51.0) | 91.8 (88.3–94.3) | 97.4 (95.0–98.7) | 6.9 (1.9–22.0) | 0.57 (0.39–0.74) | 2.4 | 0.9 | 2.79 | ||
| Colorectal cancer | No | 0.5 | 5.1 | 66.7 (20.8–93.9) | 95.2 (93.3–96.6) | 99.8 (99.1–99.97) | 6.1 (1.7–19.6) | 0.77 (0.42–1.0) | 14.0 | 0.4 | 39.88 | |
| Yes | 0.0 | 8.6 | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Non-GI cancer | No | 1.2 | 5.1 | 37.5 (13.7–69.4) | 95.3 (93.4–96.7) | 99.2 (98.1–99.7) | 9.1 (3.1–23.6) | 0.60 (0.32–0.87) | 8.1 | 0.7 | 12.27 | |
| Yes | 3.6 | 8.6 | 16.7 (4.7–44.8) | 91.7 (88.2–94.2) | 96.8 (94.1–98.2) | 6.9 (1.9–22.0) | 0.49 (0.28–0.70) | 2.0 | 0.9 | 2.21 | ||
| Cancer | No | 1.8 | 5.1 | 41.7 (19.3–68.0) | 95.6 (93.8–97.0) | 98.9 (97.7–99.5) | 15.2 (6.7–30.9) | 0.63 (0.42–0.84) | 9.5 | 0.6 | 15.61 | |
| Yes | 6.5 | 8.6 | 18.2 (7.3–38.5) | 92.1 (88.6–94.6) | 94.2 (91.0–96.3) | 13.8 (5.5–30.6) | 0.53 (0.38–0.67) | 2.3 | 0.9 | 2.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pin-Vieito, N.; Iglesias, M.J.; Remedios, D.; Álvarez-Sánchez, V.; Fernández-Bañares, F.; Boadas, J.; Martínez-Bauer, E.; Campo, R.; Bujanda, L.; Ferrández, Á.; et al. Predictive Value of Carcinoembryonic Antigen in Symptomatic Patients without Colorectal Cancer: A Post-Hoc Analysis within the COLONPREDICT Cohort. Diagnostics 2020, 10, 1036. https://doi.org/10.3390/diagnostics10121036
Pin-Vieito N, Iglesias MJ, Remedios D, Álvarez-Sánchez V, Fernández-Bañares F, Boadas J, Martínez-Bauer E, Campo R, Bujanda L, Ferrández Á, et al. Predictive Value of Carcinoembryonic Antigen in Symptomatic Patients without Colorectal Cancer: A Post-Hoc Analysis within the COLONPREDICT Cohort. Diagnostics. 2020; 10(12):1036. https://doi.org/10.3390/diagnostics10121036
Chicago/Turabian StylePin-Vieito, Noel, María José Iglesias, David Remedios, Victoria Álvarez-Sánchez, Fernando Fernández-Bañares, Jaume Boadas, Eva Martínez-Bauer, Rafael Campo, Luis Bujanda, Ángel Ferrández, and et al. 2020. "Predictive Value of Carcinoembryonic Antigen in Symptomatic Patients without Colorectal Cancer: A Post-Hoc Analysis within the COLONPREDICT Cohort" Diagnostics 10, no. 12: 1036. https://doi.org/10.3390/diagnostics10121036
APA StylePin-Vieito, N., Iglesias, M. J., Remedios, D., Álvarez-Sánchez, V., Fernández-Bañares, F., Boadas, J., Martínez-Bauer, E., Campo, R., Bujanda, L., Ferrández, Á., Piñol, V., Rodríguez-Alcalde, D., Menéndez-Rodríguez, M., García-Morales, N., Pérez-Mosquera, C., & Cubiella, J. (2020). Predictive Value of Carcinoembryonic Antigen in Symptomatic Patients without Colorectal Cancer: A Post-Hoc Analysis within the COLONPREDICT Cohort. Diagnostics, 10(12), 1036. https://doi.org/10.3390/diagnostics10121036








