Incorporating Point-of-Care Bacterial Fluorescence into a Wound Clinic Antimicrobial Stewardship Program
Abstract
:1. Introduction
2. Instituting an Antimicrobial Stewardship Program (ASP)
- (1)
- Identifying an antimicrobial stewardship leader;
- (2)
- Establishing an annual antimicrobial stewardship goal;
- (3)
- Implementing evidence-based practice guidelines related to the antimicrobial stewardship goal;
- (4)
- Providing clinical staff with educational resources related to the antimicrobial stewardship goal;
- (5)
- Collecting, analyzing, and reporting data related to the antimicrobial stewardship goal.
- Element 1 (EP-1)
- Element 2 (EP-2)
- Element 3 (EP-3)
- Element 4 (EP-4)
- Element 5 (EP-5)
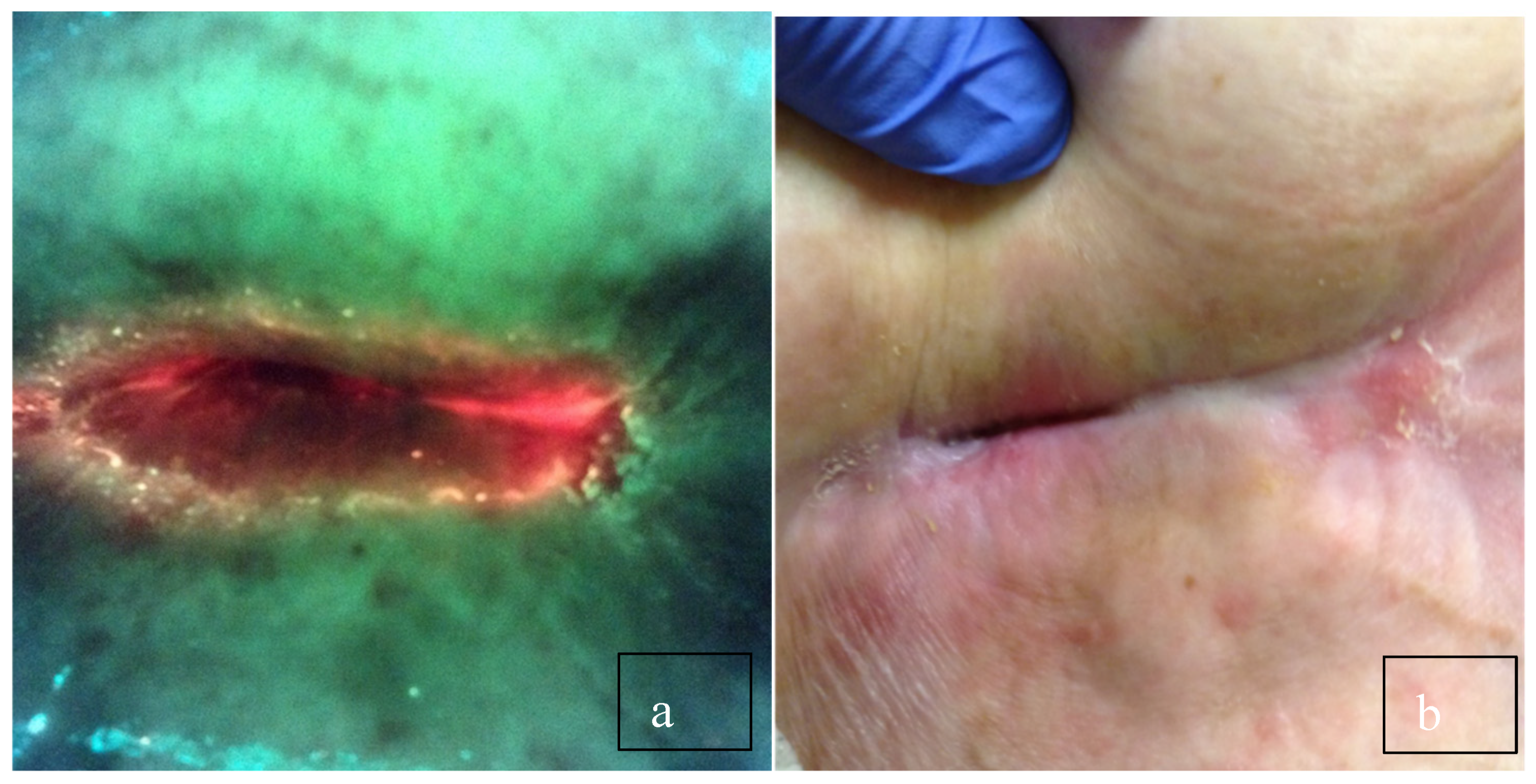
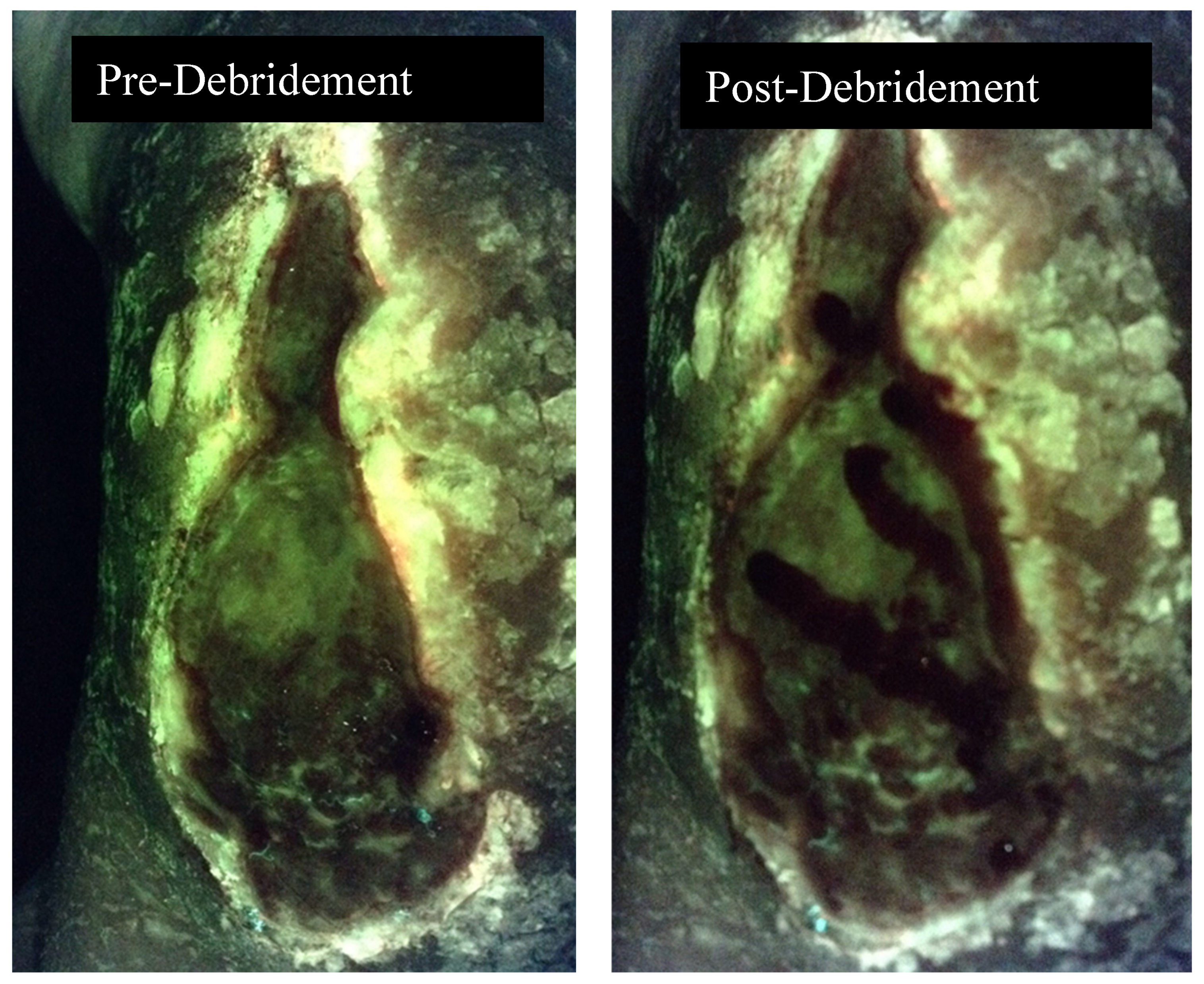
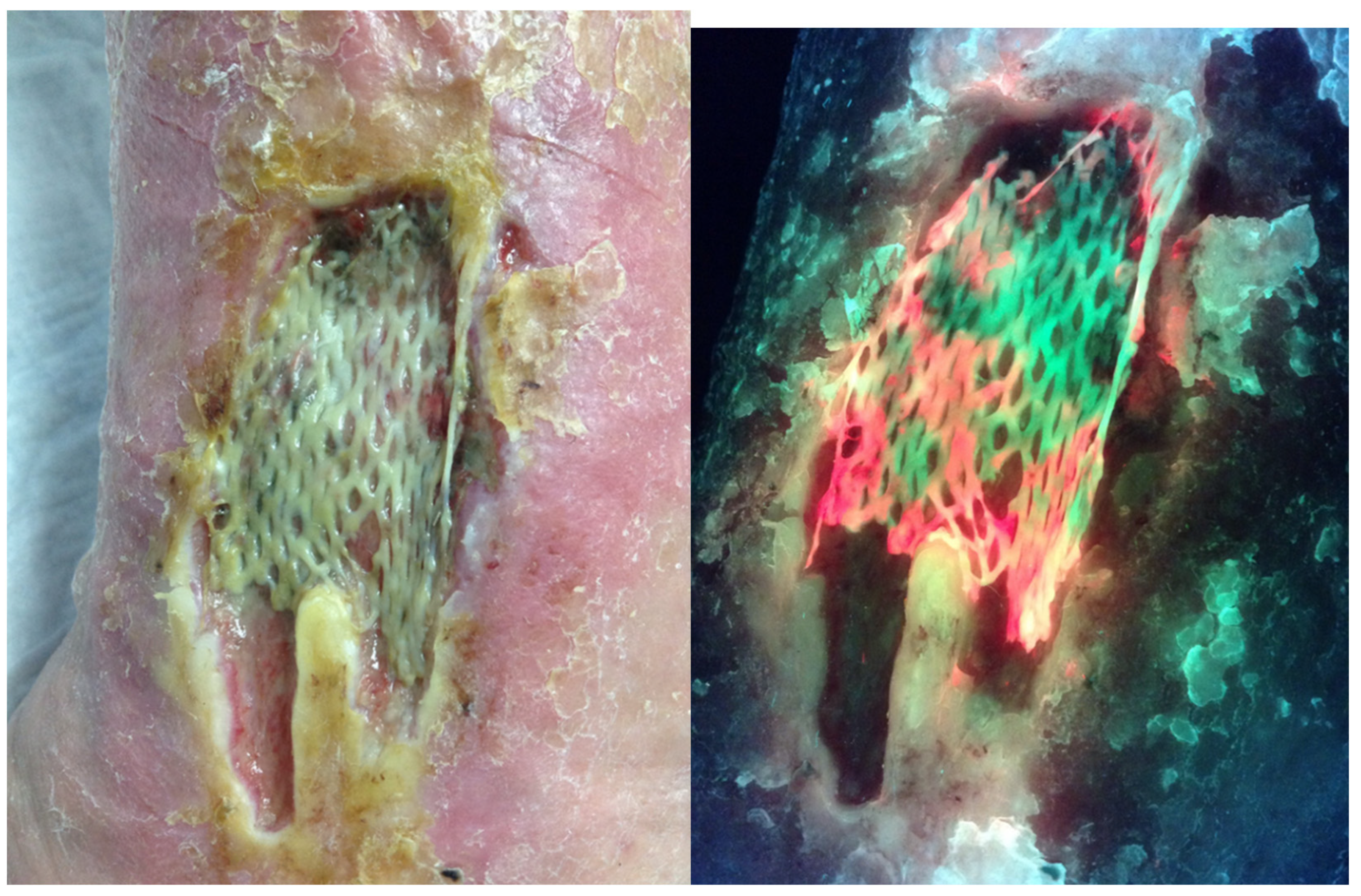
3. Discussion
Funding
Conflicts of Interest
References
- Amirthalingam, S.; Yi, K.S.; Ching, L.T.; Mun, N.Y. Topical Antibacterials and Global Challenges on Resistance Development. Trop. J. Pharm. Res. 2015, 14, 919–924. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, S.; Chattopadhyay, M.K.; Grossart, H.P. The multifaceted roles of antibiotics and antibiotic resistance in nature. Front. Microbiol. 2013, 4, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, S.I.; Rao, P.C.; Dolecek, C.; Day, N.P.J.; Stergachis, A.; Lopez, A.D.; Murray, C.J.L. Measuring and mapping the global burden of antimicrobial resistance. BMC Med. 2018, 16, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 1 November 2020).
- World Health Organization. Antimicrobial Resistance—Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Hamilton, K.W.; Gerber, J.S.; Moehring, R.; Anderson, D.J.; Calderwood, M.S.; Han, J.H.; Mehta, J.M.; Pollack, L.A.; Zaoutis, T.; Srinivasan, A.; et al. Point-of-prescription interventions to improve antimicrobial stewardship. Clin. Infect. Dis. 2015, 60, 1252–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joint Commission. R3 Report/Requirement, Rational, Reference. Issue 23. 20 June 2019. Available online: https://www.jointcommission.org/standards/r3-report/r3-report-issue-23-antimicrobial-stewardship-in-ambulatory-health-care/ (accessed on 15 June 2020).
- Hersh, A.L.; Chambers, H.F.; Maselli, J.H.; Gonzales, R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch. Intern. Med. 2008, 168, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- US Wound Registry. The Woodlands, Texas. Available online: https://uswoundregistry.com/ (accessed on 15 June 2020).
- Serena, T.; Robson, M.C.; Cooper, D.M.; Ignatius, J. Lack of reliability of clinical/visual assessment of chronic wound infection: The incidence of biopsy-proven infection in venous leg ulcers. Wounds 2006, 18, 197–202. [Google Scholar]
- Rondas, A.A.; Schols, J.M.; Halfens, R.J.; Stobberingh, E.E. Swab versus biospy for the diagnosis of chronic infected wounds. Adv. Skin Wound Care 2013, 26, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurley, C.M.; McClusky, P.; Sugrue, R.M.; Clover, J.A.; Kelly, J.E. Efficacy of a bacterial fluorescence imaging device in an outpatient wound care clinic: A pilot study. J. Wound Care 2019, 28, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Serena, T.E.; Harrell, K.; Serena, L.; Yaakov, R.A. Real-time bacterial fluorescence imaging accurately identifies wound with moderate-to-heavy bacterial burden. J. Wound Care 2019, 28, 346–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rennie, M.Y.; Dunham, D.; Lindvere-Teene, L.; Raizman, R.; Hill, R.; Linden, R. Understanding Real-Time Fluorescence Signals from Bacteria and Wound Tissues Observed with the MolecuLight i:X(TM). Diagnostics 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, L.M.; Dunham, D.; Rennie, M.Y.; Kirman, J.; Lopez, A.J.; Keim, K.C.; Little, W.; Gomez, A.; Bourke, J.; Ng, H.; et al. In vitro detection of porphyrin-producing wound bacteria with real-time fluorescence imaging. Future Microbiol. 2020, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rennie, M.Y.; Lindvere-Teene, L.; Tapang, K.; Linden, R. Point-of-care fluorescence imaging predicts the presence of pathogenic bacteria in wounds: A clinical study. J. Wound Care 2017, 26, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Le, L.; Baer, M.; Briggs, P.; Bullock, N.; Cole, W.; DiMarco, D.; Hamil, R.; Harrell, K.; Kasper, M.; Li, W.; et al. Diagnostic Accuracy of Point-of-Care Fluorescence Imaging for the Detection of Bacterial Burden in Wounds: Results from the 350-Patient FLAAG Trial. Adv. Wound Care 2020. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, M.D. Bacteria and Antibiotics in Wound Healing. Surg. Clin. N. Am. 2020, 100, 757–776. [Google Scholar] [CrossRef] [PubMed]
- Ottolino-Perry, K.; Chamma, E.; Blackmore, K.M.; Lindvere-Teene, L.; Starr, D.; Tapang, K.; Rosen, C.F.; Pitcher, B.; Panzarella, T.; Linden, R.; et al. Improved detection of clinically relevant wound bacteria using autofluorescence image-guided sampling in diabetic foot ulcers. Int. Wound J. 2017, 14, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, E.; Jeffery, S.L.A. The Use of the MolecuLight i:X in managing burns: A pilot study. J. Burn Care Res. 2018, 39, 154–161. [Google Scholar] [PubMed] [Green Version]
- Hill, R.; Rennie, M.Y.; Douglas, J. Using Bacterial Fluorescence Imaging and Antimicrobial Stewardship to Guide Wound Management Practices: A Case Series. Ostomy Wound Manag. 2018, 64, 18–28. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Dryden, M.; Gottrup, F.; Nathwani, D.; Seaton, R.A.; Stryja, J. Antimicrobial stewardship in wound care: A Position Paper from the British Society for Antimicrobial Chemotherapy and European Wound Management Association. J. Antimicrob. Chemother. 2016, 71, 3026–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serena, T.E. Incorporating Point-of-Care Bacterial Fluorescence into a Wound Clinic Antimicrobial Stewardship Program. Diagnostics 2020, 10, 1010. https://doi.org/10.3390/diagnostics10121010
Serena TE. Incorporating Point-of-Care Bacterial Fluorescence into a Wound Clinic Antimicrobial Stewardship Program. Diagnostics. 2020; 10(12):1010. https://doi.org/10.3390/diagnostics10121010
Chicago/Turabian StyleSerena, Thomas E. 2020. "Incorporating Point-of-Care Bacterial Fluorescence into a Wound Clinic Antimicrobial Stewardship Program" Diagnostics 10, no. 12: 1010. https://doi.org/10.3390/diagnostics10121010
APA StyleSerena, T. E. (2020). Incorporating Point-of-Care Bacterial Fluorescence into a Wound Clinic Antimicrobial Stewardship Program. Diagnostics, 10(12), 1010. https://doi.org/10.3390/diagnostics10121010




