Monte Carlo Modeling of Shortwave-Infrared Fluorescence Photon Migration in Voxelized Media for the Detection of Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
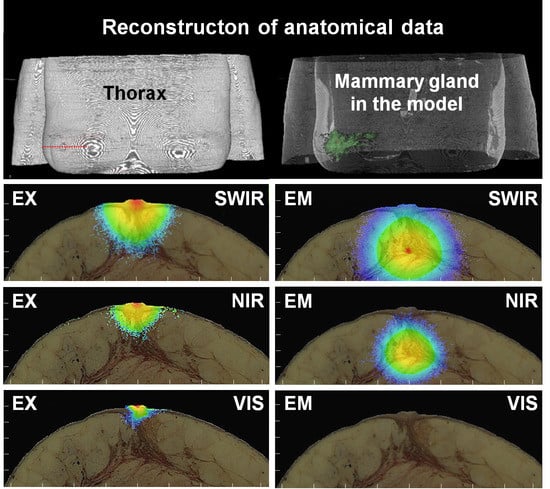
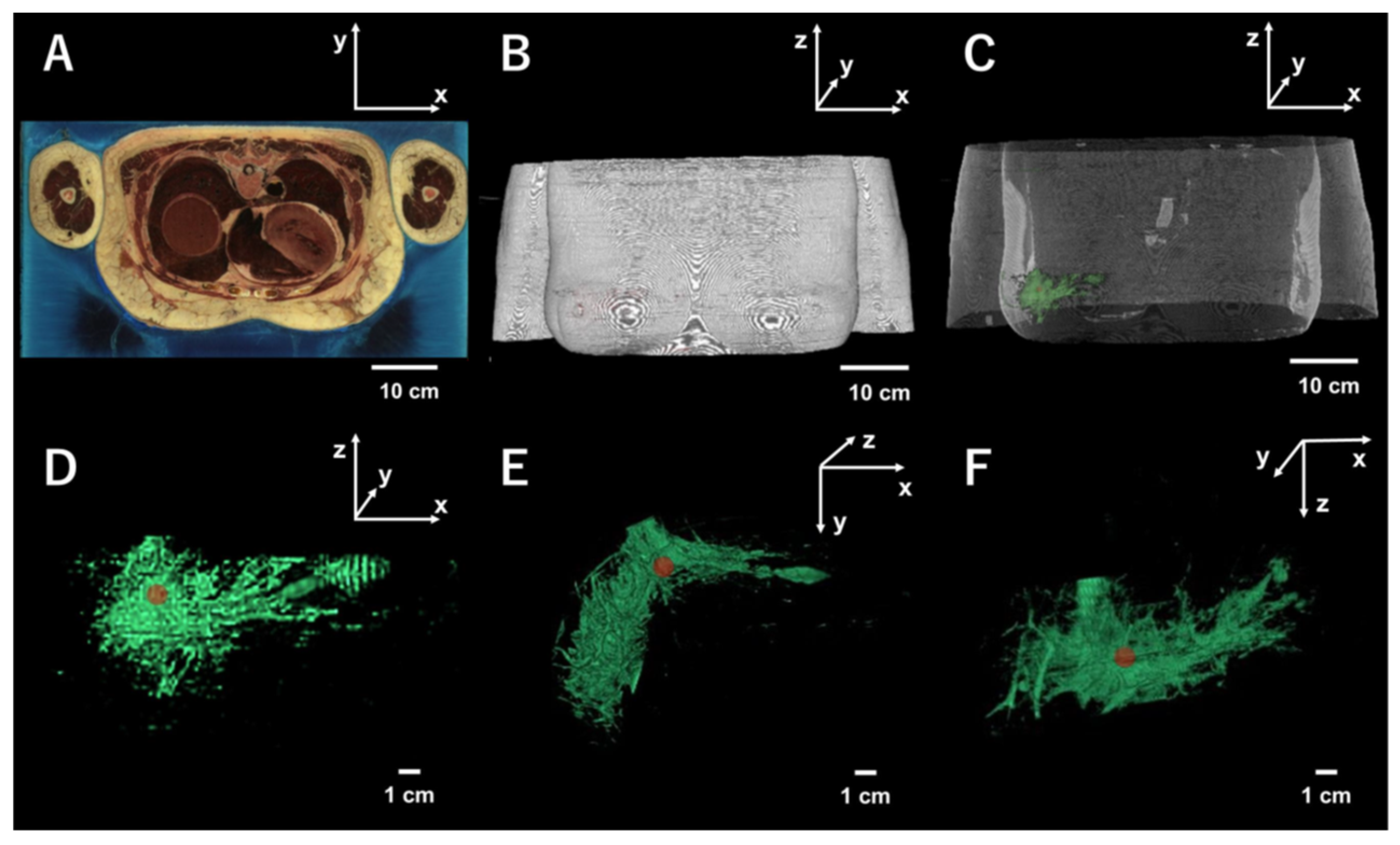
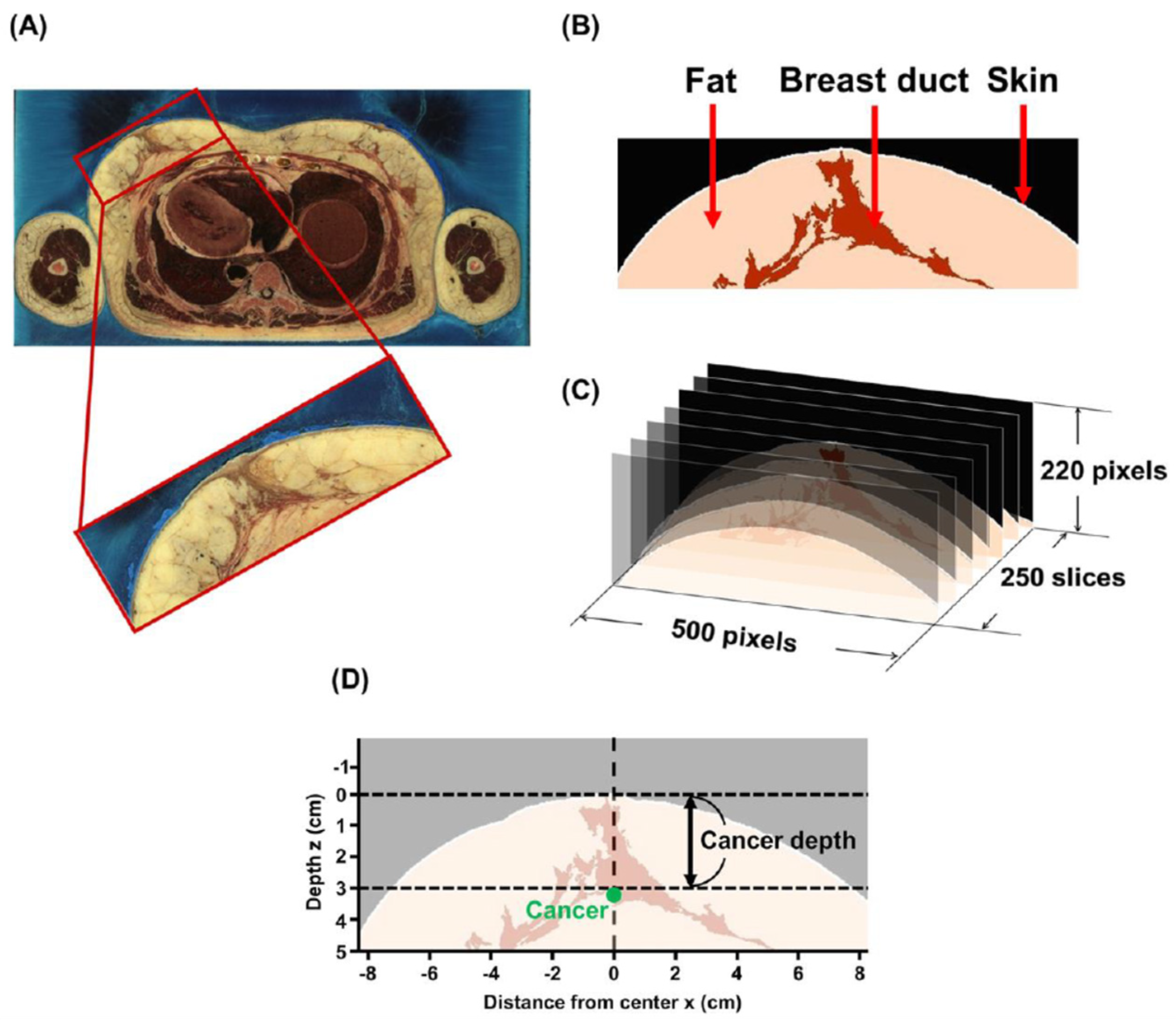
2.1. Breast Model
2.2. Image Processing and Implementation of the Model
2.3. The Monte Carlo Simulation
3. Results and Discussion
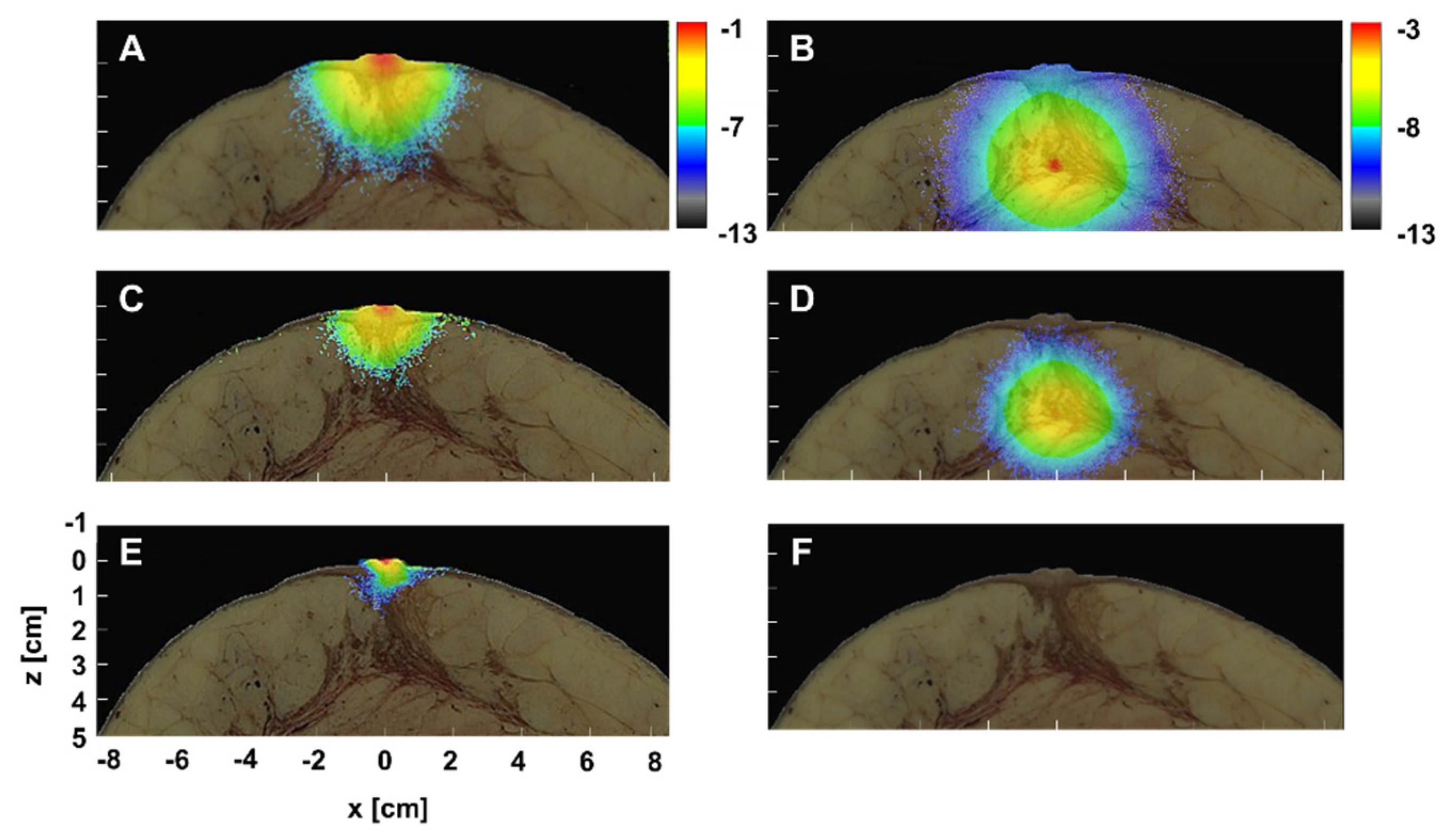
3.1. Excitation Gradient
3.2. Setting Optical Parameters Faithful to Duct Morphology
3.3. SWIR for the Detection of Small Breast Cancer in Deep Tissue
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van Dongen, J.A.; Voogd, A.C.; Fentiman, I.S.; Legrand, C.; Sylvester, R.J.; Tong, D.; van der Schueren, E.; Helle, P.A.; van Zijl, K.; Bartelink, H. Long-Term Results of a Randomized Trial Comparing Breast-Conserving Therapy with Mastectomy: European Organization for Research and Treatment of Cancer 10801 Trial. J. Natl. Cancer Inst. 2000, 92, 1143–1150. [Google Scholar] [CrossRef]
- Silverstein, M.J.; Cohlan, B.F.; Gierson, E.D.; Furmanski, M.; Gamagami, P.; Colburn, W.J.; Lewinsky, B.S.; Waisman, J.R. Duct Carcinoma in Situ: 227 Cases without Microinvasion. Eur. J. Cancer 1992, 28, 630–634. [Google Scholar] [CrossRef]
- Causer, P.A.; Piron, C.A.; Jong, R.A.; Plewes, D.B. Preliminary In Vivo Validation of a Dedicated Breast MRI and Sonographic Coregistration Imaging System. Am. J. Roentgenol. 2008, 191, 1203–1207. [Google Scholar] [CrossRef]
- Wilson, R.H.; Nadeau, K.P.; Jaworski, F.B.; Tromberg, B.J.; Durkin, A.J. Review of Short-Wave Infrared Spectroscopy and Imaging Methods for Biological Tissue Characterization. J. Biomed. Opt. 2015, 20, 030901. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, Y.; Morimatsu, M.; Nishimura, G.; Sakata, T.; Yasuda, H.; Komatsuzaki, A.; Watanabe, T.M.; Jin, T. Synthesis and Optical Properties of Emission-Tunable PbS/CdS Core/Shell Quantum Dots for In Vivo Fluorescence Imaging in the Second Near-Infrared Window. RSC. Adv. 2014, 4, 41164–41171. [Google Scholar] [CrossRef]
- Tong, M.; Guo, W.; Gao, W. Use of Fluorescence Imaging in Combination with Patent Blue Dye versus Patent Blue Dye Alone in Sentinel Lymph Node Biopsy in Breast Cancer. J. Breast Cancer 2014, 17, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Sugie, T.; Kassim, K.A.; Takeuchi, M.; Hashimoto, T.; Yamagami, K.; Masai, Y.; Toi, M. A Novel Method for Sentinel Lymph Node Biopsy by Indocyanine Green Fluorescence Technique in Breast Cancer. Cancers 2010, 2, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Pitsinis, V.; Wishart, G.C. Comparison of Indocyanine Green Fluorescence and Blue Dye Methods in Detection of Sentinel Lymph Nodes in Early-Stage Breast Cancer. Ann. Surg. Oncol. 2017, 24, 581–582. [Google Scholar] [CrossRef] [PubMed]
- Kitai, T.; Inomoto, T.; Miwa, M.; Shikayama, T. Fluorescence Navigation with Indocyanine Green for Detecting Sentinel Lymph Nodes in Breast Cancer. Breast Cancer 2005, 12, 211–215. [Google Scholar] [CrossRef]
- Tsuboi, S.; Jin, T. Shortwave-infrared (SWIR) Fluorescence Molecular Imaging Using Indocyanine Green–Antibody Conjugates for the Optical Diagnostics of Cancerous Tumours. RSC. Adv. 2020, 10, 28171–28179. [Google Scholar] [CrossRef]
- Iida, T.; Jin, T.; Nomura, Y. Monte Carlo Modeling of Near-Infrared Fluorescence Photon Migration in Breast Tissue for Tumor Prediction. Adv. Biomed. Eng. 2020, 9, 100–105. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.; Sun, Y.; Duan, M.; Peng, L. Effect of Head Model on Monte Carlo Modeling of Spatial Sensitivity Distribution for Functional Near-Infrared Spectroscopy. J. Innov. Opt. Health Sci. 2015, 8, 1550024. [Google Scholar] [CrossRef]
- Li, T.; Xue, C.; Wang, P.; Li, Y.; Wu, L. Photon Penetration Depth in Human Brain for Light Stimulation and Treatment: A Realistic Monte Carlo Simulation Study. J. Innov. Opt. Health Sci. 2017, 10, 1743002. [Google Scholar] [CrossRef]
- Iida, T.; Yamato, H.; Jin, T.; Nomura, Y. Optimal Focus Evaluated Using Monte Carlo Simulation in Non-Invasive Neuroimaging in the Second near-Infrared Window. MethodsX 2019, 6, 2367–2373. [Google Scholar] [CrossRef] [PubMed]
- Yamato, H.; Iida, T.; Jin, T.; Nomura, Y. Monte Carlo Evaluation of In Vivo Neuroimaging Using Quantum Dots with Fluorescence in the Second Window of Near Infrared Region. Adv. Biomed. Eng. 2019, 8, 105–109. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Yamada, Y.; Tamura, M.; Nomura, Y. Monte Carlo Simulation of Light Transmission through Living Tissues. Appl. Opt. 1991, 30, 4515–4520. [Google Scholar] [CrossRef]
- Visible Human Project. Available online: https://www.nlm.nih.gov/research/visible/visible_human.html (accessed on 15 August 2020).
- Salomatina, E.; Jiang, B.; Novak, J.; Yaroslavsky, A.N. Optical Properties of Normal and Cancerous Human Skin in the Visible and Near-Infrared Spectral Range. J. Biomed. Opt. 2006, 11, 064026. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical Properties of Biological Tissues: A Review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef]
- Troy, T.L.; Page, D.L.; Sevick-Muraca, E.M. Optical Properties of Normal and Diseased Breast Tissues: Prognosis for Optical Mammography. J. Biomed. Opt. 1996, 1, 342–355. [Google Scholar] [CrossRef]
- Ding, H.; Lu, J.Q.; Wooden, W.A.; Kragel, P.J.; Hu, X.H. Refractive Indices of Human Skin Tissues at Eight Wavelengths and Estimated Dispersion Relations between 300 and 1600 nm. Phys. Med. Biol. 2006, 51, 1479–1489. [Google Scholar] [CrossRef]
- Prince, S.; Malarvizhi, S. Monte Carlo Simulation of NIR Diffuse Reflectance in the Normal and Diseased Human Breast Tissues. BioFactors 2007, 30, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S. Recent Advances in Fluorescein Fundus Angiography. Br. J. Ophthalmol. 1974, 58, 391–412. [Google Scholar] [CrossRef]
- Altan-Yaycioglu, R.; Akova, Y.A.; Akca, S.; Yilmaz, G. Inflammation of the Posterior Uvea: Findings on Fundus Fluorescein and Indocyanine Green Angiography. Ocul. Immunol. Inflamm. 2006, 14, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jacques, S.L.; Zheng, L. MCML—Monte Carlo Modeling of Light Transport in Multi-Layered Tissues. Comput. Methods Programs Biomed. 1995, 47, 131–146. [Google Scholar] [CrossRef]
- Matsumoto, M.; Nishimura, T. Mersenne Twister: A 623-Dimensionally Equidistributed Uniform Pseudo-Random Number Generator. ACM Trans. Model. Comput. Simul. 1998, 8, 3–30. [Google Scholar] [CrossRef]
- Jacques, S.L. Light Distributions from Point, Line and Plane Sources for Photochemical Reactions and Fluorescence in Turbid Biological Tissues. Photochem. Photobiol. 1998, 67, 23–32. [Google Scholar] [CrossRef]
- Nan, L.; Zhiliang, H.; Ran, L. A CMOS Detector System for Fluorescent Bio-Sensing Application. In Proceedings of the 2008 IEEE Asian Solid-State Circuits Conference (ASSCC), Fukuoka, Japan, 3–5 November 2008. [Google Scholar]
- Murari, K.; Ralph, E.C.; Nitish, V.T.; Cauwenberghs, G. A CMOS In-Pixel CTIA High-Sensitivity Fluorescence Imager. IEEE. Trans. Biomed. Circuits Syst. 2011, 5, 449–458. [Google Scholar] [CrossRef]
- Guo, R.; Lu, G.; Qin, B.; Fei, B. Ultrasound Imaging Technologies for Breast Cancer Detection and Management: A Review. Ultrasound Med. Biol. 2018, 44, 37–70. [Google Scholar] [CrossRef]
- De Mattos-Arruda, L.; Cortes, J.; Santarpia, L.; Vivancos, A.; Tabernero, J.; Jorge S Reis-Filho, J.S.R.; Seoane, J. Circulating Tumour Cells and Cell-free DNA as Tools for Managing Breast Cancer. Nat. Rev. Clin. Oncol. 2013, 10, 377–389. [Google Scholar] [CrossRef]
- Loyez, M.; Hassan, E.M.; Lobry, M.; Liu, F.; Caucheteur, C.; Wattiez, R.; DeRosa, M.C.; Willmore, W.G.; Albert, J. Rapid Detection of Circulating Breast Cancer Cells using a Multiresonant Optical Fiber Aptasensor with Plasmonic Amplification. ACS Sens. 2020, 5, 454–463. [Google Scholar] [CrossRef]
- Saeed, A.A.; Sánchez, J.L.A.; O’Sullivan, C.K.; Abbas, M.N. DNA biosensors based on gold nanoparticles-modified graphene oxide for the detection of breast cancer biomarkers for early diagnosis. Bioelectrochemistry 2017, 118, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Fanizzi, A.; Basile, T.M.; Losurdo, L.; Bellotti, L.; Bottigli, U.; Campobasso, F.; Didonna, V.; Fausto, A.; Massafra, R.; Tagliafico, A.; et al. Ensemble Discrete Wavelet Transform and Gray-Level Co-Occurrence Matrix for Microcalcification Cluster Classification in Digital Mammography. Appl. Sci. 2019, 9, 5388. [Google Scholar] [CrossRef]



| Wavelength (nm) | Tissue | µa (cm−1) | µs (cm−1) | n | |
|---|---|---|---|---|---|
| VIS | Ex 488 | Skin | 6.0 | 625 | 1.37 |
| Fat | 6.0 | 310 | 1.45 | ||
| Duct | 0.2 | 317 | 1.42 | ||
| Cancer | 1.0 | 300 | 1.45 | ||
| Em 520 | Skin | 5.8 | 450 | 1.37 | |
| Fat | 4.0 | 300 | 1.45 | ||
| Duct | 0.2 | 268 | 1.42 | ||
| Cancer | 1.0 | 230 | 1.45 | ||
| NIR | Ex 780 | Skin | 2.0 | 241 | 1.37 |
| Fat | 1.4 | 136 | 1.45 | ||
| Duct | 0.2 | 169 | 1.42 | ||
| Cancer | 1.0 | 150 | 1.45 | ||
| Em 820 | Skin | 1.2 | 228 | 1.37 | |
| Fat | 1.2 | 132 | 1.45 | ||
| Duct | 0.2 | 198 | 1.42 | ||
| Cancer | 0.7 | 140 | 1.45 | ||
| SWIR | Ex 970 | Skin | 1.0 | 210 | 1.37 |
| Fat | 0.9 | 76.6 | 1.45 | ||
| Duct | 0.3 | 122 | 1.42 | ||
| Cancer | 0.9 | 75.5 | 1.45 | ||
| Em 1100 | Skin | 0.7 | 176 | 1.37 | |
| Fat | 0.6 | 72.3 | 1.45 | ||
| Duct | 0.3 | 122 | 1.42 | ||
| Cancer | 1.0 | 80.0 | 1.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iida, T.; Kiya, S.; Kubota, K.; Jin, T.; Seiyama, A.; Nomura, Y. Monte Carlo Modeling of Shortwave-Infrared Fluorescence Photon Migration in Voxelized Media for the Detection of Breast Cancer. Diagnostics 2020, 10, 961. https://doi.org/10.3390/diagnostics10110961
Iida T, Kiya S, Kubota K, Jin T, Seiyama A, Nomura Y. Monte Carlo Modeling of Shortwave-Infrared Fluorescence Photon Migration in Voxelized Media for the Detection of Breast Cancer. Diagnostics. 2020; 10(11):961. https://doi.org/10.3390/diagnostics10110961
Chicago/Turabian StyleIida, Tatsuto, Shunsuke Kiya, Kosuke Kubota, Takashi Jin, Akitoshi Seiyama, and Yasutomo Nomura. 2020. "Monte Carlo Modeling of Shortwave-Infrared Fluorescence Photon Migration in Voxelized Media for the Detection of Breast Cancer" Diagnostics 10, no. 11: 961. https://doi.org/10.3390/diagnostics10110961
APA StyleIida, T., Kiya, S., Kubota, K., Jin, T., Seiyama, A., & Nomura, Y. (2020). Monte Carlo Modeling of Shortwave-Infrared Fluorescence Photon Migration in Voxelized Media for the Detection of Breast Cancer. Diagnostics, 10(11), 961. https://doi.org/10.3390/diagnostics10110961