Validation of PfSNP-LAMP-Lateral Flow Dipstick for Detection of Single Nucleotide Polymorphism Associated with Pyrimethamine Resistance in Plasmodium falciparum
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Blood Sample Collection and Genomic DNA Extraction
2.2. DNA Sequencing
2.3. Recombinant Plasmid Construction
2.4. PfSNP-LAMP-LFD Conditions
3. Results
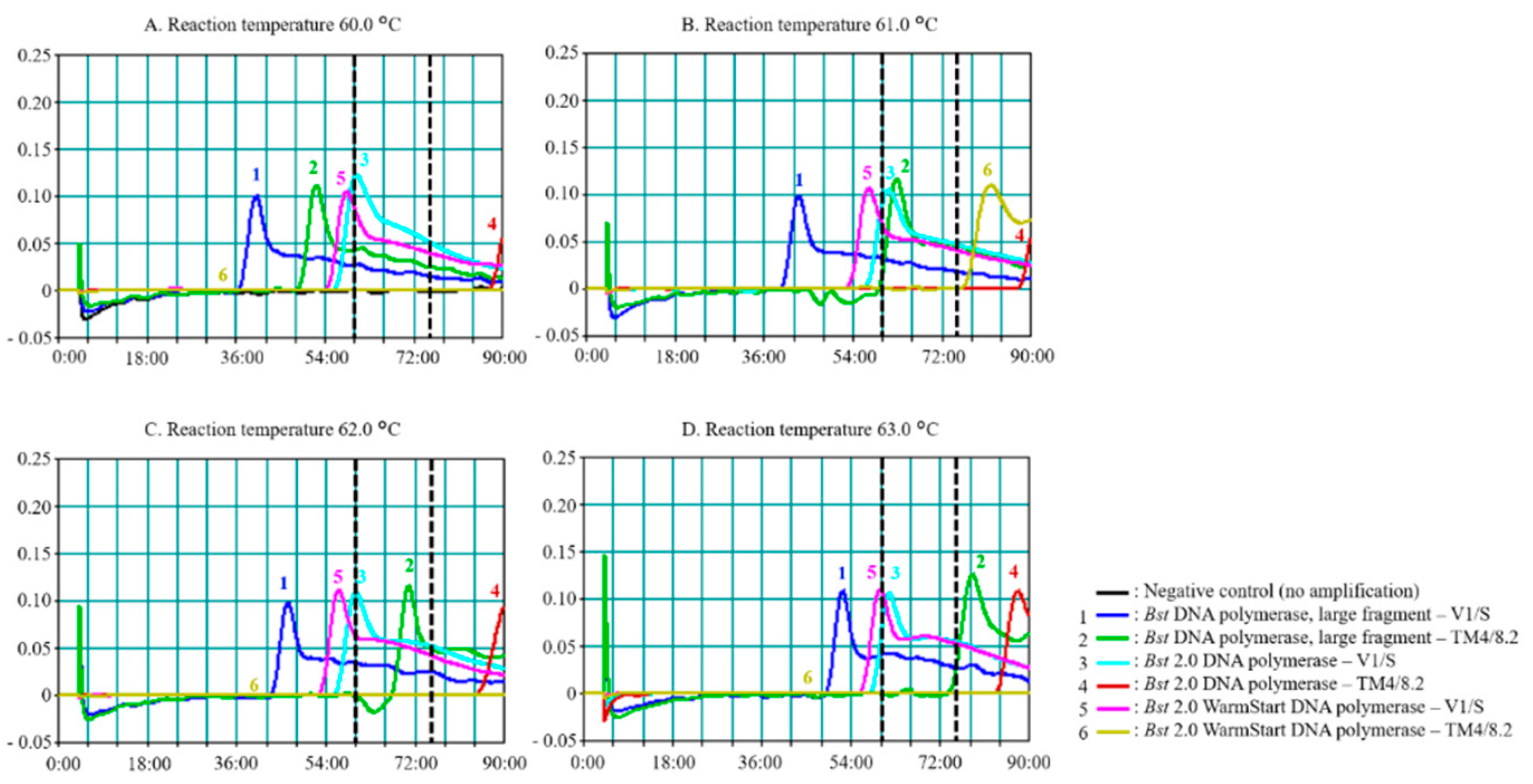
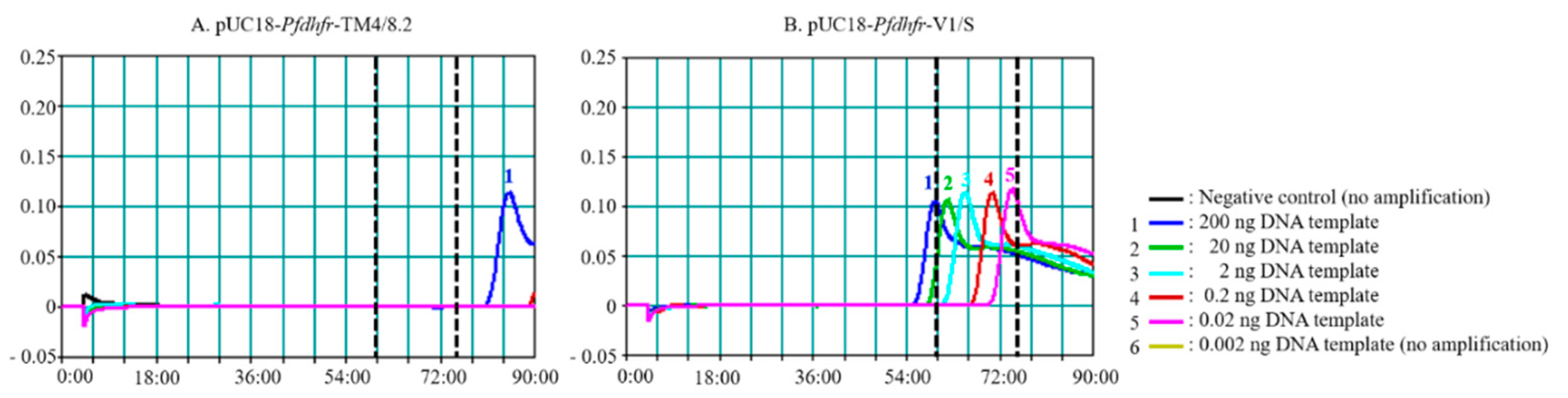
3.1. Effects of Enzyme and Reaction Time on PfSNP-LAMP Sensitivity and Specificity
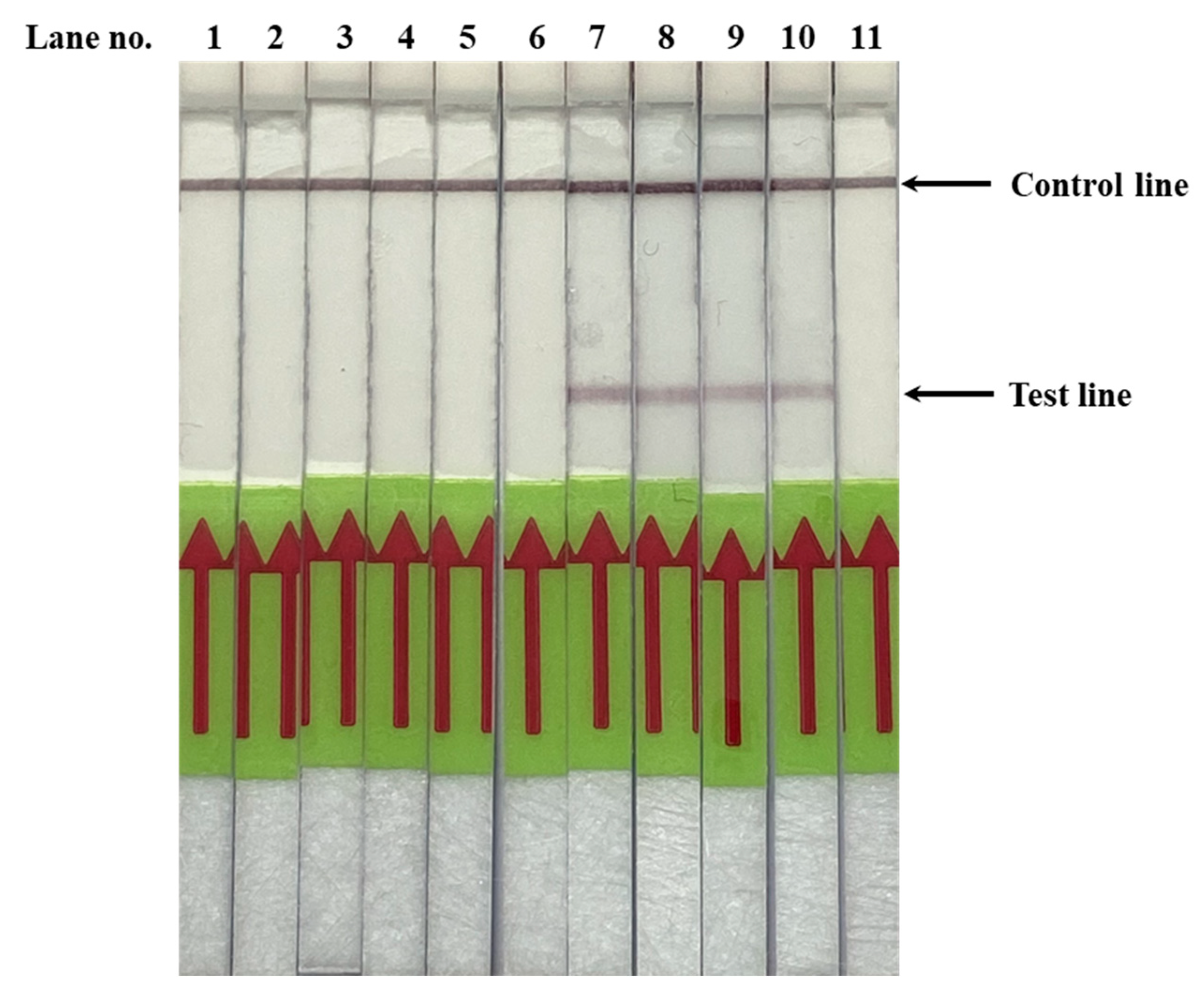
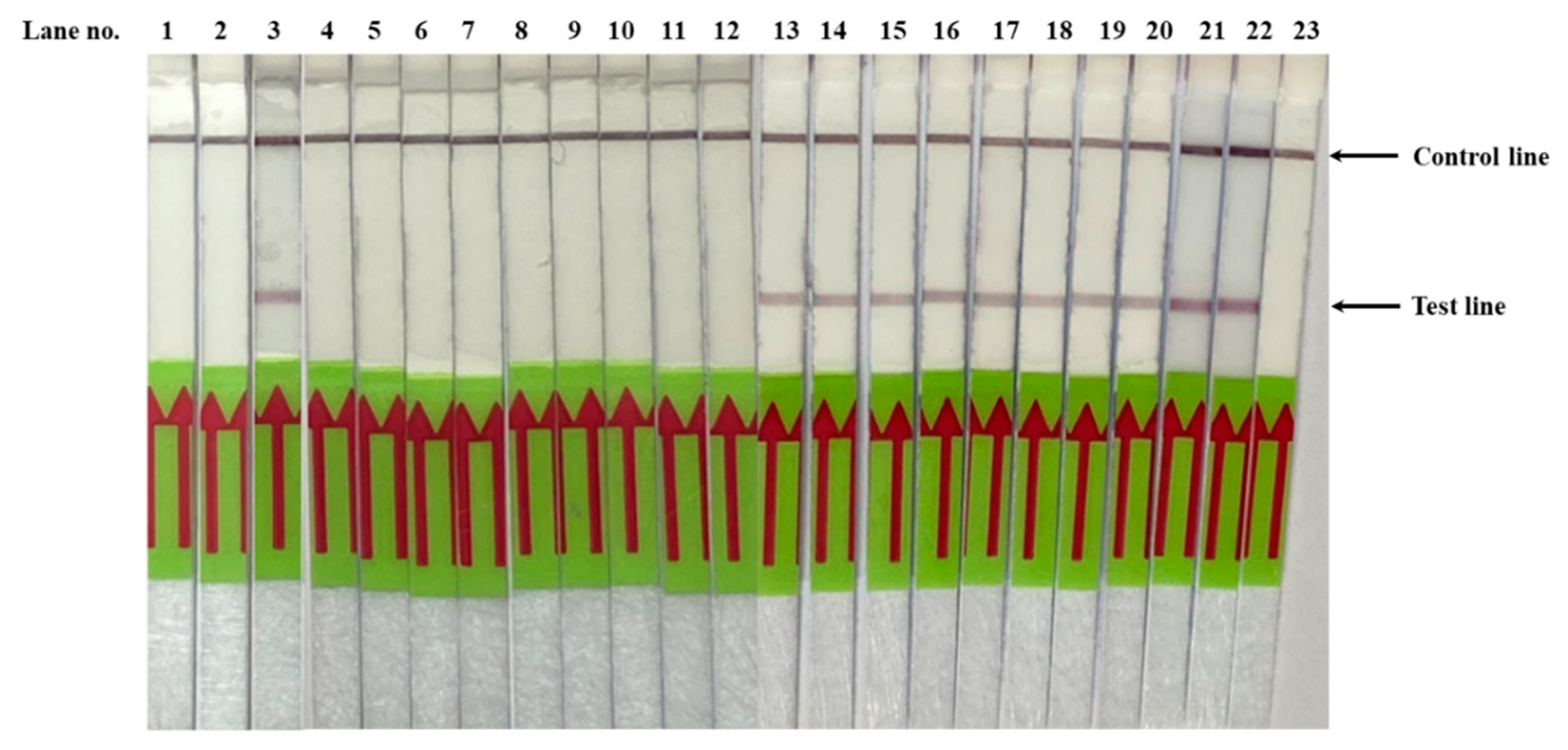
3.2. Validation of PfSNP-LAMP-LFD in Clinical Blood Samples from Malaria Patients
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- WHO. WHO Seasonal Malaria Chemoprevention with Sulfadoxine–Pyrimethamine plus Amodiaquine in Children: A Field Guide. August 2013; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- WHO. WHO Policy Brief for the Implementation of Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-Pyrimethamine (IPTp-SP), April 2013 (rev. January 2014); World health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Bzik, D.J.; Li, W.B.; Horii, T.; Inselburg, J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene. Proc. Natl. Acad. Sci. USA 1987, 84, 8360–8364. [Google Scholar] [CrossRef] [PubMed]
- Triglia, T.; Cowman, A.F. Primary structure and expression of the dihydropteroate synthetase gene of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 1994, 91, 7149–7153. [Google Scholar] [CrossRef] [PubMed]
- Cowman, A.F.; Morry, M.J.; Biggs, B.A.; Cross, G.A.; Foote, S.J. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 1988, 85, 9109–9113. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.S.; Walliker, D.; Wellems, T.E. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc. Natl. Acad. Sci. USA 1988, 85, 9114–9118. [Google Scholar] [CrossRef] [PubMed]
- Bjorkman, A.; Phillips-Howard, P.A. The epidemiology of drug-resistant malaria. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 177–180. [Google Scholar] [CrossRef][Green Version]
- Peterson, D.S.; Milhous, W.K.; Wellems, T.E. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. USA 1990, 87, 3018–3022. [Google Scholar] [CrossRef]
- Plowe, C.V.; Cortese, J.F.; Djimde, A.; Nwanyanwu, O.C.; Watkins, W.M.; Winstanley, P.A.; Estrada-Franco, J.G.; Mollinedo, R.E.; Avila, J.C.; Cespedes, J.L.; et al. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J. Infect. Dis. 1997, 176, 1590–1596. [Google Scholar] [CrossRef]
- Yuthavong, Y.; Vilaivan, T.; Chareonsethakul, N.; Kamchonwongpaisan, S.; Sirawaraporn, W.; Quarrell, R.; Lowe, G. Development of a lead inhibitor for the A16V+S108T mutant of dihydrofolate reductase from the cycloguanil-resistant strain (T9/94) of Plasmodium falciparum. J. Med. Chem. 2000, 43, 2738–2744. [Google Scholar] [CrossRef]
- Wongsrichanalai, C.; Pickard, A.L.; Wernsdorfer, W.H.; Meshnick, S.R. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2002, 2, 209–218. [Google Scholar] [CrossRef]
- Nair, S.; Williams, J.T.; Brockman, A.; Paiphun, L.; Mayxay, M.; Newton, P.N.; Guthmann, J.P.; Smithuis, F.M.; Hien, T.T.; White, N.J.; et al. A selective sweep driven by pyrimethamine treatment in southeast asian malaria parasites. Mol. Biol. Evol. 2003, 20, 1526–1536. [Google Scholar] [CrossRef]
- Roper, C.; Pearce, R.; Nair, S.; Sharp, B.; Nosten, F.; Anderson, T.J.C. Intercontinental spread of pyrimethamine—Resistant malaria. Science 2004, 305, 1124. [Google Scholar] [CrossRef] [PubMed]
- Plowe, C.V.; Kublin, J.G.; Doumbo, O.K. P. falciparum dihydrofolate reductase and dihydropteroate synthase mutations: Epidemiology and role in clinical resistance to antifolates. Drug Resist. Updat. 1998, 1, 389–396. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchai, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed]
- Eiken Chemical Co., Ltd. Available online: https://www.eiken.co.jp/en/products/lamp/ (accessed on 6 November 2020).
- Poon, L.L.; Wong, B.W.Y.; Ma, E.H.T.; Chan, K.H.; Chow, L.M.C.; Abeyewickreme, W.; Tangpukdee, N.; Yuen, K.Y.; Guan, Y.; Looareesuwan, S.; et al. Sensitive and inexpensive molecular test for falciparum malaria: Detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin. Chem. 2006, 52, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Han, E.T.; Watanabe, R.; Sattabongkot, J.; Khuntirat, B.; Sirichaisinthop, J.; Iriko, H.; Jin, L.; Takeo, S.; Tsuboi, T. Detection of four Plasmodium species by genus and species-specific loop-mediated isothermal amplification for clinical diagnosis. J. Clin. Microbiol. 2007, 45, 2521–2528. [Google Scholar] [CrossRef] [PubMed]
- Buates, S.; Bantuchai, S.; Sattabongkot, J.; Han, E.T.; Tsuboi, T.; Udomsangpetch, R.; Sirichaisinthop, J.; Tan-ariya, P. Development of a reverse transcription-loop-mediated isothermal amplification (RT-LAMP) for clinical detection of Plasmodium falciparum gametocytes. Parasitol. Int. 2010, 59, 414–420. [Google Scholar] [CrossRef]
- Yongkiettrakul, S.; Jaroenram, W.; Arunrut, N.; Chareanchim, W.; Pannehpetch, S.; Suebsing, R.; Kiatpathomchai, W.; Pornthanakasem, W.; Yuthavong, Y.; Kongkasuriyachai, D. Application of loop-mediated isothermal amplification assay combined with lateral flow dipstick for detection of Plasmodium falciparum and Plasmodium vivax. Parasitol. Int. 2014, 63, 777–784. [Google Scholar] [CrossRef]
- Jiang, Y.S.; Bhadra, S.; Li, B.; Wu, Y.R.; Milligan, J.N.; Ellington, A.D. Robust strand exchange reactions for the sequence-specific, real-time detection of nucleic acid amplicons. Anal. Chem. 2015, 87, 3314–3320. [Google Scholar] [CrossRef]
- Yamanaka, E.S.; Tortajada-Genaro, L.A.; Pastor, N.; Maquieira, A. Polymorphism genotyping based on loop-mediated isothermal amplification and smartphone detection. Biosens. Bioelectron. 2018, 109, 177–183. [Google Scholar] [CrossRef]
- Badolo, A.; Okado, K.; Guelbeogo, W.M.; Aonuma, H.; Bando, H.; Fukumoto, S.; Sagnon, N.; Kanuka, H. Development of an allele-specific, loop-mediated, isothermal amplification method (AS-LAMP) to detect the L1014F kdr-w mutation in Anopheles gambiae sl. Malar. J. 2012, 11, 227. [Google Scholar] [CrossRef]
- Ding, S.; Chen, R.; Chen, G.; Li, M.; Wang, J.; Zou, J.; Du, F.; Dong, J.; Cui, X.; Huang, X.; et al. One-step colorimetric genotyping of single nucleotide polymorphism using probe-enhanced loop-mediated isothermal amplification (PE-LAMP). Theranostics 2019, 9, 3723–3731. [Google Scholar] [CrossRef] [PubMed]
- Yongkiettrakul, S.; Kampeera, J.; Chareanchim, W.; Rattanajak, R.; Pornthanakasem, W.; Kiatpathomchai, W.; Kongkasuriyachai, D. Simple detection of single nucleotide polymorphism in Plasmodium falciparum by SNP-LAMP assay combined with lateral flow dipstick. Parasitol. Int. 2017, 66, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Itonaga, M.; Matsuzaki, I.; Warigaya, K.; Tamura, T.; Shimizu, Y.; Fujimoto, M.; Fumiyoshi, K.; Masao, I.; Murata, S. Novel methodology for rapid detection of KRAS mutation using PNA-LNA mediated loop-mediated isothermal amplification. PLoS ONE 2016, 11, e0151654. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.M.; Matthews, S.A.; Yan, G.; Zhou, G.; Lee, M.C.; Sirichaisinthop, J.; Kiattibutr, K.; Fan, Q.; Li, P.; Sattabongkot, J.; et al. Microgeography and molecular epidemiology of malaria at the Thailand-Myanmar border in the malaria pre-elimination phase. Malar. J. 2015, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Kanako, O.; Liu, Q.; Zhou, M.; Kawamoto, F.; Wataya, Y.; Otani, S.; Yamaguchi, Y.; Tanabe, K. Identification of the four species of human malaria parasites by nested PCR that targets variant sequences in the small subunit rRNA gene. Parasitol. Int. 1997, 46, 91–95. [Google Scholar] [CrossRef]
- Biswas, S.; Escalante, A.; Chaiyaroj, S.; Angkasekwinai, P.; Lal, A.A. Prevalence of point mutations in the dihydrofolate reductase and dihydropteroate synthetase genes of Plasmodium falciparum isolates from India and Thailand: A molecular epidemiologic study. Trop. Med. Int. Health 2001, 5, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Sugaram, R.; Suwannasin, K.; Kunasol, C.; Mathema, V.B.; Day, N.J.; Sudathip, P.; Prempree, P.; Dondorp, A.M.; Imwong, M. Molecular characterization of Plasmodium falciparum antifolate resistance markers in Thailand between 2008 and 2016. Malar. J. 2020, 19, 107. [Google Scholar] [CrossRef]
- Wongsrichanalai, C.; Baracus, M.J.; Muth, S.; Sutamihardja, A.; Wernsdorfer, W.H. A review of malaria diagnostic tools: Microscopy and rapid diagnostic test (RDT). In Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives; Supplement to Volume 77(6) of AJTMH; Breman, J.G., Alilio, M.S., White, N.J., Eds.; American Society of Tropical Medicine and Hygiene: Northbrook, IL, USA, 2007. [Google Scholar]
- WHO. Policy Brief on Malaria Diagnostics in Low-Transmission Settings; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Wu, L.; Van den Hoogen, L.L.; Slater, H.; Walker, P.G.T.; Ghani, A.C.; Drakeley, C.J.; Okell, L.C. Comparison of diagnostics for the detection of asymptomatic Plasmodium falciparum infections to inform control and elimination strategies. Nature 2015, 528, S86–S93. [Google Scholar] [CrossRef]
- Sattabongkot, J.; Suansomjit, C.; Nguitragool, W.; Sirichaisinthop, J.; Warit, S.; Tiensuwan, M.; Buates, S. Prevalence of asymptomatic Plasmodium infections with sub-microscopic parasite densities in the northwestern border of Thailand: A potential threat to malaria elimination. Malar. J. 2018, 17, 329. [Google Scholar] [CrossRef]
- Chahar, M.; Anvikar, A.; Valecha, N. Development and evaluation of a novel HNB based isothermal amplification assay for fast detection of Pyrimethamine resistance (S108N) in Plasmodium falciparum. Int. J. Environ. Res. Public Health 2019, 16, 1635. [Google Scholar] [CrossRef]
- Mohon, A.N.; Menard, D.; Alam, M.S.; Perera, K.; Pillai, D.R. A novel single-nucleotide polymorphism Loop Mediated Isothermal Amplification assay for detection of Artemisinin-resistant Plasmodium falciparum malaria. Open Forum Infect. Dis. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
| No. of Samples | (P %) | Parasite Density (P/µL) 1 | PfSNP-LAMP-LFD (N51I) | |
|---|---|---|---|---|
| P. falciparum2 | 2 | ND | ND | 2 |
| 5 | >0.2 | >10,000 | 5 | |
| 25 | >0.02–0.2 | >1000–10,000 | 25 | |
| 10 | >0.004–0.02 | >200–1000 | 10 | |
| 6 | >0.0002–0.004 | >100–200 | 6 | |
| 3 | 0.0001–0.0002 | >50–100 | 3 | |
| 4 | <0.0001 | <50 | 4 | |
| P. vivax3 | 1 | ND | ND | 0 |
| 72 | <0.0002–0.24 | <10–12,000 | 0 | |
| TOTAL | 128 | 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yongkiettrakul, S.; Kolié, F.R.; Kongkasuriyachai, D.; Sattabongkot, J.; Nguitragool, W.; Nawattanapaibool, N.; Suansomjit, C.; Warit, S.; Kangwanrangsan, N.; Buates, S. Validation of PfSNP-LAMP-Lateral Flow Dipstick for Detection of Single Nucleotide Polymorphism Associated with Pyrimethamine Resistance in Plasmodium falciparum. Diagnostics 2020, 10, 948. https://doi.org/10.3390/diagnostics10110948
Yongkiettrakul S, Kolié FR, Kongkasuriyachai D, Sattabongkot J, Nguitragool W, Nawattanapaibool N, Suansomjit C, Warit S, Kangwanrangsan N, Buates S. Validation of PfSNP-LAMP-Lateral Flow Dipstick for Detection of Single Nucleotide Polymorphism Associated with Pyrimethamine Resistance in Plasmodium falciparum. Diagnostics. 2020; 10(11):948. https://doi.org/10.3390/diagnostics10110948
Chicago/Turabian StyleYongkiettrakul, Suganya, Fassou René Kolié, Darin Kongkasuriyachai, Jetsumon Sattabongkot, Wang Nguitragool, Namfon Nawattanapaibool, Chayanut Suansomjit, Saradee Warit, Niwat Kangwanrangsan, and Sureemas Buates. 2020. "Validation of PfSNP-LAMP-Lateral Flow Dipstick for Detection of Single Nucleotide Polymorphism Associated with Pyrimethamine Resistance in Plasmodium falciparum" Diagnostics 10, no. 11: 948. https://doi.org/10.3390/diagnostics10110948
APA StyleYongkiettrakul, S., Kolié, F. R., Kongkasuriyachai, D., Sattabongkot, J., Nguitragool, W., Nawattanapaibool, N., Suansomjit, C., Warit, S., Kangwanrangsan, N., & Buates, S. (2020). Validation of PfSNP-LAMP-Lateral Flow Dipstick for Detection of Single Nucleotide Polymorphism Associated with Pyrimethamine Resistance in Plasmodium falciparum. Diagnostics, 10(11), 948. https://doi.org/10.3390/diagnostics10110948






