Multicentric Atrial Strain COmparison between Two Different Modalities: MASCOT HIT Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Population of the Study
2.2. Standard Echocardiography
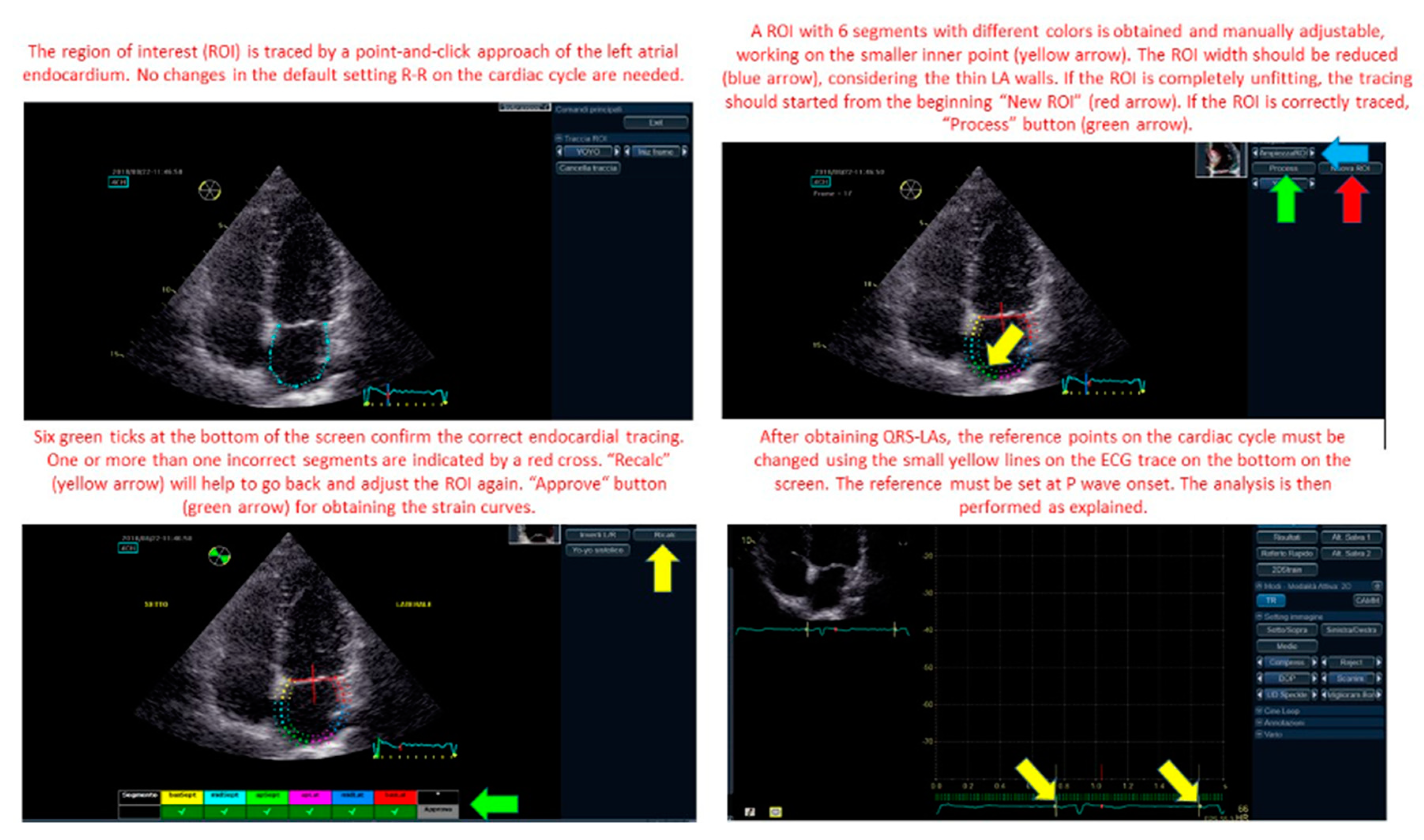
2.3. Speckle Tracking Echocardiography
2.4. Data Collection
2.5. Sample Size Justification
2.6. Statistical Analysis
3. Results
3.1. General Characteristic of the Enrolling Centres
3.2. General Characteristics of the Population
3.3. Assessment of Left Atrial Function
4. Discussion
4.1. Study Limitations
4.2. Clinical Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Blume, G.G.; McLeod, C.J.; Barnes, M.E.; Seward, J.B.; Pellikka, P.A.; Bastiansen, P.M.; Tsang, T.S.M. Left atrial function: Physiology, assessment, and clinical implications. Eur. J. Echocardiogr. 2011, 12, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameli, M.; Mandoli, G.E.; Sciaccaluga, C.; Mondillo, S. More than 10 years of speckle tracking echocardiography: Still a novel technique or a definite tool for clinical practice? Echocardiography 2019, 36, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G.; Galderisi, M.; Marwick, T.; Nagueh, S.F.; Sengupta, P.P.; et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J. Am. Soc. Echocardiogr. 2011, 24, 277–313. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, R.M.; Demirkol, S.; Buakhamsri, A.; Greenberg, N.; Popovic, Z.B.; Thomas, J.D.; Klein, A.L. Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. J. Am. Soc. Echocardiogr. 2010, 23, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [Green Version]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.; Muraru, D.; Popescu, B.A.; Sitges, M.; Rosca, M.; Pedrizzetti, G.; Henein, M.Y.; Donal, E.; Badano, L.P. Evaluation of Left Atrial Size and Function: Relevance for Clinical Practice. J. Am. Soc. Echocardiogr. 2020, 33, 934–952. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1961–1977. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Robinet, S.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Kacharava, G.; Athanassopoulos, G.D.; et al. Echocardiographic reference ranges for normal left atrial function parameters: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 630–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, E.; Fournet, M.; Chabanne, C.; Lelong, B.; Leguerrier, A.; Flecher, E.; Mabo, P.; Donal, E. Prognostic value of left atrial reservoir function in patients with severe aortic stenosis: A 2D speckle-tracking echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 533–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernigo, M.; Benfari, G.; Geremia, G.; Noni, M.; Borio, G.; Mazzali, G.; Zamboni, M.; Onorati, F.; Faggian, G.; Vassanelli, C.; et al. Atrial Function as an Independent Predictor of Postoperative Atrial Fibrillation in Patients Undergoing Aortic Valve Surgery for Severe Aortic Stenosis. J. Am. Soc. Echocardiogr. 2017, 30, 956–965.e1. [Google Scholar] [CrossRef]
- Cameli, M.; Pastore, M.C.; Righini, F.M.; Mandoli, G.E.; D’Ascenzi, F.; Lisi, M.; Nistor, D.; Sparla, S.; Curci, V.; Tommaso, C.D.; et al. Prognostic value of left atrial strain in patients with moderate asymptomatic mitral regurgitation. Int. J. Cardiovasc. Imaging 2019, 35, 1597–1604. [Google Scholar] [CrossRef]
- Yang, L.T.; Liu, Y.W.; Shih, J.Y.; Li, Y.H.; Tsai, L.M.; Luo, C.Y.; Tsai, W.C. Predictive value of left atrial deformation on prognosis in severe primary mitral regurgitation. J. Am. Soc. Echocardiogr. 2015, 28, 1309–1317.e4. [Google Scholar] [CrossRef]
- Miyoshi, H.; Oishi, Y.; Mizuguchi, Y.; Iuchi, A.; Nagase, N.; Ara, N.; Oki, T. Effect of an increase in left ventricular pressure overload on left atrial-left ventricular coupling in patients with hypertension: A two-dimensional speckle tracking echocardiographic study. Echocardiography 2013, 30, 658–666. [Google Scholar] [CrossRef]
- Miyoshi, H.; Oishi, Y.; Mizuguchi, Y.; Iuchi, A.; Nagase, N.; Ara, N.; Oki, T. Early predictors of alterations in left atrial structure and function related to left ventricular dysfunction in asymptomatic patients with hypertension. J. Am. Soc. Hypertens. 2013, 7, 206–215. [Google Scholar] [CrossRef]
- Cameli, M.; Sciaccaluga, C.; Loiacono, F.; Simova, I.; Miglioranza, M.H.; Nistor, D.; Bandera, F.; Emdin, M.; Giannoni, A.; Ciccone, M.M.; et al. The analysis of left atrial function predicts the severity of functional impairment in chronic heart failure: The FLASH multicenter study. Int. J. Cardiol. 2019, 286, 87–91. [Google Scholar] [CrossRef]
- Freed, B.H.; Daruwalla, V.; Cheng, J.Y.; Aguilar, F.G.; Beussink, L.; Choi, A.; Klein, D.A.; Dixon, D.; Baldridge, A.; Rasmussen-Torvik, L.J.; et al. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure with Preserved Ejection Fraction: Importance of Left Atrial Strain. Circ. Cardiovasc. Imaging 2016, 9, e003754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosca, M.; Popescu, B.A.; Beladan, C.C.; Calin, A.; Muraru, D.; Popa, E.C.; Lancellotti, P.; Enache, R.; Coman, I.M.; Jurcuţ, R.; et al. Left atrial dysfunction as a correlate of heart failure symptoms in hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2010, 23, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Al Saikhan, L.; Hughes, A.D.; Chung, W.S.; Alsharqi, M.; Nihoyannopoulos, P. Left atrial function in heart failure with mid-range ejection fraction differs from that of heart failure with preserved ejection fraction: A 2D speckle-tracking echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Yamada, H.; Bando, M.; Saijo, Y.; Nishio, S.; Hirata, Y.; Klein, A.L.; Sata, M. Optimal Analysis of Left Atrial Strain by Speckle Tracking Echocardiography: P-wave versus R-wave Trigger. Echocardiography 2015, 32, 1241–1249. [Google Scholar] [CrossRef]
- Wakami, K.; Ohte, N.; Asada, K.; Fukuta, H.; Goto, T.; Mukai, S.; Hitomi Narita, H.; Kimura, G. Correlation between left ventricular end-diastolic pressure and peak left atrial wall strain during left ventricular systole. J. Am. Soc. Echocardiogr. 2009, 22, 847–851. [Google Scholar] [CrossRef] [PubMed]
| Controls (n = 309) | AH and AS (n = 333) | MR and HF (n = 296) | p Value | |
|---|---|---|---|---|
| Age, years | 47.1 ± 15.6 | 65.3 ± 12.9 | 66.2 ± 13.8 | <0.0001 |
| Female, % | 47.7 | 47.4 | 38.4 | 0.029 |
| Weight, kg | 71.9 ± 13.3 | 77.8 ± 15.8 | 75 ± 15.2 | <0.0001 |
| Height, cm | 170 ± 9.6 | 167.6 ± 9.4 | 168.4 ± 9.3 | 0.0013 |
| BMI, kg/m2 | 24.8 ± 3.9 | 27.7 ± 4.8 | 26.4 ± 4.7 | <0.0001 |
| BSA, m2 | 1.82 ± 0.2 | 1.86 ± 0.2 | 1.84 ± 0.2 | 0.044 |
| HR, bpm | 68.9 ± 11.2 | 68.9 ± 10.5 | 70.2 ± 13 | 0.273 |
| SBP, mmHg | 123.4 ± 14 | 135.7 ± 18.4 | 127.4 ± 20.7 | <0.0001 |
| DBP, mmHg | 76 ± 8.5 | 79.6 ± 11.4 | 76.2 ± 12.7 | <0.0001 |
| Controls (n = 309) | AH and AS (n = 333) | MR and HF (n = 296) | p Value | |
|---|---|---|---|---|
| IVS, mm | 9.0 ± 1.6 | 12 ± 2.4 | 10.8 ± 2.2 | <0.0001 |
| LV PW, mm | 8.6 ± 1.6 | 10.9 ± 1.9 | 10.1 ± 2.0 | <0.0001 |
| LV mass index, g/m2 | 75.6 ± 19.1 | 107.6 ± 31.9 | 120.4 ± 35 | <0.0001 |
| LV EDD, mm | 47.3 ± 5.7 | 47.4 ± 6.2 | 55.0 ± 8.6 | <0.0001 |
| LV ESD, mm | 30.6 ± 5.7 | 31.0 ± 6.6 | 39.8 ± 11.0 | <0.0001 |
| LV EDV index, mL/m2 | 51.2 ± 12.5 | 51.9 ± 14.4 | 75.2 ± 30.2 | <0.0001 |
| LV ESV index, mL/m2 | 20.4 ± 6.5 | 21.9 ± 9.2 | 41.7 ± 28.9 | <0.0001 |
| LV EF, % | 60.4 ± 6.7 | 58.5 ± 9.2 | 47.0 ± 14.6 | <0.0001 |
| LA max volume index, mL/m2 | 26.0 ± 6.7 | 35.1 ± 13.4 | 45.9 ± 19.0 | <0.0001 |
| LA preA volume index, mL/m2 | 16.7 ± 5.8 | 25.1 ± 12.5 | 33.3 ± 15.7 | <0.0001 |
| LA min volume index, mL/m2 | 10.3 ± 4.3 | 16.3 ± 10.4 | 24.2 ± 14.4 | <0.0001 |
| E/A ratio | 1.3 ± 0.5 | 1.0 ± 0.5 | 1.48 ± 1.0 | <0.0001 |
| Mitral E DT, ms | 196.8 ± 53.4 | 223.5 ± 76.8 | 199.4 ± 76.7 | <0.0001 |
| E/e’ ratio | 7.0 ± 2.8 | 11.8 ± 6.3 | 13.4 ± 7.9 | <0.0001 |
| Controls (n = 309) | AH and AS (n = 333) | MR and HF (n = 296) | p Value | |
|---|---|---|---|---|
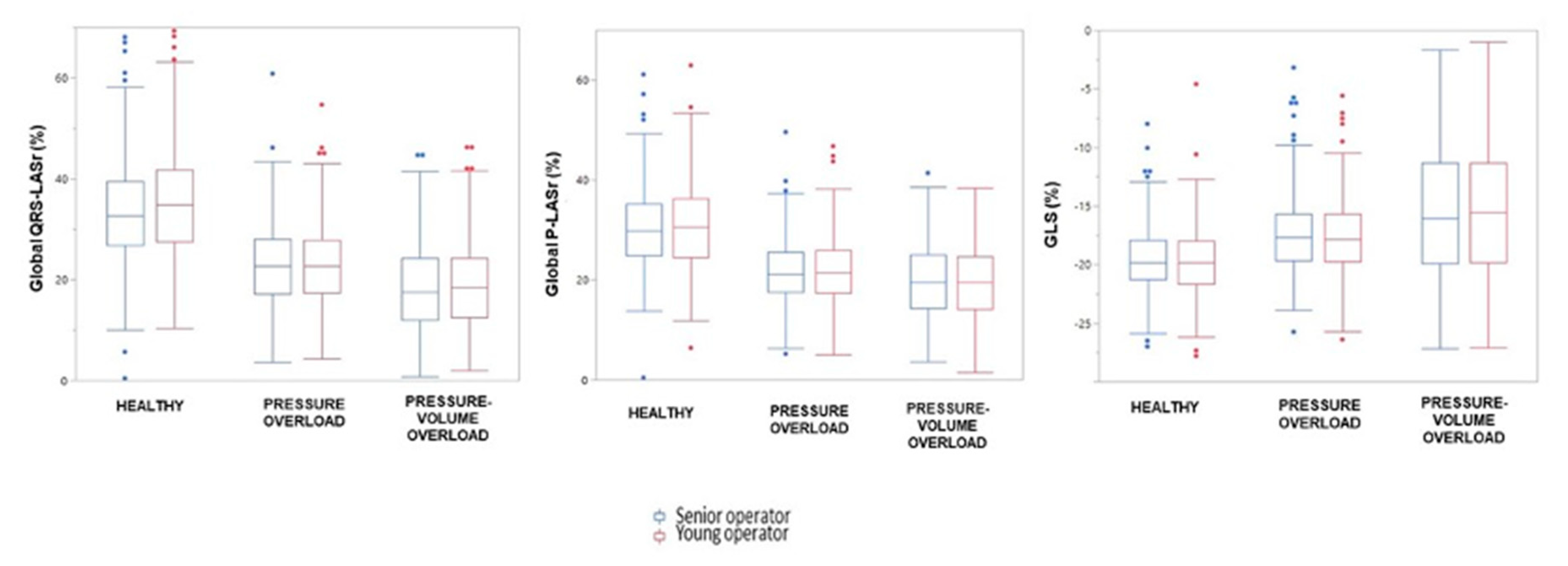
| Global QRS-LASr y, % | 35.4 ± 11.7 | 22.9 ± 8.4 | 19.1 ± 8.9 | <0.0001 |
| Global QRS-LASr s, % | 33.5 ± 10.9 | 23.0 ± 8.5 | 18.9 ± 9.2 | <0.0001 |
| Global QRS-LASct y, % | 15.5 ± 5.4 | 13.3 ± 5.5 | 10.1 ± 5.7 | <0.0001 |
| Global QRS-LASct s, % | 15 ± 5.3 | 13.4 ± 5.7 | 10 ± 5.7 | <0.0001 |
| Global P-LASr y, % | 31.2 ± 8.5 | 21.8 ± 6.9 | 19.2 ± 7.6 | <0.0001 |
| Global P-LASr s, % | 30.5 ± 8 | 21.9 ± 6.8 | 19.2 ± 7.4 | <0.0001 |
| LV GLS y, % | −19.9 ± 3.1 | −17.6 ± 3.3 | −15.5 ± 5.7 | <0.0001 |
| LV GLS s, % | −19.7 ± 3.0 | −17.4 ± 3.3 | −15.3 ± 5.4 | <0.0001 |
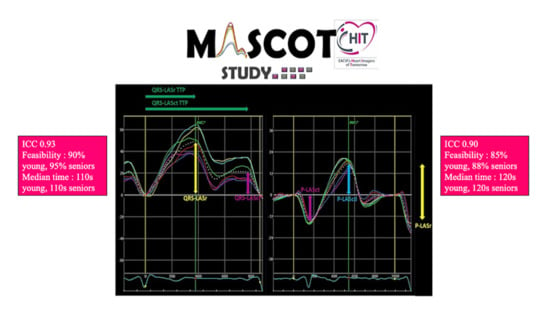
| ICC | 95% CI Lower Bound | 95% CI Upper Bound | |
|---|---|---|---|
| Study population | |||
| Global QRS-LASr | |||
| Average measures | 0.93 | 0.92 | 0.94 |
| Global P-LASr | |||
| Average measures | 0.90 | 0.89 | 0.92 |
| GLS | |||
| Average measures | 0.96 | 0.95 | 0.96 |
| Controls | |||
| Global QRS-LASr | |||
| Average measures | 0.84 | 0.80 | 0.88 |
| Global P-LASr | |||
| Average measures | 0.80 | 0.75 | 0.85 |
| LA pressure overload | |||
| Global QRS-LASr | |||
| Average measures | 0.92 | 0.90 | 0.94 |
| Global P-LASr | |||
| Average measures | 0.90 | 0.87 | 0.92 |
| LA volume-pressure overload | |||
| Global QRS-LASr | |||
| Average measures | 0.95 | 0.93 | 0.96 |
| Global P-LASr | |||
| Average measures | 0.94 | 0.92 | 0.95 |
| P-LASr | P-LASct | p | |
|---|---|---|---|
| CONTROLS measured by young operators | |||
| QRS-LASr | 0.76 | <0.001 | |
| QRS-LASct | −0.47 | <0.001 | |
| CONTROLS measured by senior operators | |||
| QRS-LASr | 0.71 | <0.001 | |
| QRS-LASct | −0.52 | <0.001 | |
| LA PRESSURE OVERLOAD measured by young operators | |||
| QRS-LASr | 0.82 | <0.001 | |
| QRS-LASct | −0.71 | <0.001 | |
| LA PRESSURE OVERLOAD measured by senior operators | |||
| QRS-LASr | 0.80 | <0.001 | |
| QRS-LASct | −0.67 | <0.001 | |
| LA PRESSURE–VOLUME OVERLOAD measured by young operators | |||
| QRS-LASr | 0.87 | <0.001 | |
| QRS-LASct | −0.69 | <0.001 | |
| LA PRESSURE–VOLUME OVERLOAD measured by senior operators | |||
| QRS-LASr | 0.88 | <0.001 | |
| QRS-LASct | −0.82 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cameli, M.; Miglioranza, M.H.; Magne, J.; Mandoli, G.E.; Benfari, G.; Ancona, R.; Sibilio, G.; Reskovic Luksic, V.; Dejan, D.; Griseli, L.; et al. Multicentric Atrial Strain COmparison between Two Different Modalities: MASCOT HIT Study. Diagnostics 2020, 10, 946. https://doi.org/10.3390/diagnostics10110946
Cameli M, Miglioranza MH, Magne J, Mandoli GE, Benfari G, Ancona R, Sibilio G, Reskovic Luksic V, Dejan D, Griseli L, et al. Multicentric Atrial Strain COmparison between Two Different Modalities: MASCOT HIT Study. Diagnostics. 2020; 10(11):946. https://doi.org/10.3390/diagnostics10110946
Chicago/Turabian StyleCameli, Matteo, Marcelo Haertel Miglioranza, Julien Magne, Giulia Elena Mandoli, Giovanni Benfari, Roberta Ancona, Gerolamo Sibilio, Vlatka Reskovic Luksic, Dosen Dejan, Leonardo Griseli, and et al. 2020. "Multicentric Atrial Strain COmparison between Two Different Modalities: MASCOT HIT Study" Diagnostics 10, no. 11: 946. https://doi.org/10.3390/diagnostics10110946
APA StyleCameli, M., Miglioranza, M. H., Magne, J., Mandoli, G. E., Benfari, G., Ancona, R., Sibilio, G., Reskovic Luksic, V., Dejan, D., Griseli, L., Van De Heyning, C. M., Mortelmans, P., Michalski, B., Kupczynska, K., Di Giannuario, G., Devito, F., Dulgheru, R., Ilardi, F., Salustri, A., ... Popescu, B. A. (2020). Multicentric Atrial Strain COmparison between Two Different Modalities: MASCOT HIT Study. Diagnostics, 10(11), 946. https://doi.org/10.3390/diagnostics10110946