Metal Ion Release, Clinical and Radiological Outcomes in Large Diameter Metal-on-Metal Total Hip Arthroplasty at Long-Term Follow-Up
Abstract
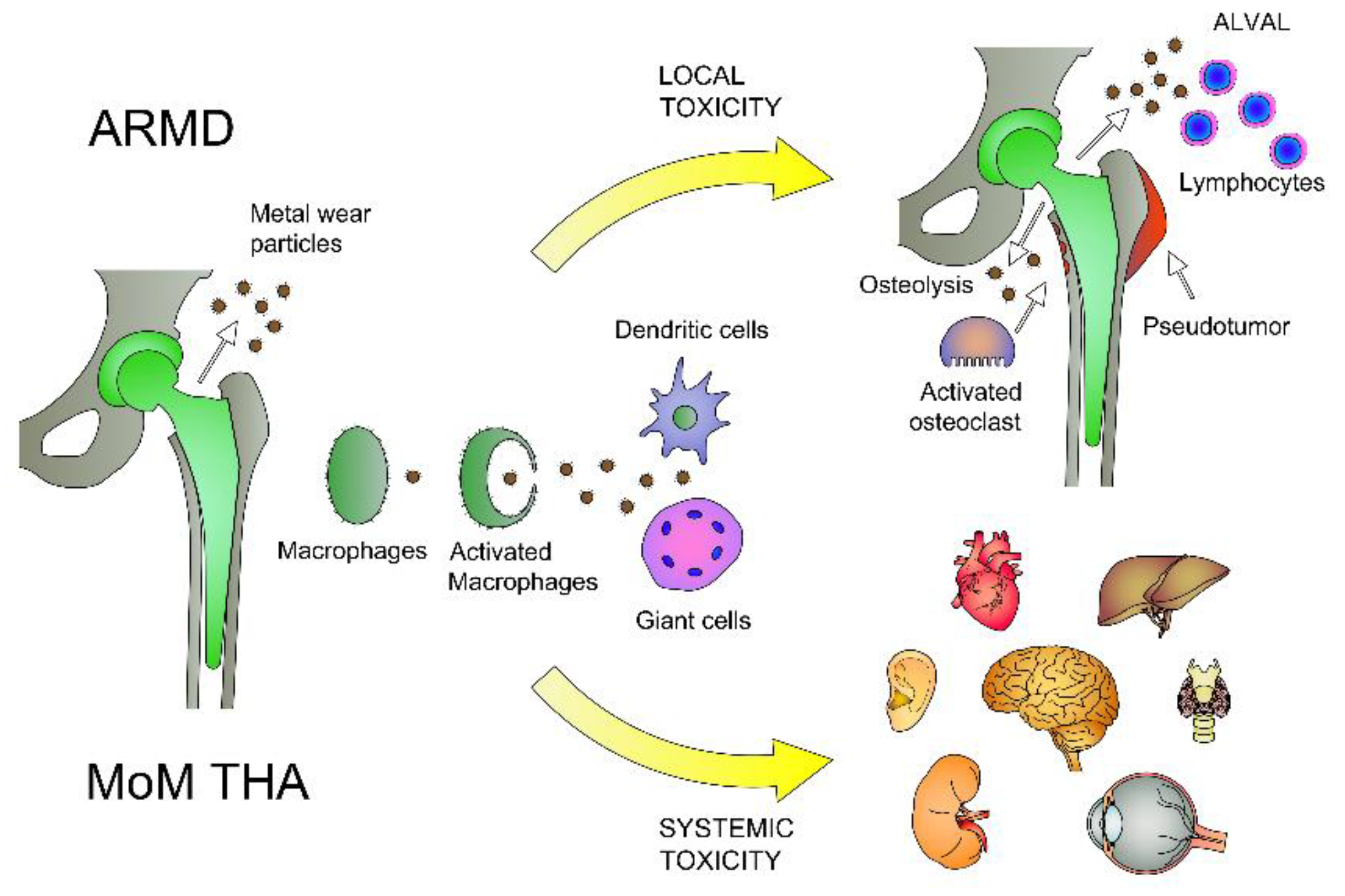
1. Introduction
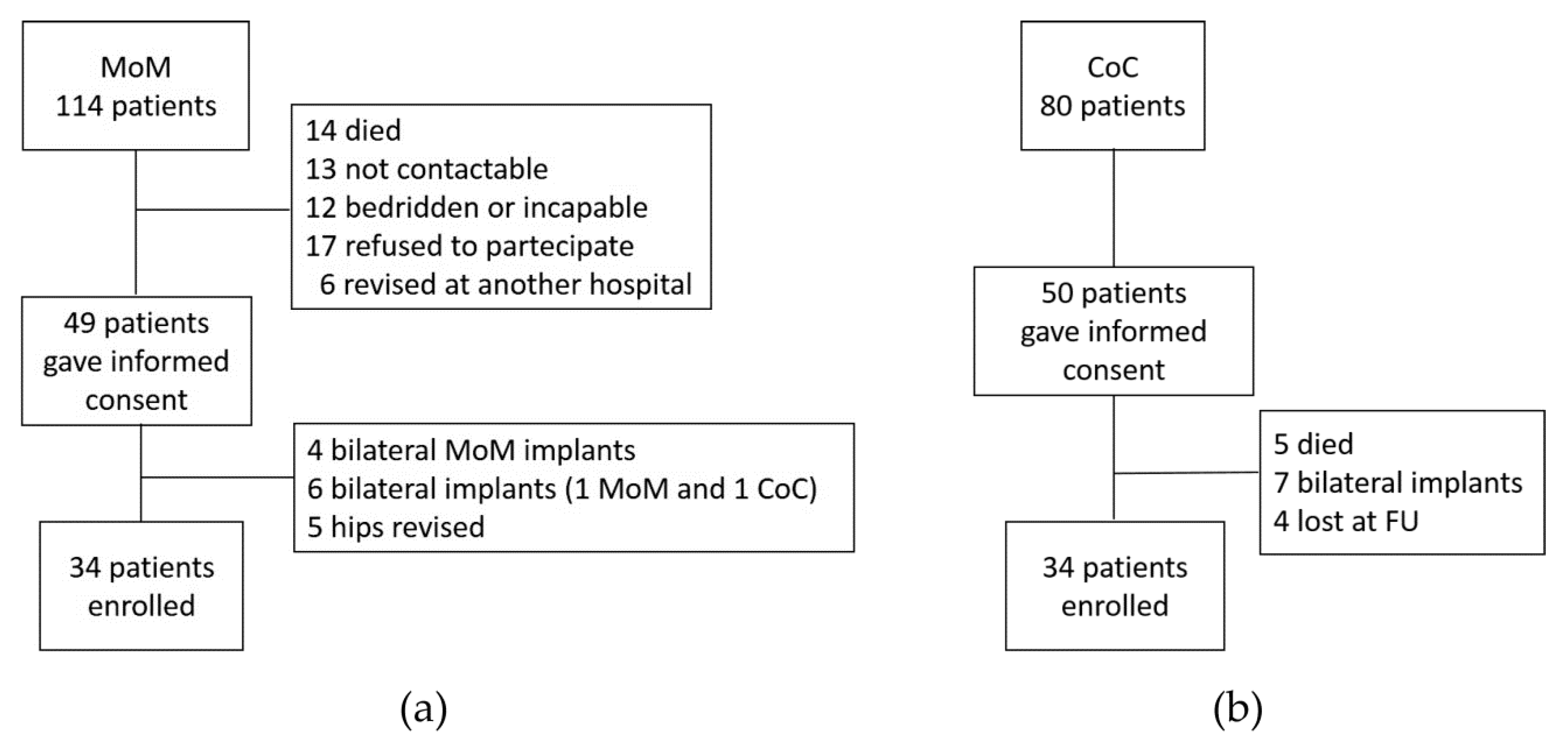
1.1. Study Population
1.2. Hip Implants
1.3. Surveillance Follow-Up Program
1.4. Clinical Evaluation
1.5. Radiological Evaluation
1.6. Metal Ion Measurement
1.7. Statistical Analysis
2. Results
2.1. Clinical Outcomes
2.2. Radiological Outcomes
2.3. Metal Ion Release in Blood and Urine Samples
2.4. Comparison Between Asymptomatic and Symptomatic Patients
2.5. Influence of Patient Demographic and Clinical Features on Metal Ion Release
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Learmonth, I.D.; Young, C.; Rorabeck, C. The operation of the century: Total hip replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef]
- OECD iLibrary. Hip and Knee Replacement. Available online: https://www.oecd-ilibrary.org/sites/2fc83b9a-en/index.html?itemId=/content/component/2fc83b9a-en (accessed on 10 November 2020).
- Kurtz, S.; Ong, K.; Lau, E.C.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. Vol. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Knight, S.R.; Aujla, R.; Biswas, S.P. Total Hip Arthroplasty—Over 100 years of operative history. Orthop. Rev. 2011, 3, e16. [Google Scholar] [CrossRef] [PubMed]
- Bitar, D. Biological response to prosthetic debris. World J. Orthop. 2015, 6, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Briggs, T.W.R.; Hanna, S.A.; Kayani, B.; Tai, S.; Pollock, R.C.; Cannon, S.R.; Blunn, G.W.; Carrington, R.W.J. Metal-on-polyethylene versus metal-on-metal bearing surfaces in total hip arthroplasty. Bone Jt. J. 2015, 1183–1191. [Google Scholar] [CrossRef]
- Putame, G.; Pascoletti, G.; Franceschini, G.; Dichio, G.; Terzini, M. Prosthetic Hip ROM from Multibody Software Simulation. In Proceedings of the IEEE 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 5386–5389. [Google Scholar]
- Kwon, Y.-M.; Lombardi, A.V.; Jacobs, J.J.; Fehring, T.K.; Lewis, C.G.; Cabanela, M.E. Risk Stratification Algorithm for Management of Patients with Metal-on-Metal Hip Arthroplasty. J. Bone Jt. Surg. Am. Vol. 2014, 96, e4. [Google Scholar] [CrossRef]
- Langton, D.J.; Joyce, T.J.; Jameson, S.S.; Lord, J.; Van Orsouw, M.; Holland, J.P.; Nargol, A.V.F.; De Smet, K.A. Adverse reaction to metal debris following hip resurfacing. J. Bone Jt. Surg. Br. Vol. 2011, 93, 164–171. [Google Scholar] [CrossRef]
- Catelas, I.; Wimmer, M.A. New Insights into Wear and Biological Effects of Metal-on-Metal Bearings. J. Bone Jt. Surg. Am. Vol. 2011, 93, 76–83. [Google Scholar] [CrossRef]
- Granchi, D.; Savarino, L.M.; Ciapetti, G.; Baldini, N. Biological effects of metal degradation in hip arthroplasties. Crit. Rev. Toxicol. 2017, 48, 170–193. [Google Scholar] [CrossRef]
- Bijukumar, D.R.; Segu, A.; De Souza, J.C.M.; Li, X.; Barba, M.; Mercuri, L.G.; Jacobs, J.J.; Mathew, M.T. Systemic and local toxicity of metal debris released from hip prostheses: A review of experimental approaches. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 951–963. [Google Scholar] [CrossRef]
- Carlson, B.C.; Bryan, A.J.; Carrillo-Villamizar, N.T.; Sierra, R.J. The Utility of Metal Ion Trends in Predicting Revision in Metal-on-Metal Total Hip Arthroplasty. J. Arthroplast. 2017, 32, S214–S219. [Google Scholar] [CrossRef] [PubMed]
- Nicolli, A.; Bisinella, G.; Padovani, G.; Vitella, A.; Chiara, F.; Trevisan, A. Predictivity and Fate of Metal Ion Release From Metal-On-Metal Total Hip Prostheses. J. Arthroplast. 2014, 29, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Matharu, G.S.; Judge, A.; Eskelinen, A.; Murray, D.W.; Pandit, H.G. What is appropriate surveillance for metal-on-metal hip arthroplasty patients? Acta Orthop. 2017, 89, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Hutt, J.; Lavigne, M.; Lungu, E.; Belzile, E.; Morin, F.; Vendittoli, P.-A. Comparison of Whole-Blood Metal Ion Levels Among Four Types of Large-Head, Metal-on-Metal Total Hip Arthroplasty Implants. J. Bone Jt. Surg. Am. Vol. 2016, 98, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Jantzen, C.; Jørgensen, H.L.; Duus, B.R.; Sporring, S.L.; Lauritzen, J.B. Chromium and cobalt ion concentrations in blood and serum following various types of metal-on-metal hip arthroplasties. Acta Orthop. 2013, 84, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, F.; Hartmann, A.; Schmitt, J.; Lützner, J.; Seidler, A.; Campbell, P.; Delaunay, C.; Drexler, H.; Ettema, H.; García-Cimbrelo, E.; et al. European multidisciplinary consensus statement on the use and monitoring of metal-on-metal bearings for total hip replacement and hip resurfacing. Orthop. Traumatol. Surg. Res. 2013, 99, 263–271. [Google Scholar] [CrossRef]
- Lainiala, O.S.; Moilanen, T.P.; Hart, A.J.; Huhtala, H.S.; Sabah, S.A.; Eskelinen, A. Higher Blood Cobalt and Chromium Levels in Patients With Unilateral Metal-on-Metal Total Hip Arthroplasties Compared to Hip Resurfacings. J. Arthroplast. 2016, 31, 1261–1266. [Google Scholar] [CrossRef]
- MHRA. Medical Device Alert: MDA/2017/018: All Metal-on-Metal (MoM) hip Replacements—Updated Advice for Follow-up of Patients. Available online: https://www.gov.uk/drug-device-alerts/all-metal-on-metal-mom-hip-replacements-updated-advice-for-follow-up-of-patients (accessed on 10 November 2020).
- U.S. Food and Drug Administration. Concerns about Metal-on-Metal hip Implants. Available online: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/ucm241604.htm (accessed on 10 November 2020).
- Scientific Committee on Emerging and Newly Identified Health Risks. Final Opinion on the Safety of Metal-on-Metal Joint Replacements with a Particular Focus on hip Implants. Available online: http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_042.pdf (accessed on 10 November 2020).
- Iacobellis, C.; Berizzi, A.; Pozzuoli, A.; Biz, C. Normalization of chromium and cobalt values after femoral head replacement. Int. J. Surg. Case Rep. 2015, 10, 146–150. [Google Scholar] [CrossRef]
- Bistolfi, A.; Cimino, A.; Lee, G.-C.; Ferracini, R.; Maina, G.; Berchialla, P.; Massazza, G.; Massè, A. Does metal porosity affect metal ion release in blood and urine following total hip arthroplasty? A short term study. HIP Int. 2018, 28, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Apolone, G.; Mosconi, P. The Italian SF-36 Health Survey: Translation, validation and norming. J. Clin. Epidemiol. 1998, 51, 1025–1036. [Google Scholar] [CrossRef]
- Dettoni, F.; Pellegrino, P.; La Russa, M.R.; Bonasia, D.E.; Blonna, D.; Bruzzone, M.; Castoldi, F.; Rossi, R. Validation and Cross Cultural Adaptation of the Italian Version of the Harris Hip Score. HIP Int. 2015, 25, 91–97. [Google Scholar] [CrossRef] [PubMed]
- DeLee, J.G.; Charnley, J. Radiological Demarcation of Cemented Sockets in Total Hip Replacement. Clin. Orthop. Relat. Res. 1976, 20–32. [Google Scholar] [CrossRef]
- Gruen, T.; McNeice, G.M.; Amstutz, H.C. “Modes of failure” of cemented stem-type femoral components: A radiographic analysis of loosening. Clin. Orthop. Relat. Res. 1979, 141, 17–27. [Google Scholar] [CrossRef]
- Sharp, I.K. ACETABULAR DYSPLASIA. J. Bone Jt. Surg. Br. Vol. 1961, 1961, 268–272. [Google Scholar] [CrossRef]
- De Haan, R.; Pattyn, C.; Gill, H.S.; Murray, D.W.; Campbell, P.A.; De Smet, K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J. Bone Jt. Surg. Br. Vol. 2008, 90, 1291–1297. [Google Scholar] [CrossRef]
- Pomeroy, E.; MacNamara, F.; Murphy, E.P.; McGoldrick, N.; Mahapatra, A.; Awan, N. Femoral offset found not to affect metal ion levels in metal-on-metal total hip arthroplasty. Ir. J. Med Sci. 2018, 188, 149–153. [Google Scholar] [CrossRef]
- Bernstein, M.; Walsh, A.; Petit, A.; Zukor, D.J.; Antoniou, J. Femoral Head Size Does Not Affect Ion Values in Metal-on-Metal Total Hips. Clin. Orthop. Relat. Res. 2010, 469, 1642–1650. [Google Scholar] [CrossRef]
- Vendittoli, P.-A.; Amzica, T.; Roy, A.G.; Lusignan, D.; Girard, J.; Lavigne, M. Metal Ion Release With Large-Diameter Metal-on-Metal Hip Arthroplasty. J. Arthroplast. 2011, 26, 282–288. [Google Scholar] [CrossRef]
- Sands, D.; Schemitsch, E.H. The Role of Metal-on-Metal Bearings in Total Hip Arthroplasty and Hip Resurfacing. HSS J. 2016, 13, 2–6. [Google Scholar] [CrossRef]
- Lavigne, M.; Belzile, E.L.; Roy, A.; Morin, F.; Amzica, T.; Vendittoli, P.-A. Comparison of Whole-Blood Metal Ion Levels in Four Types of Metal-on-Metal Large-Diameter Femoral Head Total Hip Arthroplasty: The Potential Influence of the Adapter Sleeve. J. Bone Jt. Surg. Am. Vol. 2011, 93, 128–136. [Google Scholar] [CrossRef]
- Reito, A.; Lainiala, O.; Elo, P.; Eskelinen, A. Prevalence of Failure due to Adverse Reaction to Metal Debris in Modern, Medium and Large Diameter Metal-on-Metal Hip Replacements – The Effect of Novel Screening Methods: Systematic Review and Metaregression Analysis. PLoS ONE 2016, 11, e0147872. [Google Scholar] [CrossRef] [PubMed]
- Lass, R.; Grübl, A.; Kolb, A.; Stelzeneder, D.; Pilger, A.; Kubista, B.; Giurea, A.; Windhager, R. Comparison of synovial fluid, urine, and serum ion levels in metal-on-metal total hip arthroplasty at a minimum follow-up of 18 years. J. Orthop. Res. 2014, 32, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Maurer-Ertl, W.; Friesenbichler, J.; Sadoghi, P.; Pechmann, M.; Trennheuser, M.; Leithner, A. Metal ion levels in large-diameter total hip and resurfacing hip arthroplasty-Preliminary results of a prospective five year study after two years of follow-up. BMC Musculoskelet. Disord. 2012, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Lee, D.; Bali, K.; Railton, P.; Kinniburgh, D.; Faris, P.; Marshall, D.A.; Burkart, B.; Powell, J.N. Does bearing size influence metal ion levels in large-head metal-on-metal total hip arthroplasty? A comparison of three total hip systems. J. Orthop. Surg. Res. 2014, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Matharu, G.S.; Berryman, F.; Judge, A.; Reito, A.; McConnell, J.S.; Lainiala, O.; Young, S.K.; Eskelinen, A.; Pandit, H.; Murray, D. Blood Metal Ion Thresholds to Identify Patients with Metal-on-Metal Hip Implants at Risk of Adverse Reactions to Metal Debris. J. Bone Jt. Surg. Am. Vol. 2017, 99, 1532–1539. [Google Scholar] [CrossRef]
- Van Lingen, C.P.; Zagra, L.M.; Ettema, H.B.; Verheyen, C.C. Sequelae of large-head metal-on-metal hip arthroplasties. EFORT Open Rev. 2016, 1, 345–353. [Google Scholar] [CrossRef]
- Reito, A.; Moilanen, T.; Puolakka, T.; Pajamäki, J.; Eskelinen, A. Repeated metal ion measurements in patients with high risk metal-on-metal hip replacement. Int. Orthop. 2014, 38, 1353–1361. [Google Scholar] [CrossRef]
- Higuchi, Y.; Seki, T.; Hasegawa, Y.; Morita, D.; Komatsu, D.; Takegami, Y.; Ishiguro, N. Comparison of cementless total hip arthroplasty survivorship between metal-on-highly cross-linked polyethylene and ceramic on ceramic bearings: A case control study with a 5-9-year follow-up. Orthop. Traumatol. Surg. Res. 2018, 104, 663–669. [Google Scholar] [CrossRef]
- Pattyn, C.A.; Lauwagie, S.N.; Verdonk, R.C. Whole Blood Metal Ion Concentrations in Correlation with Activity Level in Three Different Metal-On-Metal Bearings. J. Arthroplast. 2011, 26, 58–64. [Google Scholar] [CrossRef]
- Weissinger, M.; Grübl, A.; Pöll, G. Serum-cobalt levels with metal-on-metal bearings in the cement-free total hip arthroplasty results covering two years; prospective study. Acta Chir. Orthop. et Traumatol. Cechoslov. 2011, 78, 410–415. [Google Scholar]
- Desmarchelier, R.; Viste, A.; Chouteau, J.; Lerat, J.-L.; Fessy, M.-H. Metasul vs Cerasul Bearings. J. Arthroplast. 2013, 28, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.R.; Jennings, J.M.; Watters, T.S.; Levy, D.L.; Miner, T.M.; Dennis, D.A. Midterm Prospective Comparative Analysis of 2 Hard-on-Hard Bearing Total Hip Arthroplasty Designs. J. Arthroplast. 2018, 33, 1820–1825. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-K.; Yoon, B.-H.; Choi, Y.S.; Jo, W.-L.; Ha, Y.-C.; Koo, K.-H. Metal on Metal or Ceramic on Ceramic for Cementless Total Hip Arthroplasty: A Meta-Analysis. J. Arthroplast. 2016, 31, 2637–2645.e1. [Google Scholar] [CrossRef] [PubMed]
- Hussey, D.K.; Madanat, R.; Donahue, G.S.; Rolfson, O.; Bragdon, C.R.; Muratoglu, O.K.; Malchau, H. Scoring the Current Risk Stratification Guidelines in Follow-up Evaluation of Patients After Metal-on-Metal Hip Arthroplasty. J. Bone Jt. Surg. Am. Vol. 2016, 98, 1905–1912. [Google Scholar] [CrossRef]
- Higuchi, Y.; Seki, T.; Takegami, Y.; Komatsu, D.; Morita, D.; Ishiguro, N. Same survival but higher rate of osteolysis for metal-on-metal Ultamet versus ceramic-on-ceramic in patients undergoing primary total hip arthroplasty after 8 years of follow-up. Orthop. Traumatol. Surg. Res. 2018, 104, 1155–1161. [Google Scholar] [CrossRef]
- Donaldson, F.E.; Coburn, J.C.; Siegel, K.L. Total hip arthroplasty head–neck contact mechanics: A stochastic investigation of key parameters. J. Biomech. 2014, 47, 1634–1641. [Google Scholar] [CrossRef]
- Valente, G.; Lanting, B.; Macdonald, S.; Teeter, M.G.; Van Citters, D.; Howard, J. Femoral head material loss at the head-neck junction in total hip arthroplasty: The effect of head size, stem material and stem offset. HIP Int. 2018, 29, 647–651. [Google Scholar] [CrossRef]
- White, P.B.; Meftah, M.; Ranawat, A.S.; Ranawat, C.S. A Comparison of Blood Metal Ions in Total Hip Arthroplasty Using Metal and Ceramic Heads. J. Arthroplast. 2016, 31, 2215–2220. [Google Scholar] [CrossRef]
- Barlow, B.T.; Ortiz, P.A.; Boles, J.W.; Lee, Y.-Y.; Padgett, D.E.; Westrich, G.H. What Are Normal Metal Ion Levels After Total Hip Arthroplasty? A Serologic Analysis of Four Bearing Surfaces. J. Arthroplast. 2017, 32, 1535–1542. [Google Scholar] [CrossRef]
- Hartmann, A.; Hannemann, F.; Lützner, J.; Seidler, A.; Drexler, H.; Günther, K.-P.; Schmitt, J. Metal Ion Concentrations in Body Fluids after Implantation of Hip Replacements with Metal-on-Metal Bearing – Systematic Review of Clinical and Epidemiological Studies. PLoS ONE 2013, 8, e70359. [Google Scholar] [CrossRef]
- Bayley, N.; Khan, H.; Grosso, P.; Hupel, T.; Stevens, D.; Snider, M.; Schemitsch, E.; Kuzyk, P. What Are the Predictors and Prevalence of Pseudotumor and Elevated Metal Ions After Large-diameter Metal-on-metal THA? Clin. Orthop. Relat. Res. 2015, 473, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.Y.; McAnally, J.L.; Van Horne, J.R.; Wolfson, T.; Gamst, A.; Chung, C.B. Relationship of Plasma Metal Ions and Clinical and Imaging Findings in Patients with ASR XL Metal-on-Metal Total Hip Replacements. J. Bone Jt. Surg. Am. Vol. 2013, 95, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, A.R.; Bergin, K.M.; Kelly, G.E.; McCoy, G.F.; Wozniak, A.P.; Quinlan, J.F. The Effect of Acetabular Inclination on Metal Ion Levels Following Metal-on-Metal Hip Arthroplasty. J. Arthroplast. 2014, 29, 186–191. [Google Scholar] [CrossRef]
- Hallows, R.K.; Pelt, C.E.; Erickson, J.A.; Peters, C.L. Serum Metal Ion Concentration: Comparison Between Small and Large Head Metal-on-Metal Total Hip Arthroplasty. J. Arthroplast. 2011, 26, 1176–1181. [Google Scholar] [CrossRef]
- Langton, D.J.; Sprowson, A.P.; Mahadeva, D.; Bhatnagar, S.; Holland, J.P.; Nargol, A.V. Cup Anteversion in Hip Resurfacing: Validation of EBRA and the Presentation of a Simple Clinical Grading System. J. Arthroplast. 2010, 25, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Moroni, A.; Savarino, L.; Hoque, M.; Cadossi, M.; Baldini, N. Do Ion Levels In Hip Resurfacing Differ From Metal-on-metal THA at Midterm? Clin. Orthop. Relat. Res. 2010, 469, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Van Der Straeten, C.; Van Quickenborne, D.; De Roest, B.; Calistri, A.; Victor, J.; De Smet, K. Metal ion levels from well-functioning Birmingham Hip Resurfacings decline significantly at ten years. Bone Jt. J. 2013, 95, 1332–1338. [Google Scholar] [CrossRef]
- Hasegawa, M.; Yoshida, K.; Wakabayashi, H.; Sudo, A. Cobalt and Chromium Ion Release After Large-Diameter Metal-on-Metal Total Hip Arthroplasty. J. Arthroplast. 2012, 27, 990–996. [Google Scholar] [CrossRef]
- Brodner, W.; Grübl, A.; Jankovsky, R.; Meisinger, V.; Lehr, S.; Gottsauner-Wolf, F. Cup inclination and serum concentration of cobalt and chromium after metal-on-metal total hip arthroplasty. J. Arthroplast. 2004, 19, 66–70. [Google Scholar] [CrossRef]
- Jelsma, J.; Schotanus, M.G.M.; Senden, R.; Heyligers, I.C.; Grimm, B. Metal ion concentrations after metal-on-metal hip arthroplasty are not correlated with habitual physical activity levels. HIP Int. 2018, 29, 638–646. [Google Scholar] [CrossRef]
- Hunt, L.P.; Blom, A.W.; Matharu, G.S.; Porter, M.L.; Whitehouse, M.R. The risk of developing cancer following metal-on-metal hip replacement compared with non metal-on-metal hip bearings: Findings from a prospective national registry “The National Joint Registry of England, Wales, Northern Ireland and the Isle of Man”. PLoS ONE 2018, 13, e0204356. [Google Scholar] [CrossRef] [PubMed]
- Visuri, T.; Pulkkinen, P.; Paavolainen, P.; Pukkala, E. Cancer risk is not increased after conventional hip arthroplasty. Acta Orthop. 2010, 81, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.H.; Leikin, J.B.; Dargan, P.I.; Archer, J.R.H.; Wood, D.M.; Brent, J. Metal-on-Metal Hip Joint Prostheses: A Retrospective Case Series Investigating the Association of Systemic Toxicity with Serum Cobalt and Chromium Concentrations. J. Med. Toxicol. 2017, 13, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Savarino, L.; Padovani, G.; Ferretti, M.; Greco, M.; Cenni, E.; Perrone, G.; Greco, F.; Baldini, N.; Giunti, A. Serum ion levels after ceramic-on-ceramic and metal-on-metal total hip arthroplasty: 8-year minimum follow-up. J. Orthop. Res. 2008, 26, 1569–1576. [Google Scholar] [CrossRef]
- Swiatkowska, I.; Martin, N.G.; Henckel, J.; Apthorp, H.; Hamshere, J.; Hart, A.J. Blood and plasma titanium levels associated with well-functioning hip implants. J. Trace Elem. Med. Biol. 2020, 57, 9–17. [Google Scholar] [CrossRef]
- Pijls, B.G.; Meessen, J.M.T.A.; Tucker, K.; Stea, S.; Steenbergen, L.; Fenstad, A.M.; Mäkelä, K.; Stoica, I.C.; Goncharov, M.; Overgaard, S.; et al. MoM total hip replacements in Europe: A NORE report. EFORT Open Rev. 2019, 4, 423–429. [Google Scholar] [CrossRef]
- Sidaginamale, R.P.; Joyce, T.J.; Lord, J.K.; Jefferson, R.; Blain, P.G.; Nargol, A.V.F.; Langton, D.J. Blood metal ion testing is an effective screening tool to identify poorly performing metal-on-metal bearing surfaces. Bone Jt. Res. 2013, 2, 84–95. [Google Scholar] [CrossRef]
- Saini, R.; Railton, P.; Boyd, J.; Sadrzadeh, H.; Powell, J.N. Concordance between laboratories in metal ion testing in patients with metal-on-metal hip implants. Can. J. Surg. 2019, 62, 9–13. [Google Scholar] [CrossRef]



| Characteristics | MoM (n = 34) | CoC (n = 34) | p-Value |
|---|---|---|---|
| Women n (%) | 23 (67.6) | 21 (61.8) | 0.80 * |
| Men n (%) | 11 (32.4) | 13 (38.2) | |
| Age (years) Mean (SD) | 66.1 (10.4) | 68.6 (7.6) | 0.26 ¶ |
| Left side n (%) | 19 (55.9) | 18 (52.9) | 1.00 * |
| Femoral head (mm) Median (Range) | 46.0 (42.0–58.0) | 36.0 (32.0–40.0) | <0.0001 ° |
| BMI (Kg/m²) Median (Range) | 24.3 (18.9–38.2) | 25.5 (19.5–38.9) | 0.14 ° |
| Dietary supplements n (%) | 6 (17.6) | 4 (11.8) | 0.73 * |
| Smoke n (%) | 10 (29.4) | 13 (38.2) | 0.61 * |
| Allergies n (%) | 11 (32.4) | 5 (14.7) | 0.15 * |
| Drugs n (%) | 29 (85.3) | 32 (94.1) | 0.43 * |
| Job exposure n (%) | 4 (11.8) | 2 (5.9) | 0.67 * |
| Physical activity n (%) | 18 (54.9) | 13 (38.2) | 0.33 * |
| Diagnosis n (%) | 0.12 * | ||
| Bone fracture | 17 (50.0) | 22 (64.7) | |
| Osteoarthritis | 13 (38.2) | 12 (35.3) | |
| Necrosis | 4 (11.8) | 0 (0.0) | |
| Follow-up time after surgery (years) Median (Range) | 7.4 (3.2–10.0) | 7.4 (3.8–9.3) | 0.61 ° |
| Outcomes | MoM (n = 34) | CoC (n = 34) | p-Value |
|---|---|---|---|
| Harris Hip score Median (Range) | 89.9 (50.9–97.0) | 88.0 (48.9–97.0) | 0.70 ° |
| Pain n (%) | 0.24 * | ||
| Grade 0 | 14 (41.2) | 19 (55.9) | |
| Grade 1 | 12 (35.3) | 11 (32.4) | |
| Grade 2 | 6 (17.6) | 1 (2.9) | |
| Grade 3 | 2 (5.9) | 2 (5.9) | |
| Grade 4 | 0 (0.0) | 1 (2.9) | |
| Grade 5 SF-36 Median (Range) | 0 (0.0) | 0 (0.0) | |
| PCS Scale | 41.0 (20.7–58.4) | 47.7 (23.9–60.4) | 0.20 ° |
| MCS Scale | 53.9 (12.0–64.1) | 51.7 (23.3–66.1) | 0.94 ° |
| Outcomes | MoM (n = 34) | CoC (n = 34) | p-Value |
|---|---|---|---|
| Inclination angle (°) Median (Range) | 40.0 (9.0–55.0) | 40.5 (12.0–55.0) | 0.93 ° |
| Periprosthetic osteolysis n (%) | 23 (67.6) | 6 (17.6) | <0.0001 * |
| Postoperative Femoral offset (mm) Median (Range) DFO | 41.9 (32.5–55.3) 1.6 (−4.9–8) | 41.8 (33.6–54.7) 1.1 (−6–5) | 0.565 ° 0.749 ° |
| Metals | MoM (n = 34) | CoC (n = 34) | p-Value |
|---|---|---|---|
| Cobalt B (µg/L) | 1.2 (0.6–13.6) | 0.6 (0.6–2.5) | 0.0002 * |
| Cobalt U/Creatinine (µg/g) | 4.8 (0.4–71.0) | 1.4 (0.2–11.8) | <0.0001 * |
| Chromium B (µg/L) | 0.8 (0.1–7.3) | 0.3 (0.1–2.5) | 0.0029 * |
| Chromium U/Creatinine (µg/g) | 2.9 (0.7–34.9) | 0.6 (0.1–10.0) | <0.0001 * |
| Metals | MoM | CoC | p–Value | ||
|---|---|---|---|---|---|
| Asymptomatic (n = 14) | Symptomatic (n = 20) | Asymptomatic (n = 19) | Symptomatic (n = 15) | ||
| Cobalt B (µg/L) | 0.7 (0.56–9.25) | 1.2 (0.6–13.6) | 0.6 (0.56–2.5) | 0.6 (0.6–1.9) | a 0.030 b 0.003 c 0.458 d 0.950 |
| Cobalt U/Creatinine (µg/g) | 4.4 (0.85–23.9) | 4.9 (0.4–71.0) | 1.2 (0.3–9.3) | 1.8 (0.2–11.8) | a 0.001 b 0.012 c 0.649 d 0.107 |
| Chromium B (µg/L) | 0.9 (0.1–6.5) | 0.6 (0.1–7.3) | 0.3 (0.1–1.5) | 0.3 (0.1–2.5) | a <0.0001 b 0.261 c 0.292 d 0.617 |
| Chromium U/Creatinine (µg/g) Median (Range) | 2.9 (1.24–23.4) | 3.1 (0.7–34.9) | 0.3 (0.1–10.0) | 1.2 (0.1–7.5) | a <0.0001 b 0.003 c 0.834 d 0.054 |
| Outcomes | MoM | CoC | p–Value | ||
|---|---|---|---|---|---|
| Asymptomatic (n = 14) | Symptomatic (n = 20) | Asymptomatic (n = 19) | Symptomatic (n = 15) | ||
| HHS | 94.4 (86.8–97.0) | 81.5 (50.9–92.9) | 92.1 (64.3–97.0) | 81.8 (48.8–94.9) | a 0.412 b 0.641 c 0.000029 d 0.006 |
| SF-36 | |||||
| PCS Scale | 47.9 (29.7–57.7) | 38.7 (20.7–58.4) | 52.7 (23.8–60.4) | 39.5 (27.7–59.1) | a 0.274 b 0.841 c 0.107 d 0.028 |
| MCS Scale | 55.4 (49.8–55.4) | 42.4 (12.0–64.1) | 52.6 (31.3–59.8) | 50.1 (23.3–66.1) | a 0.032 b 0.182 c 0.013 d 0.959 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozzuoli, A.; Berizzi, A.; Crimì, A.; Belluzzi, E.; Frigo, A.C.; Conti, G.D.; Nicolli, A.; Trevisan, A.; Biz, C.; Ruggieri, P. Metal Ion Release, Clinical and Radiological Outcomes in Large Diameter Metal-on-Metal Total Hip Arthroplasty at Long-Term Follow-Up. Diagnostics 2020, 10, 941. https://doi.org/10.3390/diagnostics10110941
Pozzuoli A, Berizzi A, Crimì A, Belluzzi E, Frigo AC, Conti GD, Nicolli A, Trevisan A, Biz C, Ruggieri P. Metal Ion Release, Clinical and Radiological Outcomes in Large Diameter Metal-on-Metal Total Hip Arthroplasty at Long-Term Follow-Up. Diagnostics. 2020; 10(11):941. https://doi.org/10.3390/diagnostics10110941
Chicago/Turabian StylePozzuoli, Assunta, Antonio Berizzi, Alberto Crimì, Elisa Belluzzi, Anna Chiara Frigo, Giorgio De Conti, Annamaria Nicolli, Andrea Trevisan, Carlo Biz, and Pietro Ruggieri. 2020. "Metal Ion Release, Clinical and Radiological Outcomes in Large Diameter Metal-on-Metal Total Hip Arthroplasty at Long-Term Follow-Up" Diagnostics 10, no. 11: 941. https://doi.org/10.3390/diagnostics10110941
APA StylePozzuoli, A., Berizzi, A., Crimì, A., Belluzzi, E., Frigo, A. C., Conti, G. D., Nicolli, A., Trevisan, A., Biz, C., & Ruggieri, P. (2020). Metal Ion Release, Clinical and Radiological Outcomes in Large Diameter Metal-on-Metal Total Hip Arthroplasty at Long-Term Follow-Up. Diagnostics, 10(11), 941. https://doi.org/10.3390/diagnostics10110941










