Bilateral Phyllodes Giant Tumor. A Case Report Analyzed by Array-CGH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Presentation
2.2. Histological and Immunohistochemical Findings
2.3. CGH-Array Analysis
3. Results
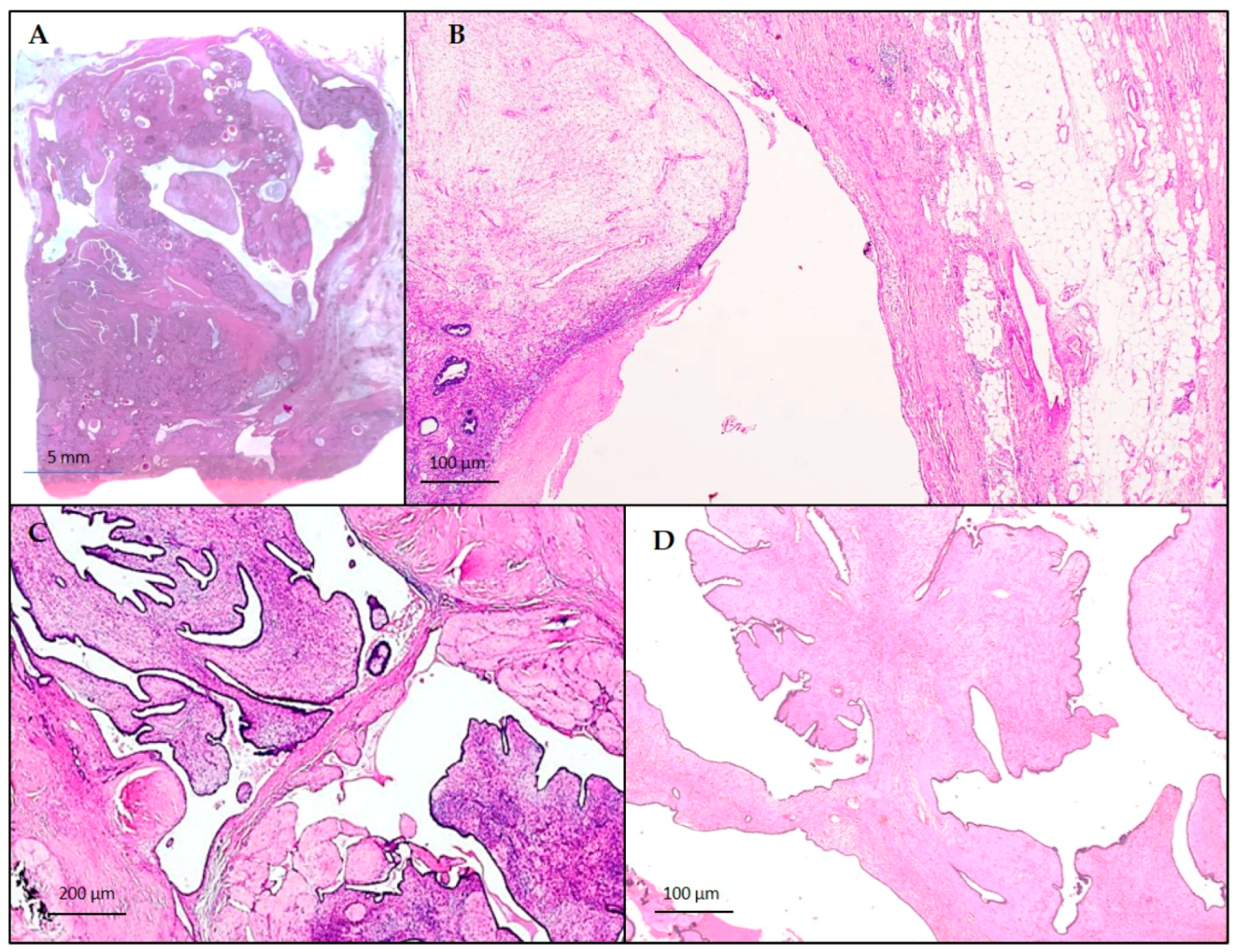
3.1. Macroscopic Examination
3.2. Histological and Immunohistochemical Findings
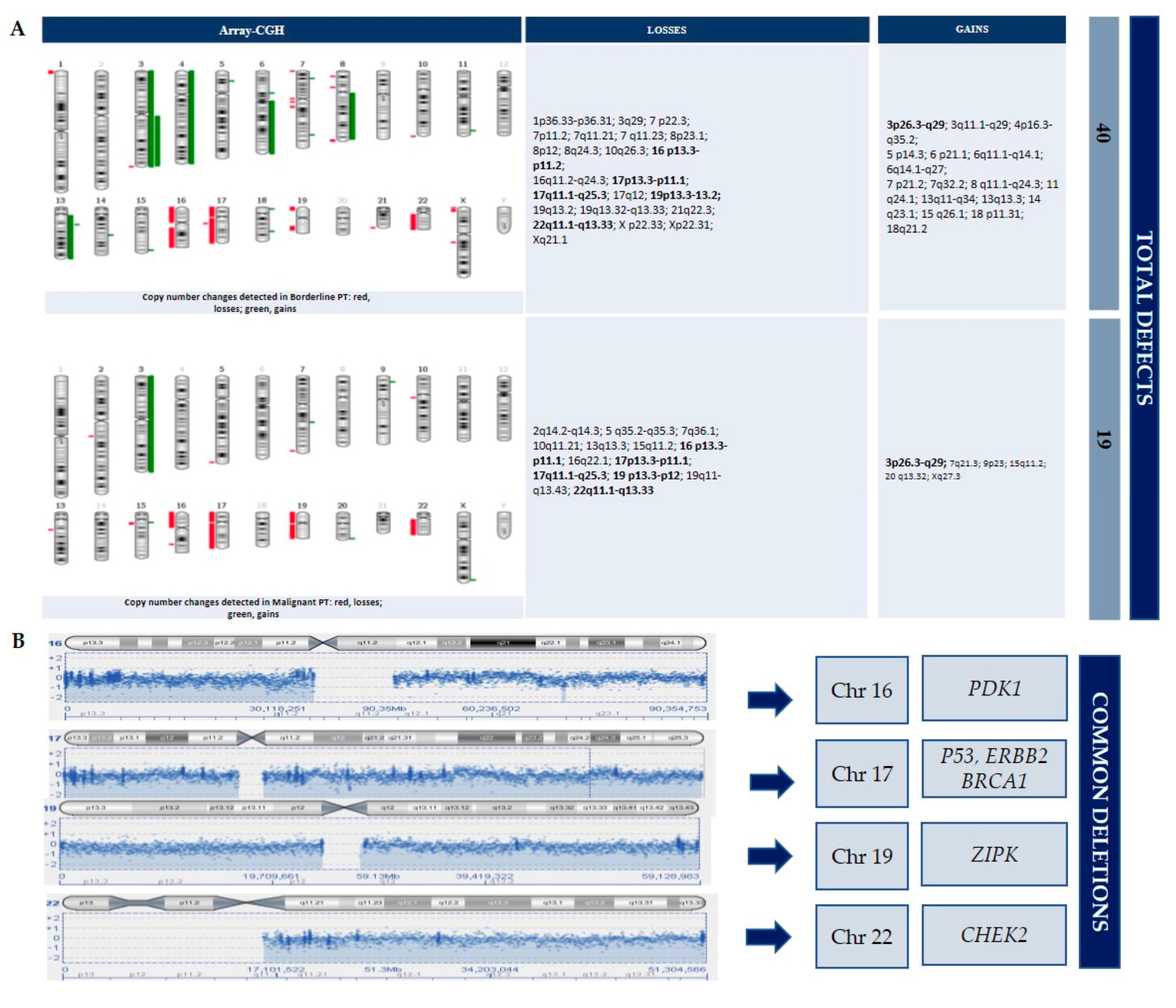
3.3. CGH-Array Analysis
4. Discussion
4.1. PIK3CA Gene
4.2. PDK1 Gene
4.3. ZIPK Gene
4.4. BRCA1, CHEK2 and Other Tumor Suppressor Genes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Punzo, C.; Fortarezza, F.; De Ruvo, V.; Minafra, M.; Laforgia, R.; Casamassima, G.; Pezzuto, F.; Punzi, A.; Caporusso, C.; Angelelli, G.; et al. Primitive squamous cell carcinoma of the breast (SCCB): Case report of an uncommon variant of metaplastic carcinoma. G. Chir. 2017, 38, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Breast Tumours. WHO Classification of Tumours, 5th ed.; IARC Press: Lyon, France, 2019. [Google Scholar]
- Krings, G.; Bean, G.R.; Chen, Y.-Y. Fibroepithelial lesions; The WHO spectrum. Semin. Diagn. Pathol. 2017, 34, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.M.; Alston, R.D.; McNally, R.J.; Evans, D.G.R.; Kelsey, A.M.; Harris, M.; Eden, O.B.; Varley, J.M. Relative frequency and morphology of cancers in carriers of germline TP53 mutations. Oncogene 2001, 20, 4621–4628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuijper, A.; Snijders, A.M.; Berns, E.M.J.J.; Kuenen-Boumeester, V.; Van Der Wall, E.; Albertson, N.G.; Van Diest, P.J. Genomic profiling by array comparative genomic hybridization reveals novel DNA copy number changes in breast phyllodes tumours. Cell. Oncol. 2008, 31, 31–39. [Google Scholar]
- Jones, A.M.; Mitter, R.; Poulsom, R.; Gillett, C.; Hanby, A.M.; Phyllodes Tumour Consortium; Tomlinson, I.P.M.; Sawyer, E.J. mRNA expression profiling of phyllodes tumours of the breast: Identification of genes important in the development of borderline and malignant phyllodes tumours. J. Pathol. 2008, 216, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.; Joseph, N.M.; Ravindranathan, A.; Stohr, B.A.; Greenland, N.Y.; Vohra, P.; Hosfield, E.; Yeh, I.; Talevich, E.; Onodera, C.; et al. Genomic profiling of malignant phyllodes tumors reveals aberrations in FGFR1 and PI-3 kinase/RAS signaling pathways and provides insights into intratumoral heterogeneity. Mod. Pathol. 2016, 29, 1012–1027. [Google Scholar] [CrossRef] [PubMed]
- Yeong, J.; Thike, A.A.; Ng, C.C.Y.; Nasir, N.D.M.; Loh, K.; Teh, B.T.; Tan, P.H. A genetic mutation panel for differentiating malignant phyllodes tumour from metaplastic breast carcinoma. Pathology 2017, 49, 786–789. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhang, E.; Ye, H.; Nandakumar, V.; Wang, Z.; Chen, L.; Tang, C.; Li, J.; Li, H.; Zhang, W.; et al. PIK3CA and TP53 Gene mutations in human breast cancer tumors frequently detected by Ion Torrent DNA sequencing. PLoS ONE 2014, 9, e99306. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.Y.; Nasir, N.D.M.; Chang, H.Y.; Ng, C.C.Y.; Guan, P.; Nagarajan, S.; Rajasegaran, V.; Lee, J.Y.; Lim, J.Q.; Thike, A.A.; et al. Morphologic and genetic heterogeneity in breast fibroepithelial lesions—A comprehensive mapping study. Mod. Pathol. 2020, 33, 1732–1745. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Xing, M.; Mambo, E.; Huang, X.; Liu, J.; Guo, Z.; Chatterjee, A.; Goldenberg, D.; Gollin, S.M.; Sukumar, S.; et al. Somatic mutation and gain of copy number of PIK3CA in human breast cancer. Breast Cancer Res. 2005, 7, R609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.-H.; Cho, J.-H.; Kwon, S.-Y.; Jung, S.-J.; Lee, J.-H. Clinicopathological characteristics of PIK3CA mutation and amplification in Korean patients with breast cancers. Int. J. Med. Sci. 2020, 17, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.; Yue, S.; Jiang, Y.; Zhang, D.; Shi, H.; Qian, H.; Zhou, T.; Fang, W.; Yao, M.; Zuo, G.; et al. Nucleus-located PDK1 regulates growth, invasion and migration of breast cancer cells. Life Sci. 2020, 253, 117722. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Lau, S.-H.; Hu, L.; Rao, H.-L.; Liu, H.-B.; Zhan, W.-H.; Chen, G.; Wen, J.-M.; Wang, Q.; Li, B.; et al. Downregulation of ZIP kinase is associated with tumor invasion, metastasis and poor prognosis in gastric cancer. Int. J. Cancer 2009, 124, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, O.; Johnson, N.; Dos-Santos-Silva, I.; Kilpivaara, O.; Aittomäki, K.; Blomqvist, C.; Nevanlinna, H.; Wasielewski, M.; Meijers-Heijerboer, H.; Broeks, A.; et al. Family history, genetic testing, and clinical risk prediction: Pooled analysis of CHEK2 1100delC in 1,828 bilateral breast cancers and 7,030 controls. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 230–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, N.; Lin, N.U.; Kidd, J.; Allen, B.A.; Singh, N.; Wenstrup, R.J.; Hartman, A.-R.; Winer, E.P.; Garber, J.E. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J. Clin. Oncol. 2016, 34, 1460–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shpitz, B.; Bomstein, Y.; Sternberg, A.; Klein, E.; Tiomkin, V.; Kaufman, A.; Groisman, G.; Bernheim, J. Immunoreactivity of p53, Ki-67, and c-erbB-2 in phyllodes tumors of the breast in correlation with clinical and morphologic features. J. Surg. Oncol. 2002, 79, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, U.; Bayol, U.; Pala, E.E.; Cumurcu, S. Importance of P53, Ki-67 expression in the differential diagnosis of benign/malignant phyllodes tumors of the breast. Indian J. Pathol. Microbiol. 2013, 56, 129. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortarezza, F.; Pezzuto, F.; Cazzato, G.; Punzo, C.; d’Amati, A.; Lettini, T.; Gentile, M.; Buonadonna, A.L.; Mariano, M.; Pezzolla, A.; et al. Bilateral Phyllodes Giant Tumor. A Case Report Analyzed by Array-CGH. Diagnostics 2020, 10, 825. https://doi.org/10.3390/diagnostics10100825
Fortarezza F, Pezzuto F, Cazzato G, Punzo C, d’Amati A, Lettini T, Gentile M, Buonadonna AL, Mariano M, Pezzolla A, et al. Bilateral Phyllodes Giant Tumor. A Case Report Analyzed by Array-CGH. Diagnostics. 2020; 10(10):825. https://doi.org/10.3390/diagnostics10100825
Chicago/Turabian StyleFortarezza, Francesco, Federica Pezzuto, Gerardo Cazzato, Clelia Punzo, Antonio d’Amati, Teresa Lettini, Mattia Gentile, Antonia Lucia Buonadonna, Marta Mariano, Angela Pezzolla, and et al. 2020. "Bilateral Phyllodes Giant Tumor. A Case Report Analyzed by Array-CGH" Diagnostics 10, no. 10: 825. https://doi.org/10.3390/diagnostics10100825
APA StyleFortarezza, F., Pezzuto, F., Cazzato, G., Punzo, C., d’Amati, A., Lettini, T., Gentile, M., Buonadonna, A. L., Mariano, M., Pezzolla, A., & Serio, G. (2020). Bilateral Phyllodes Giant Tumor. A Case Report Analyzed by Array-CGH. Diagnostics, 10(10), 825. https://doi.org/10.3390/diagnostics10100825






