Clinical and Imaging Features of Primary Cardiac Angiosarcoma
Abstract
1. Introduction
2. Methods
2.1. Clinical Data
2.2. Imaging Data
2.3. Image Analysis
2.4. Pathological Analysis
3. Results
3.1. General Results and Follow-Up
3.2. Imaging Findings
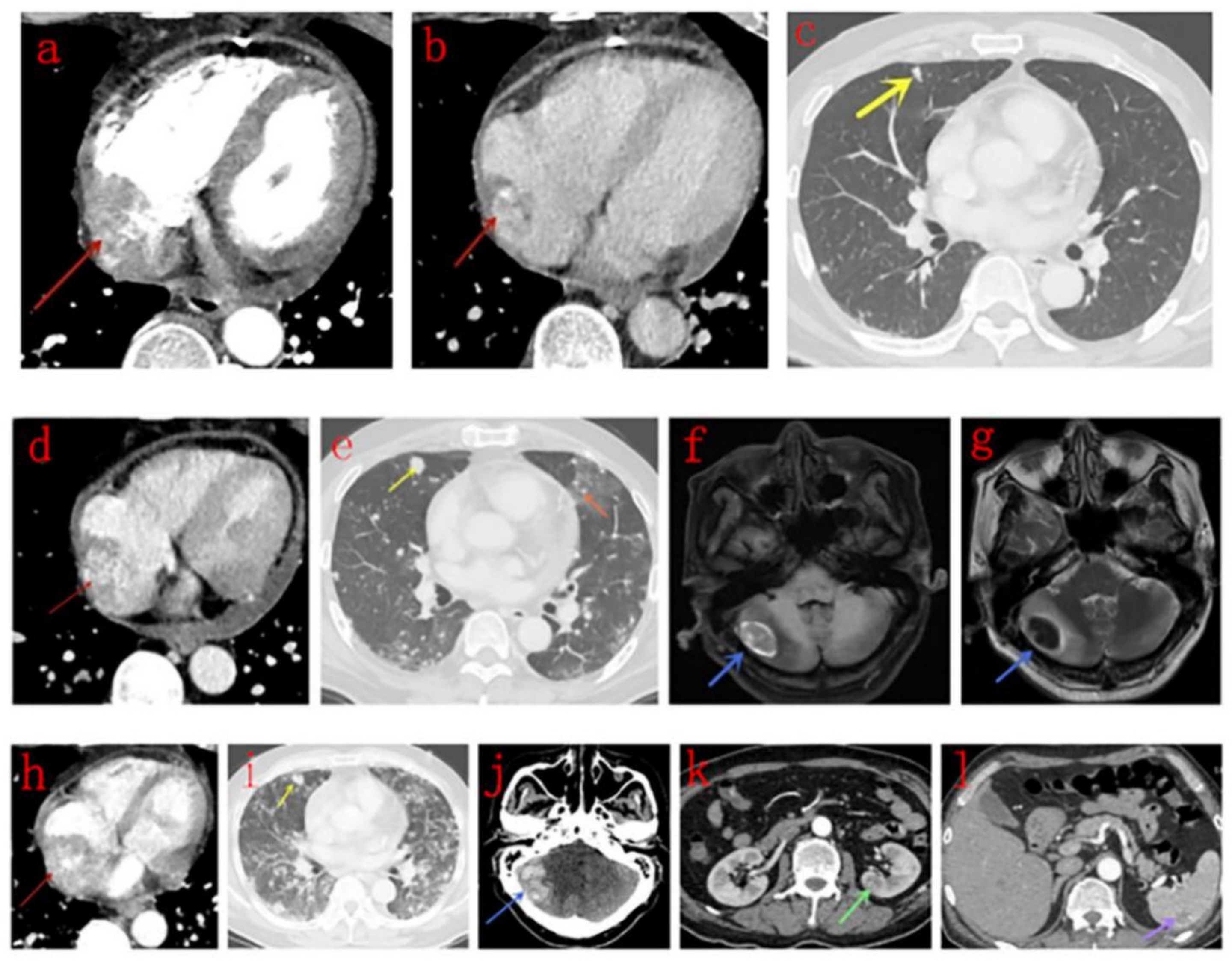
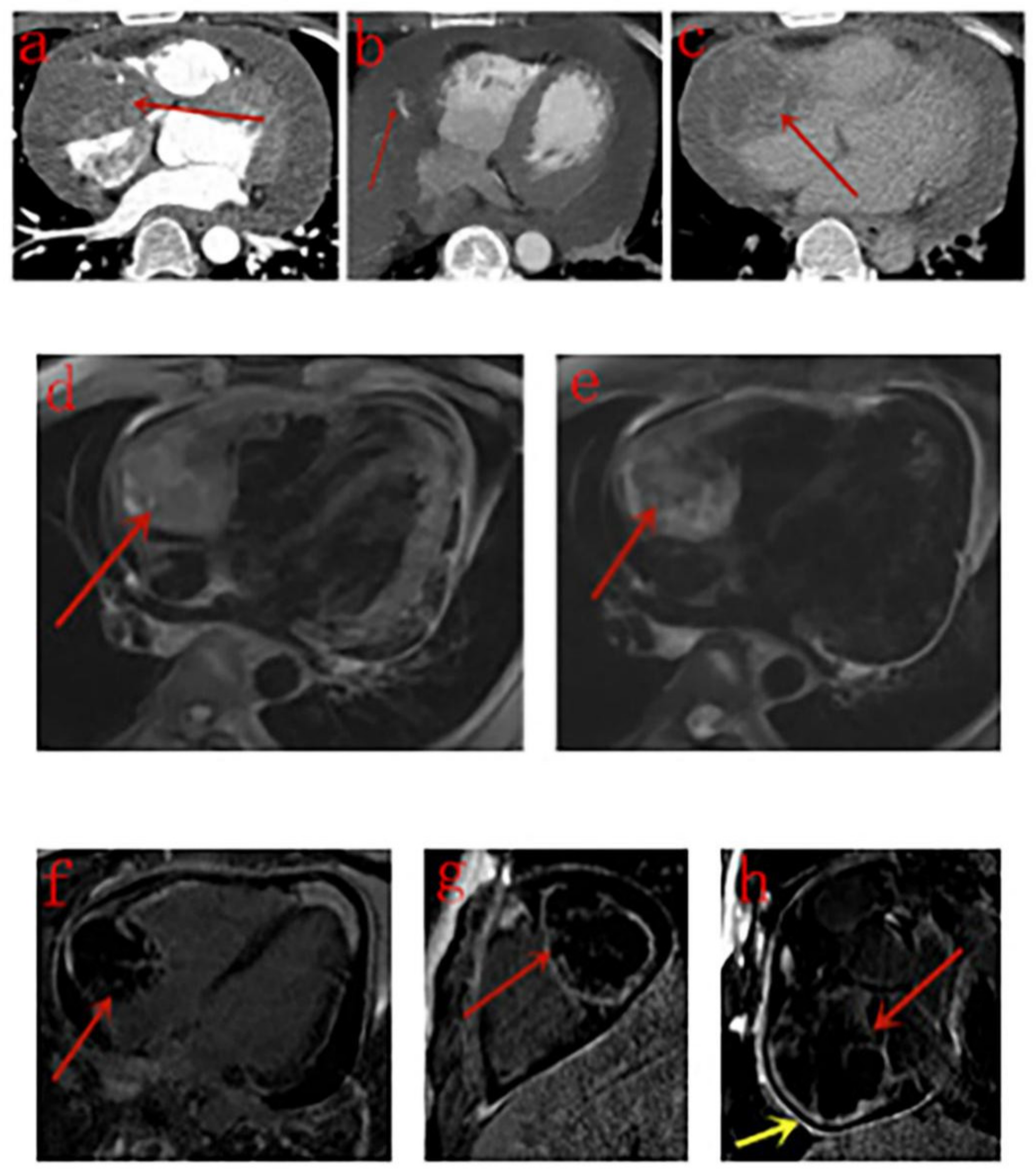
3.3. CT Findings
3.4. MRI Findings
3.5. PET/CT Findings
3.6. Pathological Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, A.; Ferlosio, A.; Roselli, M.; Chiariello, L.; Spagnoli, L.G. Cardiac Sarcomas—An Update. J. Thorac. Oncol. 2010, 5, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.R.; Walsh, G.L. Sarcomas of the heart and great vessels. In Soft Tissue Sarcomas; Pollock, R.E., Ed.; CB Decker Inc.: Hamilton, ON, Canada, 2002; pp. 155–156, 158–160. [Google Scholar]
- Antonuzzo, L.; Rotella, V.; Mazzoni, F.; Doni, L.; Bianchini, D.; Garbini, F.; Maio, V.; Di Costanzo, F. Primary cardiac angiosarcoma: A fatal disease. Case Rep. Med. 2009, 2009, 591512. [Google Scholar] [CrossRef] [PubMed]
- Leduc, C.; Jenkins, S.M.; Sukov, W.R.; Rustin, J.G.; Maleszewski, J.J. Cardiac angiosarcoma histopathologic, immunohistochemical, and cytogenetic analysis of 10 cases. Hum. Pathol. 2017, 60, 199–207. [Google Scholar] [CrossRef]
- Patel, S.D.; Peterson, A.; Bartczak, A.; Lee, S.; Chojnowski, S.; Gajewski, P.; Loukas, M. Primary cardiac angiosarcoma—A review. Med. Sci. Monit. 2014, 20, 103–109. [Google Scholar]
- Chen, T.W.; Loong, H.H.; Srikanthan, A.; Zer, A.; Barua, R.; Butany, J.; Cusimano, R.J.; Liang, Y.C.; Chang, C.H.; Iakobishvili, Z.; et al. Primary cardiac sarcomas: A multi-national retrospective review. Cancer Med. 2019, 8, 104–110. [Google Scholar] [CrossRef]
- Yu, J.F.; Cui, H.; Ji, G.M.; Li, S.Q.; Huang, Y.; Wang, R.N.; Xiaop, W.F. Clinical and imaging manifestations of primary cardiac angiosarcoma. BMC Med. Imaging 2019, 19, 16. [Google Scholar] [CrossRef]
- Rao, J.N.; Gowda, G.D.; Anand, R.; Desai, N.B. Angiosarcoma of the right atrium and right ventricle. J. Card. Surg. 2017, 32, 807–808. [Google Scholar] [CrossRef]
- Chaves, V.M.; Pereira, C.; Andrade, M.; von Hafe, P.; Almeida, J.S. Cardiac Angiosarcoma From Cardiac Tamponade to Ischaemic Stroke—A Diagnostic Challenge. Eur. J. Case. Rep. Intern. Med. 2019, 6, 001079. [Google Scholar] [CrossRef]
- Look Hong, N.J.; Pandalai, P.K.; Hornick, J.L.; Shekar, P.S.; Harmon, D.C.; Chen, Y.L.; Butrynski, J.E.; Baldini, E.H.; Raut, C.P. Cardiac Angiosarcoma Management and Outcomes: 20-Year Single-institution Experience. Ann. Surg. Oncol. 2012, 19, 2707–2715. [Google Scholar] [CrossRef]
- Cao, J.; Wang, J.; He, C.; Fang, M. Angiosarcoma—A review of diagnosis and current treatment. Am. J. Cancer Res. 2019, 9, 2303–2313. [Google Scholar] [PubMed]
- Casha, A.R.; Davidson, L.A.; Roberts, P.; Nair, R.U. Familial angiosarcoma of the heart. J. Thorac. Cardiovasc. Surg. 2002, 124, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Keeling, I.P.; Ploner, F.; Rigler, B. Familial Cardiac Angiosarcoma. Ann. Thorac. Surg. 2006, 82, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Buamah, P. Benign conditions associated with raised serum CA-125 concentration. J. Surg. Oncol. 2000, 75, 264–265. [Google Scholar] [CrossRef]
- Gulati, G.; Sharma, S.; Kothari, S.S.; Juneja, R.; Saxena, A.; Talwar, K.K. Comparison of echo and MRI in the imaging evaluation of intracardiac masses. Cardiovasc. Interv. Radiol. 2004, 27, 459–469. [Google Scholar] [CrossRef]
- Yi, L.L.; Zhang, J.X.; Zhou, S.G.; Wang, J.; Huang, Y.Q.; Li, J.; Yu, X.; Wang, R.N. CT and MRI studies of hepatic angiosarcoma. Clin. Radiol. 2019, 74, 406.e1–406.e8. [Google Scholar] [CrossRef]
- Tang, K.; Shang, Q.L.; Zhou, Q.C.; Zhou, J.W.; She, X.L.; Zhang, M. Primary Cardiac Angiosarcoma with Spontaneous Ruptures of the Right Atrium and Right Coronary Artery. Echocardiography 2013, 30, 156–160. [Google Scholar] [CrossRef]
- Cottini, M.; Pergolini, A.; Gentile, P.; Musumeci, F. Primary cardiac angiosarcoma. J. Card. Surg. 2016, 31, 63–64. [Google Scholar] [CrossRef]
- Hoey, E.T.; Shahid, M.; Ganeshan, A.; Baijal, S.; Simpson, H.; Watkin, R.W. MRI assessment of cardiac tumours—Spectrum of appearances of histologically malignant lesions and tumour mimics. Quant. Imaging Med. Surg. 2014, 4, 489–497. [Google Scholar]
- Mader, M.T.; Poulton, T.B.; White, R.D. Malignant tumors of the heart and great vessels- MRI imaging appearance. Radiographics 1997, 17, 145–153. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Liu, J.; Xu, L.; Li, Y.; Liu, D.; Sun, Z.; Wen, Z. Cardiac magnetic resonance imaging of primary cardiac tumors. Quant. Imaging Med. Surg. 2020, 10, 294–313. [Google Scholar] [CrossRef] [PubMed]
- Yahata, S.; Endo, T.; Honma, H.; Ino, T.; Hayakawa, H.; Ogawa, M.; Kumazkai, T. Sunray appearance on enhanced magnetic resonance image of cardiac angiosarcoma with pericardial obliteration. Am. Heart J. 1994, 127, 468–471. [Google Scholar] [CrossRef]
- Tüdös, Z.; Köcher, M.; Černá, M.; Odstrcil, F.; Prouzova, Z.; Formanek, R.; Precek, J. “Sun Ray” Appearance in a Case of Cardiac Angiosarcoma: A Comparison of MRI and PET/CT. Magn. Reson. Med. Sci. 2017, 16, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, J.; Stacey, R.B. Cardiac magnetic resonance in cardiac angiosarcoma. Echocardiography 2017, 34, 1077–1081. [Google Scholar] [CrossRef]
- Tüdös, Z.; Hutyra, M. Cardiac magnetic resonance in cardiac angiosarcoma -Which contrast- enhancement pattern is typical? Echocardiography 2018, 35, 287. [Google Scholar] [CrossRef]
- Colin, G.C.; Symons, R.; Dymarkowski, S.; Gerber, B.; Bogaert, J. Value of CMR to differentiate cardiac angiosarcoma from cardiac lymphoma. JACC. Cardiovasc. Imaging 2015, 8, 744–746. [Google Scholar] [CrossRef]
- Sun, W.Z.; Wu, Q.J.; Wang, Y.; Chen, M.; Zhang, J.; Zhang, G.; Zhou, Y.F. A rare case of primary well-differentiated angiosarcoma of the right atrium. J. Geriatr. Cardiol. 2019, 16, 164–167. [Google Scholar]
- Park, K.S.; Song, B.G.; Ok, K.S.; Park, D.W.; Jung, H.J.; Kwak, M.O.; Cho, W.H.; Choi, S.K. Primary cardiac angiosarcoma treated by complete tumor resection with cardiac reconstruction. Heart Lung. 2011, 40, e41–e43. [Google Scholar] [CrossRef]
- Qin, L.F.; Xu, X.J.; Davies, H.; Wan, Z.D.; Xu, H.F.; Zhao, H.G. Cardiac angiosarcoma: A case report and review of current treatment. Medicine 2019, 98, e18193. [Google Scholar]

| Case | Age a | Sex | Serum CA-125 (U/mL) | Attachment | Maximum Size (mm) b | Adjacent Invasion c | Metastases | Treatment | Surviving (mo) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 23 | F | Not Known | Lateral wall | 80.0 | AP RCA | None | Surgery | 6(DOD) |
| 2 | 74 | M | Normal | Lateral wall | 51.4 | AP RCA RV | Lung Lymph nodes | Surgery | 7(DOOD) |
| 3 | 47 | F | 37.9 | Lateral wall | 93.0 | Limited | None | Surgery | 15(AFOD) |
| 4 | 34 | M | Not Known | Lateral wall | 66.1 | RCA | None | Surgery | Not Known |
| 5 | 50 | M | Not Known | Lateral wall | 69.7 | AP | None | Surgery | Not Known |
| 6 | 62 | M | 99.0 | Lateral wall | 66.4 | AP RCA | Pericardium Lung | Surgery | Not Known |
| 7 | 61 | M | Not Known | Atrial septum | 54.4 | AP SVC | Lung | Thoracotomy | 1(DOD) |
| 8 | 17 | M | Not Known | Lateral wall | 47.4 | AP | Lung | Thoracotomy, Chemotherapy | 7(AWD) |
| 9 | 26 | M | Normal | Lateral wall | 79.5 | AP RCA RV | Lung | Chemotherapy | 9(AWD) |
| 10 | 61 | M | Not Known | Lateral wall | 38.4 | AP RCA | Lung | Chemotherapy | 6(DOD) |
| 11 | 31 | M | Not Known | Lateral wall | 81.2 | AP RCA | Pericardium Lung Lymph nodes | None | 3(AWD) |
| 12 | 52 | M | 1339.0 | Posterior wall | 50.0 | AP IVC | Pericardium Lung Brain Kidney Spleen | None | 3(AWD) |
| Case | Scan Type | Density | Enhancement Pattern | Differentiation (Ki-67 Index) | Lung Metastasis | GGO Peripheries |
|---|---|---|---|---|---|---|
| 1 | E | - | Heterogeneous | Moderate (>50%) | N | - |
| 2 | P + E | Uneven | Strong Heterogeneous Centripetal | Low (80%) | Y | N |
| 3 | P | Uneven | - | Low (85%) | N | - |
| 4 | E | - | Heterogeneous Centripetal | Moderate (30%) | N | - |
| 5 | P + E | Uneven | Moderate Heterogeneous Centripetal | Low (30%) | N | - |
| 6 | E | - | Heterogeneous Centripetal | Moderate (25%+) | Y | N |
| 7 | P | Uneven | - | High (10%+) | Y | N |
| 8 | P + E | Even | Strong Heterogeneous Centripetal | NK (30%) | Y | Y |
| 9 | P + E | Uneven | Strong Heterogeneous Centripetal | NK (50%+) | Y | Y |
| 10 | P + E | Even | Mild Heterogeneous Centripetal | High (50%+) | Y * | Y |
| 11 | P + E | Uneven | Strong Heterogeneous | NK (50%+) | Y * | Y |
| 12 | E | - | Heterogeneous Centripetal | NK (20–25%+) | Y | Y |
| Case | Boundary | Intensity on T1WI | Intensity on T2WI | Signal Void | Enhancement Pattern | Differentiation | Pericardium Enhancement |
|---|---|---|---|---|---|---|---|
| 2 | Regular | Uneven high | Uneven high | Y | Heterogeneous, patchy | Low | N |
| 4 | Regular | Uneven high | Uneven high | N | Heterogeneous, rim | Moderate | Y |
| 7 | Irregular | Uneven high | Uneven high | N | Heterogeneous, rim | High | N |
| 11 | Irregular | Uneven high | Uneven high | N | Heterogeneous, rim | NK | N |
| Study | Number of Patients | CT Features | MRI Features |
|---|---|---|---|
| Antonuzzo et al. [4] | 1 | None | A mass (53 mm) extending from the free wall of the RA to the anterior mediastinum |
| Yu et al. [8] | 9 | Masses in the RA invading pericardium, RV, SVC, and tricuspid valve, presented as homogeneous or inhomogeneous on plain CT scans, and most showed inhomogeneous centripetal enhancement on enhanced CT scans. Pulmonary metastases with GGO peripheries | None |
| Rao et al. [9] | 1 | A mass in the RA and RV extending into the pericardium | A large heterogeneous mass in the RA and RV extending into the myocardium. |
| Yahata et al. [23] | 1 | None | An extensive mass in pericardial cavity protruding into the RA of heterogeneous signal intensity on T1WI and T2WI, “sunray appearance” enhancement was seen on LGE images. |
| Tüdös et al. [24] | 1 | None | A cauliflower mass protruding to the RA with extension toward RV, right appendage, and diffuse obliteration of pericardial cavity around the ventricles, RV motion was severely impaired. Signaling of tumor tissue in T1WI and T2WI was inhomogeneous, mostly isointense to slightly hyperintense. Pattern of enhancement previously described as “sunray appearance” was seen both on T1WI and LGE images, especially LGE images. |
| Lindsey et al. [25] | 1 | None | A well-circumscribed, hyperemic mass (27 × 18 mm) located at the supero-posterior wall of the RA and possibly extending from the IVC, presented as hyperintensity on T2WI and LGE. Using first-pass perfusion, the mass did not show contrast as it transited the right side, but, as contrast passed through the coronary arteries, the contrast filled the mass. |
| Colin et al. [27] | 7 | None | Tumors located in the right atrial wall and atrial appendage extending to the pericardium, right atrial cavity, and right coronary artery with central necrosis and possibly pulmonary metastasis, and with strong rim enhancement on LGE. |
| Sun et al. [28] | 1 | A large mass about 8 × 6 × 5 cm3 occupying the RA, SVC and IVC with pericardial and bilateral pleural effusion, with heterogeneous enhancement. | None |
| Park et al. [29] | 1 | Multiple-septated fluid collection in right pericardial space. | Diffuse nodular enhancing mass involving the right atrium. |
| Qian et al. [30] | 1 | A multifocal high-density shadow and lymph node enlargement (including the right upper lobe) with bilateral pleural effusions, a pericardial effusion and an enlargement of the cardiac area. Pulmonary artery computed tomography angiography (CTA) demonstrated a micro-embolism in the right upper lobe of the lung. | None |
| Our study | 12 | Masses in the right atrium invading pericardium, RCA, RV, SVC and IVC, presented as homogeneous or inhomogeneous on unenhanced CT scans and heterogeneous centripetal enhancement on enhanced CT scans, The enhancement pattern shows no exact correlation with the differentiation degree of the tumor. Pulmonary metastases with halo sign was common. | Masses in the right atrium invading pericardium, RCA, RV, SVC and IVC, presented as cauliflower-like appearance on T1WI and T2WI, signal void was noticed in a lesion with strong enhancement for arterial phase on CT scan. RA and RV motion was severely impaired. Rim enhancement was noticed in 3 patients and patchy enhancement was noticed in one patient. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, Y.; Zhang, N.; Shang, J.; Li, X.; Liu, J.; Xu, L.; Liu, D.; Sun, Z.; Wen, Z. Clinical and Imaging Features of Primary Cardiac Angiosarcoma. Diagnostics 2020, 10, 776. https://doi.org/10.3390/diagnostics10100776
Chen Y, Li Y, Zhang N, Shang J, Li X, Liu J, Xu L, Liu D, Sun Z, Wen Z. Clinical and Imaging Features of Primary Cardiac Angiosarcoma. Diagnostics. 2020; 10(10):776. https://doi.org/10.3390/diagnostics10100776
Chicago/Turabian StyleChen, Yan, Yu Li, Nan Zhang, Jianfeng Shang, Xiaodan Li, Jiayi Liu, Lei Xu, Dongting Liu, Zhonghua Sun, and Zhaoying Wen. 2020. "Clinical and Imaging Features of Primary Cardiac Angiosarcoma" Diagnostics 10, no. 10: 776. https://doi.org/10.3390/diagnostics10100776
APA StyleChen, Y., Li, Y., Zhang, N., Shang, J., Li, X., Liu, J., Xu, L., Liu, D., Sun, Z., & Wen, Z. (2020). Clinical and Imaging Features of Primary Cardiac Angiosarcoma. Diagnostics, 10(10), 776. https://doi.org/10.3390/diagnostics10100776









