Association of Major Histocompatibility Complex Class I Related Chain A/B Positive Microparticles with Acute Myocardial Infarction and Disease Severity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Samples
2.3. MICA/B+ MPs’ Analysis
2.4. Statistical Analysis
3. Results
3.1. Subject Characteristics
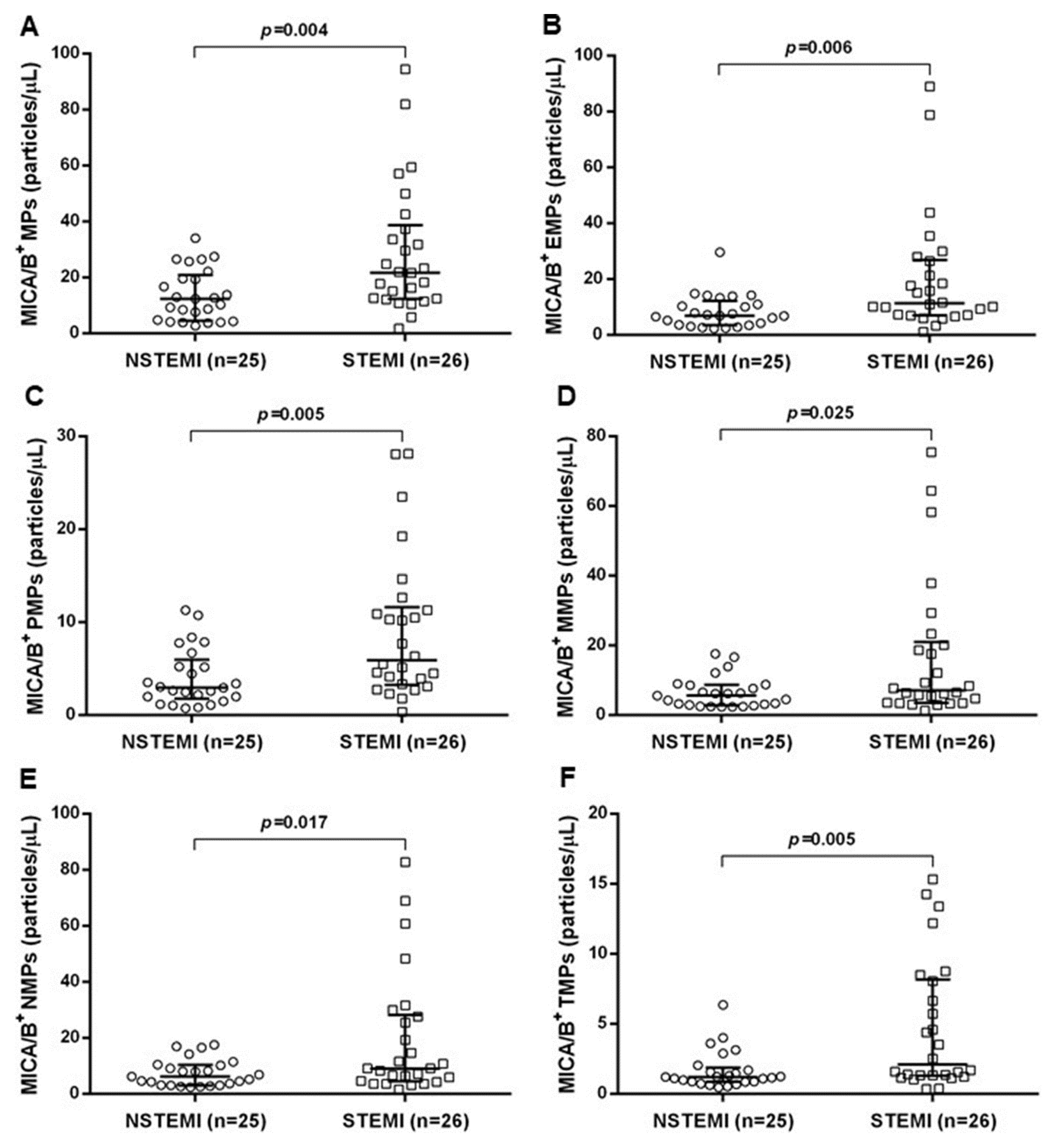
3.2. MICA/B+ MPs’ Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campos-Silva, C.; Kramer, M.K.; Vales-Gomez, M. NKG2D-ligands: Putting everything under the same umbrella can be misleading. HLA 2018, 91, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, B.Z.; Eerligh, P.; van der Slik, A.R.; Shastry, A.; Zhernakova, A.; Valdigem, G.; Bruining, J.G.; Sanjeevi, C.B.; Wijmenga, C.; Roep, B.O.; et al. MICA marks additional risk factors for Type 1 diabetes on extended HLA haplotypes: An association and meta-analysis. Mol. Immunol. 2007, 44, 2806–2812. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, Y.L.; Bondar, C.; Guzman, L.; Cueto Rua, E.; Chopita, N.; Fuertes, M.; Zwirner, N.W.; Chirdo, F.G. Broad MICA/B expression in the small bowel mucosa: A link between cellular stress and celiac disease. PLoS ONE 2013, 8, e73658. [Google Scholar] [CrossRef] [Green Version]
- Vadstrup, K.; Galsgaard, E.D.; Jensen, H.; Lanier, L.L.; Ryan, J.C.; Chen, S.Y.; Nolan, G.P.; Vester-Andersen, M.K.; Pedersen, J.S.; Gerwien, J.; et al. NKG2D ligand expression in Crohn’s disease and NKG2D-dependent stimulation of CD8(+) T cell migration. Exp. Mol. Pathol. 2017, 103, 56–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeshita, S.; Miyatake, Y.; Otsuka, N.; Kasahara, M. MICA/B expression in macrophage foam cells infiltrating atherosclerotic plaques. Exp. Mol. Pathol. 2014, 97, 171–175. [Google Scholar] [CrossRef]
- Abediankenari, S.; Yousefzadeh, Y.; Majidi, M.; Ghasemi, M.; Nasehi, M.M.; Ghaffari, J.; Habibi Saravi, R.; Abedini, M.; Elyasi, M. MICB gene expression on peripheral blood mononuclear cells and susceptibility to multiple sclerosis in north of Iran. Iran. J. Allergy Asthma Immunol. 2011, 10, 261–265. [Google Scholar]
- Groh, V.; Bruhl, A.; El-Gabalawy, H.; Nelson, J.L.; Spies, T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2003, 100, 9452–9457. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Z.; Wang, Y.X.; Jiang, C.L. Inflammation: The Common Pathway of Stress-Related Diseases. Front. Hum. Neurosci. 2017, 11, 316. [Google Scholar] [CrossRef]
- Xia, M.; Guerra, N.; Sukhova, G.K.; Yang, K.; Miller, C.K.; Shi, G.P.; Raulet, D.H.; Xiong, N. Immune activation resulting from NKG2D/ligand interaction promotes atherosclerosis. Circulation 2011, 124, 2933–2943. [Google Scholar] [CrossRef] [Green Version]
- Passacquale, G.; Di Giosia, P.; Ferro, A. The role of inflammatory biomarkers in developing targeted cardiovascular therapies: Lessons from the cardiovascular inflammation reduction trials. Cardiovasc. Res. 2016, 109, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Pauli, N.; Puchalowicz, K.; Kuligowska, A.; Krzystolik, A.; Dziedziejko, V.; Safranow, K.; Rac, M.; Chlubek, D.; Ewa Rac, M. Associations between IL-6 and Echo-Parameters in Patients with Early Onset Coronary Artery Disease. Diagnostics (Basel) 2019, 9, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anouar, H.; Daskalopoulou, S.S. Extracellular vesicles characteristics and emerging roles in atherosclerotic cardiovascular disease. Metabolism 2018. [Google Scholar] [CrossRef]
- Sluijter, J.P.G.; Davidson, S.M.; Boulanger, C.M.; Buzas, E.I.; de Kleijn, D.P.V.; Engel, F.B.; Giricz, Z.; Hausenloy, D.J.; Kishore, R.; Lecour, S.; et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 19–34. [Google Scholar] [CrossRef]
- Ridger, V.C.; Boulanger, C.M.; Angelillo-Scherrer, A.; Badimon, L.; Blanc-Brude, O.; Bochaton-Piallat, M.L.; Boilard, E.; Buzas, E.I.; Caporali, A.; Dignat-George, F.; et al. Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb. Haemost. 2017, 117, 1296–1316. [Google Scholar]
- Lv, J.X.; Kong, Q.; Ma, X. Current advances in circulating inflammatory biomarkers in atherosclerosis and related cardio-cerebrovascular diseases. Chronic Dis. Transl. Med. 2017, 3, 207–212. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Katus, H.A.; Apple, F.S.; Lindahl, B.; Morrow, D.A.; et al. Third universal definition of myocardial infarction. Eur. Heart J. 2012, 33, 2551–2567. [Google Scholar] [CrossRef] [Green Version]
- Bansal, D.; Eigenbrodt, M.; Gupta, E.; Mehta, J.L. Traditional risk factors and acute myocardial infarction in patients hospitalized with cocaine-associated chest pain. Clin. Cardiol. 2007, 30, 290–294. [Google Scholar] [CrossRef]
- Tousoulis, D.; Antoniades, C.; Koumallos, N.; Stefanadis, C. Pro-inflammatory cytokines in acute coronary syndromes: From bench to bedside. Cytokine Growth Factor Rev. 2006, 17, 225–233. [Google Scholar] [CrossRef]
- Badimon, L.; Suades, R.; Arderiu, G.; Pena, E.; Chiva-Blanch, G.; Padro, T. Microvesicles in Atherosclerosis and Angiogenesis: From Bench to Bedside and Reverse. Front. Cardiovasc. Med. 2017, 4, 77. [Google Scholar] [CrossRef] [Green Version]
- Gleimer, M.; Parham, P. Stress management: MHC class I and class I-like molecules as reporters of cellular stress. Immunity 2003, 19, 469–477. [Google Scholar] [CrossRef] [Green Version]
- McCann, F.E.; Eissmann, P.; Onfelt, B.; Leung, R.; Davis, D.M. The activating NKG2D ligand MHC class I-related chain A transfers from target cells to NK cells in a manner that allows functional consequences. J. Immunol. 2007, 178, 3418–3426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grivel, J.C.; Ivanova, O.; Pinegina, N.; Blank, P.S.; Shpektor, A.; Margolis, L.B.; Vasilieva, E. Activation of T lymphocytes in atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2929–2937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N. CD4+ T cells in atherosclerosis: Regulation by platelets. Thromb. Haemost. 2013, 109, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Flego, D.; Liuzzo, G.; Weyand, C.M.; Crea, F. Adaptive Immunity Dysregulation in Acute Coronary Syndromes: From Cellular and Molecular Basis to Clinical Implications. J. Am. Coll. Cardiol. 2016, 68, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Bullenkamp, J.; Dinkla, S.; Kaski, J.C.; Dumitriu, I.E. Targeting T cells to treat atherosclerosis: Odyssey from bench to bedside. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 194–199. [Google Scholar] [CrossRef] [Green Version]
- Holdenrieder, S.; Stieber, P.; Peterfi, A.; Nagel, D.; Steinle, A.; Salih, H.R. Soluble MICB in malignant diseases: Analysis of diagnostic significance and correlation with soluble MICA. Cancer Immunol. Immunother. 2006, 55, 1584–1589. [Google Scholar] [CrossRef]
- Fernandez-Morera, J.L.; Rodriguez-Rodero, S.; Lahoz, C.; Tunon, A.; Astudillo, A.; Garcia-Suarez, O.; Martinez-Borra, J.; Lopez-Vazquez, A.; Rodrigo, L.; Gonzalez, S.; et al. Soluble MHC class I chain-related protein B serum levels correlate with disease activity in relapsing-remitting multiple sclerosis. Hum. Immunol. 2008, 69, 235–240. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, J.F.; Huo, Z.; Zhang, Q.; Jiang, Y.; Wu, X.; Li, Y.; Jiang, G.; Zeng, L.; Yan, X.X.; et al. Elevation of soluble major histocompatibility complex class I related chain A protein in malignant and infectious diseases in Chinese patients. BMC Immunol. 2012, 13, 62. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Chen, N.; Yu, Y.; Zhou, L.; Niu, C.; Liu, Y.; Tian, H.; Lv, Z.; Han, F.; Cui, J. Prognostic value of MICA/B in cancers: A systematic review and meta-analysis. Oncotarget 2017, 8, 96384–96395. [Google Scholar] [CrossRef] [Green Version]
- Rosinska, J.; Lukasik, M.; Kozubski, W. The Impact of Vascular Disease Treatment on Platelet-Derived Microvesicles. Cardiovasc. Drugs Ther. 2017, 31, 627–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Stefano, R.; Di Bello, V.; Barsotti, M.C.; Grigoratos, C.; Armani, C.; Dell’Omodarme, M.; Carpi, A.; Balbarini, A. Inflammatory markers and cardiac function in acute coronary syndrome: Difference in ST-segment elevation myocardial infarction (STEMI) and in non-STEMI models. Biomed. Pharmacother. 2009, 63, 773–780. [Google Scholar] [CrossRef] [PubMed]
- El-Mesallamy, H.O.; Hamdy, N.M.; El-Etriby, A.K.; Wasfey, E.F. Plasma granzyme B in ST elevation myocardial infarction versus non-ST elevation acute coronary syndrome: Comparisons with IL-18 and fractalkine. Mediators Inflamm. 2013, 2013, 343268. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, S.; Banu, S.; Jabir, N.R.; Khan, M.S.; Ashraf, G.M.; Manjunath, N.C.; Tabrez, S. Clinical and angiographic correlation of high-sensitivity C-reactive protein with acute ST elevation myocardial infarction. Exp. Ther. Med. 2016, 12, 4089–4098. [Google Scholar] [CrossRef]







| Parameters | Control (n = 46) | AMI (n = 51) | p-Value |
|---|---|---|---|
| Age (years) | 59.93 ± 7.50 | 61.86 ± 9.48 | 0.268 |
| Gender (male: female) | 14:32 | 35:16 | <0.001 |
| BMI (kg/m2) | 25.41 ± 3.82 | 23.86 ± 3.83 | 0.051 |
| SBP (mmHg) | 134.41 ± 18.74 | 128.06 ± 23.57 | 0.148 |
| DBP (mmHg) | 80.71 ± 10.28 | 72.37 ± 13.84 | 0.001 |
| Total cholesterol (mg/dL) | 203.52 ± 44.47 | 167.28 ± 53.61 | 0.001 |
| Triglyceride (mg/dL) | 137.19 ± 90.49 | 142.81 ± 53.47 | 0.711 |
| LDL-C (mg/dL) | 128.28 ± 37.08 | 114.59 ± 48.47 | 0.127 |
| HDL-C (mg/dL) | 47.69 ± 15.57 | 40.14 ± 10.73 | 0.007 |
| FBS (mg/dL) | 95.80 ± 1.26 | 130.84 ± 1.39 | <0.001 |
| hs-CRP (mg/L) | 1.51 ± 3.72 | 9.52 ± 4.77 | <0.001 |
| Cardiovascular risk factors | |||
| Diabetes mellitus, n (%) | 11 (23.91) | 31 (60.78) | <0.001 |
| Hypertension, n (%) | 17 (36.95) | 33 (64.71) | 0.006 |
| Dyslipidemia, n (%) | 23 (50.00) | 46 (90.20) | <0.001 |
| Smoking, n (%) | 8 (17.39) | 29 (56.86) | <0.001 |
| BMI ≥ 25 kg/m2, n (%) | 25 (54.34) | 21 (41.17) | 0.195 |
| hs-CRP > 3 mg/L, n (%) | 13 (28.26) | 41 (80.39) | <0.001 |
| Parameters | NSTEMI (n = 25) | STEMI (n = 26) | p-Value |
|---|---|---|---|
| Age (years) | 62.80 ± 8.79 | 61.00 ± 10.21 | 0.495 |
| Gender (male: female) | 16:9 | 19:7 | 0.485 |
| BMI (kg/m2) | 23.98 ± 3.94 | 23.73 ± 3.78 | 0.818 |
| SBP (mmHg) | 134.16 ± 29.24 | 122.19 ± 14.71 | 0.075 |
| DBP (mmHg) | 73.40 ± 17.36 | 71.38 ± 9.57 | 0.613 |
| Total cholesterol (mg/dL) | 176.60 ± 47.35 | 159.03 ± 58.26 | 0.256 |
| Triglyceride (mg/dL) | 147.60 ± 57.04 | 138.57 ± 50.85 | 0.561 |
| LDL-C (mg/dL) | 116.60 ± 43.39 | 112.80 ± 53.35 | 0.785 |
| HDL-C (mg/dL) | 43.13 ± 12.94 | 37.50 ± 7.63 | 0.077 |
| FBS (mg/dL) | 124.09 ± 35.29 | 151.15 ± 58.92 | 0.061 |
| hs-CRP (mg/L) | 17.59 ± 26.74 | 29.05 ± 39.12 | 0.236 |
| Cardiovascular risk factors | |||
| Diabetes mellitus, n (%) | 15 (60.00) | 16 (61.53) | 0.910 |
| Hypertension, n (%) | 12 (48.00) | 21 (80.77) | 0.005 |
| Dyslipidemia, n (%) | 23 (92.00) | 23 (88.46) | 0.671 |
| Smoking, n (%) | 12 (48.00) | 17 (65.38) | 0.348 |
| BMI ≥ 25 kg/m2, n (%) | 10 (40.00) | 11 (42.20) | 0.867 |
| hs-CRP > 3 mg/L, n (%) | 18 (72.00) | 23 (88.46) | 0.216 |
| Medications | NSTEMI (n = 25) | STEMI (n = 26) | p-Value |
|---|---|---|---|
| Aspirin, n (%) | 25 (100.00) | 26 (100.00) | - |
| Clopidogrel, n (%) | 25 (100.00) | 26 (100.00) | - |
| Statin, n (%) | 16 (64.00) | 13 (50.00) | 0.313 |
| ACEI/ARB, n (%) | 9 (36.00) | 4 (15.38) | 0.091 |
| MPs | hs-CRP | |
|---|---|---|
| r | p-Value | |
| MICA/B+ MPs | 0.336 | 0.001 |
| MICA/B+ EMPs | 0.362 | <0.001 |
| MICA/B+ PMPs | 0.360 | 0.001 |
| MICA/B+ MMPs | 0.348 | 0.001 |
| MICA/B+ NMPs | 0.342 | 0.001 |
| MICA/B+ TMPs | 0.391 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haohan, S.; Pussadhamma, B.; Jumnainsong, A.; Leuangwatthananon, W.; Makarawate, P.; Leelayuwat, C.; Komanasin, N. Association of Major Histocompatibility Complex Class I Related Chain A/B Positive Microparticles with Acute Myocardial Infarction and Disease Severity. Diagnostics 2020, 10, 766. https://doi.org/10.3390/diagnostics10100766
Haohan S, Pussadhamma B, Jumnainsong A, Leuangwatthananon W, Makarawate P, Leelayuwat C, Komanasin N. Association of Major Histocompatibility Complex Class I Related Chain A/B Positive Microparticles with Acute Myocardial Infarction and Disease Severity. Diagnostics. 2020; 10(10):766. https://doi.org/10.3390/diagnostics10100766
Chicago/Turabian StyleHaohan, Songpol, Burabha Pussadhamma, Amonrat Jumnainsong, Wit Leuangwatthananon, Pattarapong Makarawate, Chanvit Leelayuwat, and Nantarat Komanasin. 2020. "Association of Major Histocompatibility Complex Class I Related Chain A/B Positive Microparticles with Acute Myocardial Infarction and Disease Severity" Diagnostics 10, no. 10: 766. https://doi.org/10.3390/diagnostics10100766







