UV Fluorescence-Based Determination of Urinary Advanced Glycation End Products in Patients with Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Fluorescence
2.3. Routine Laboratory Parameters
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Busch, M.; Franke, S.; Ruster, C.; Wolf, G. Advanced glycation end-products and the kidney. Eur. J. Clin. Investig. 2010, 40, 742–755. [Google Scholar] [CrossRef]
- Byun, K.; Yoo, Y.; Son, M.; Lee, J.; Jeong, G.B.; Park, Y.M.; Salekdeh, G.H.; Lee, B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017, 177, 44–55. [Google Scholar] [CrossRef]
- Munch, G.; Keis, R.; Wessels, A.; Riederer, P.; Bahner, U.; Heidland, A.; Niwa, T.; Lemke, H.D.; Schinzel, R. Determination of advanced glycation end products in serum by fluorescence spectroscopy and competitive ELISA. Eur. J. Clin. Chem. Clin. Biochem. 1997, 35, 669–677. [Google Scholar] [CrossRef]
- Schmitt, A.; Schmitt, J.; Münch, G.; Gasic-Milencovic, J. Characterization of advanced glycation end products for biochemical studies: Side chain modifications and fluorescence characteristics. Anal. Biochem. 2005, 338, 201–215. [Google Scholar] [CrossRef]
- Hohmann, C.; Liehr, K.; Henning, C.; Fiedler, R.; Girndt, M.; Gebert, M.; Hulko, M.; Storr, M.; Glomb, M.A. Detection of free advanced glycation end products in vivo during hemodialysis. J. Agric. Food Chem. 2017, 65, 930–937. [Google Scholar] [CrossRef]
- Noordzij, M.J.; Lefrandt, J.D.; Smit, A.J. Advanced glycation end products in renal failure: An overview. J. Ren. Care. 2008, 34, 207–212. [Google Scholar] [CrossRef]
- Delgado-Andrade, C. Carboxymethyl-lysine: Thirty years of investigation in the field of AGE formation. Food Funct. 2016, 7, 46–57. [Google Scholar] [CrossRef]
- Galler, A.; Muller, G.; Schinzel, R.; Kratzsch, J.; Kiess, W.; Munch, G. Impact of metabolic control and serum lipids on the concentration of advanced glycation end products in the serum of children and adolescents with type 1 diabetes, as determined by fluorescence spectroscopy and nepsilon-(carboxymethyl)lysine ELISA. Diabetes Care 2003, 26, 2609–2615. [Google Scholar] [CrossRef]
- Sanaka, T.; Funaki, T.; Tanaka, T.; Hoshi, S.; Niwayama, J.; Taitoh, T.; Nishimura, H.; Higuchi, C. Plasma pentosidine levels measured by a newly developed method using ELISA in patients with chronic renal failure. Nephron 2002, 91, 64–73. [Google Scholar] [CrossRef]
- Hurtado-Sánchez Mdel, C.; Espinosa-Mansilla, A.; Rodríguez-Cáceres, M.I.; Martín-Tornero, E.; Durán-Merás, I. Development of a method for the determination of advanced glycation end products precursors by liquid chromatography and its application in human urine samples. J. Sep. Sci. 2012, 35, 2575–2584. [Google Scholar] [CrossRef]
- Verzijl, N.; De Groot, J.; Thorpe, S.R.; Bank, R.A.; Shaw, J.N.; Lyons, T.J.; Bijlsma, J.W.J.; Lafeberi, F.P.J.G.; Baynes, J.W.; Te Koppele, J.M. Effect of collagen turnover on the accumulation of advanced glycation endproducts. J. Biol. Chem. 2000, 275, 39027–39031. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Battah, S.; Ahmed, N.; Karachalias, N.; Agalou, S.; Babaei-Jadidi, R.; Dawnay, A. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem. J. 2003, 375, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, J.; Shi, B.; He, S.; Yao, X.; Willcox, D.M.P. Advanced glycation end product (AGE) modified proteins in tears of diabetic patients. Mol. Vis. 2010, 16, 1576–1584. [Google Scholar] [PubMed]
- Makita, Z.; Radoff, S.; Rayfield, E.J.; Yang, Z.; Skolnik, E.; Delaney, V.; Friedman, E.A.; Cerami, A.; Vlassara, H. Advanced glycosylation end products in patients with diabetic nephropathy. N. Engl. J. Med. 1991, 325, 836–842. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Makita, Z.; Shiroshita, K.; Ueda, T.; Fusegawa, T.; Kuwajima, S.; Takeuchi, M.; Koike, T. Specific fluorescence assay for advanced glycation end products in blood and urine of diabetic patients. Metabolism 1998, 47, 1348–1853. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakazawa, Y.; Ienaga, K. Acid-stable fluorescent advanced glycation end products: Vesperlysines A, B, and C are formed as crosslinked products in the Maillard reaction between lysine or proteins with glucose. Biochem. Biophys. Res. Commun. 1997, 232, 227–230. [Google Scholar] [CrossRef]
- Monnier, V.M.; Kohn, R.R.; Cerami, A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc. Natl. Acad. Sci. USA 1984, 82, 583–587. [Google Scholar] [CrossRef]
- Suehiro, A.; Uchida, K.; Nakanishi, M.; Wakabayashi, I. Measurement of urinary advanced glycation end-products (AGEs) using a fluorescence assay for metabolic syndrome-related screening tests. Diabetes Metab. Syndr. 2016, 10, S110–S113. [Google Scholar] [CrossRef]
- Bucala, R.; Cerami, A. Advanced glycosylation: Chemistry, biology, and implications for diabetes and aging. Adv. Pharmacol. 1992, 23, 1–34. [Google Scholar]
- Forbes, J.M.; Cooper, M.E.; Oldfield, M.D.; Thomas, M.C. Role of advanced glycation end products in diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14, S254–S258. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Frederickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
- Wagner, Z.; Wittmann, I.; Mazak, I.; Schinzel, R.; Heidland, A.; Kientsch-Engel, R.; Nagy, J. N(epsilon)-(carboxymethyl)lysine levels in patients with type 2 diabetes: Role of renal function. Am. J. Kidney Dis. 2001, 38, 785–791. [Google Scholar] [CrossRef]
- Kuzan, A.; Chwiłkowska1, A.; Maksymowicz, K.; Szydełko-Bronowicka, A.; Stach, K.; Pezowicz, C.; Gamian, A. Advanced glycation end products as a source of artifactsin immunoenzymatic methods. Glycoconj. J. 2018, 35, 95–103. [Google Scholar] [CrossRef]
- Bohlender, J.M.; Franke, S.; Stein, G.; Wolf, G. Advanced glycation end products and the kidney. Am. J. Physiol. Ren. Physiol. 2005, 289, F645–F659. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018, 93, 803–813. [Google Scholar] [CrossRef]
- Nishino, T. Immunohistochemical detection of advanced glycosylation end products within the vascular lesions and glomeruli in diabetic nephropathy. Hum. Pathol. 1995, 26, 308–313. [Google Scholar] [CrossRef]
- Sakata, N.; Imanaga, Y.; Meng, J.; Tachikawa, Y.; Takebayashi, S.; Nagai, R.; Horiuchi, S. Increased advanced glycation end products in atherosclerotic lesions of patients with end-stage renal disease. Atherosclerosis 1999, 142, 67–77. [Google Scholar] [CrossRef]
- Stitt, A.W. Elevated AGE-modified ApoB in sera of euglycemic, normolipidemic patients with atherosclerosis: Relationship to tissue AGEs. Mol. Med. 1997, 3, 617–627. [Google Scholar] [CrossRef]
- Li, J.; Hou, F.; Guo, Z.; Shan, Y.; Zhang, X.; Liu, Z. Advanced glycation end products upregulate C-reactive protein synthesis by human hepatocytes through stimulation of monocyte IL-6 and IL-1 beta production. Scand. J. Immunol. 2007, 66, 555–562. [Google Scholar] [CrossRef]
- Chen, J.; Huang, L.; Song, M.; Yu, S.; Gao, P.; Jing, J. C-reactive protein upregulates receptor for advanced glycation end products expression and alters antioxidant defenses in rat endothelial progenitor cells. J. Cardiovasc. Pharmacol. 2009, 53, 359–367. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Fournet, M.; Bonté, F.; Desmoulière, A. Glycation damage: A possible hub for major pathophysiological disorders and aging. Aging Dis. 2018, 9, 880–900. [Google Scholar] [CrossRef]
- Tsukahara, H.; Sekine, K.; Uchiyama, M.; Kawakami, H.; Hata, I.; Todoroki, Y.; Hiraoka, M.; Kaji, M.; Yorifuji, T.; Momoi, T.; et al. Formation of advanced glycosylation end products and oxidative stress in young patients with type 1 diabetes. Pediatr. Res. 2003, 54, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Jud, P.; Sourij, H. Therapeutic options to reduce advanced glycation end products in patients with diabetes mellitus: A review. Diabetes Res. Clin. Pract. 2019, 148, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; He, J.C. The low AGE diet: A neglected aspect of clinical nephrology practice? Nephron 2015, 130, 48–53. [Google Scholar] [CrossRef]
- Tessier, F.J. The Maillard reaction in the human body. The main discoveries and factors that affect glycation. Pathol. Biol. 2010, 58, 214–219. [Google Scholar] [CrossRef]
- Kankova, K. Diabetic threesome (hyperglycaemia, renal function and nutrition) and advanced glycation end products: Evidence for the multiple-hit agent? Proc. Nutr. Soc. 2008, 67, 60–74. [Google Scholar] [CrossRef]
- Ramasamy, R.; Vannucci, S.J.; Yan, S.S.; Herold, K.; Yan, S.F.; Schmidt, A.M. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005, 15, 16r–28r. [Google Scholar] [CrossRef]
- Uribarri, J.; Peppa, M.; Cai, W.; Goldberg, T.; Lu, M.; He, C.; Vlassara, H. Restriction of dietary glycotoxins reduces excessive advanced glycation end products in renal failure patients. J. Am. Soc. Nephrol. 2003, 14, 728–731. [Google Scholar] [CrossRef]
- Thomas, M.C.; Tsalamandris, C.; MacIsaac, R.; Medley, T.; Kingwell, B.; Cooper, M.E.; Jerums, G. Low-molecular-weight AGEs are associated with GFR and anemia in patients with type 2 diabetes. Kidney Int. 2004, 66, 1167–1172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uribarri, J.; Cai, W.; Sandu, O.; Peppa, M.; Goldberg, T.; Vlassara, H. Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann. N. Y. Acad. Sci. 2005, 1043, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Henle, T.; Deppisch, R.; Beck, W.; Hergesell, O.; Hansch, G.M.; Ritz, E. Advanced glycated end-products (AGE) during haemodialysis treatment: Discrepant results with different methodologies reflecting the heterogeneity of AGE compounds. Nephrol. Dial. Transplant. 1999, 14, 1968–1975. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sivabalan, S.; Vedeswari, C.P.; Jayachandran, S.; Koteeswaran, D.; Pravda, C.; Aruna, P.R.; Ganesan, S. In vivo native fluorescence spectroscopy and nicotinamide adinine dinucleotide/flavin adenine dinucleotide reduction and oxidation states of oral submucous fibrosis for chemopreventive drug monitoring. J. Biomed. Opt. 2010, 15, 017010. [Google Scholar] [CrossRef] [PubMed]
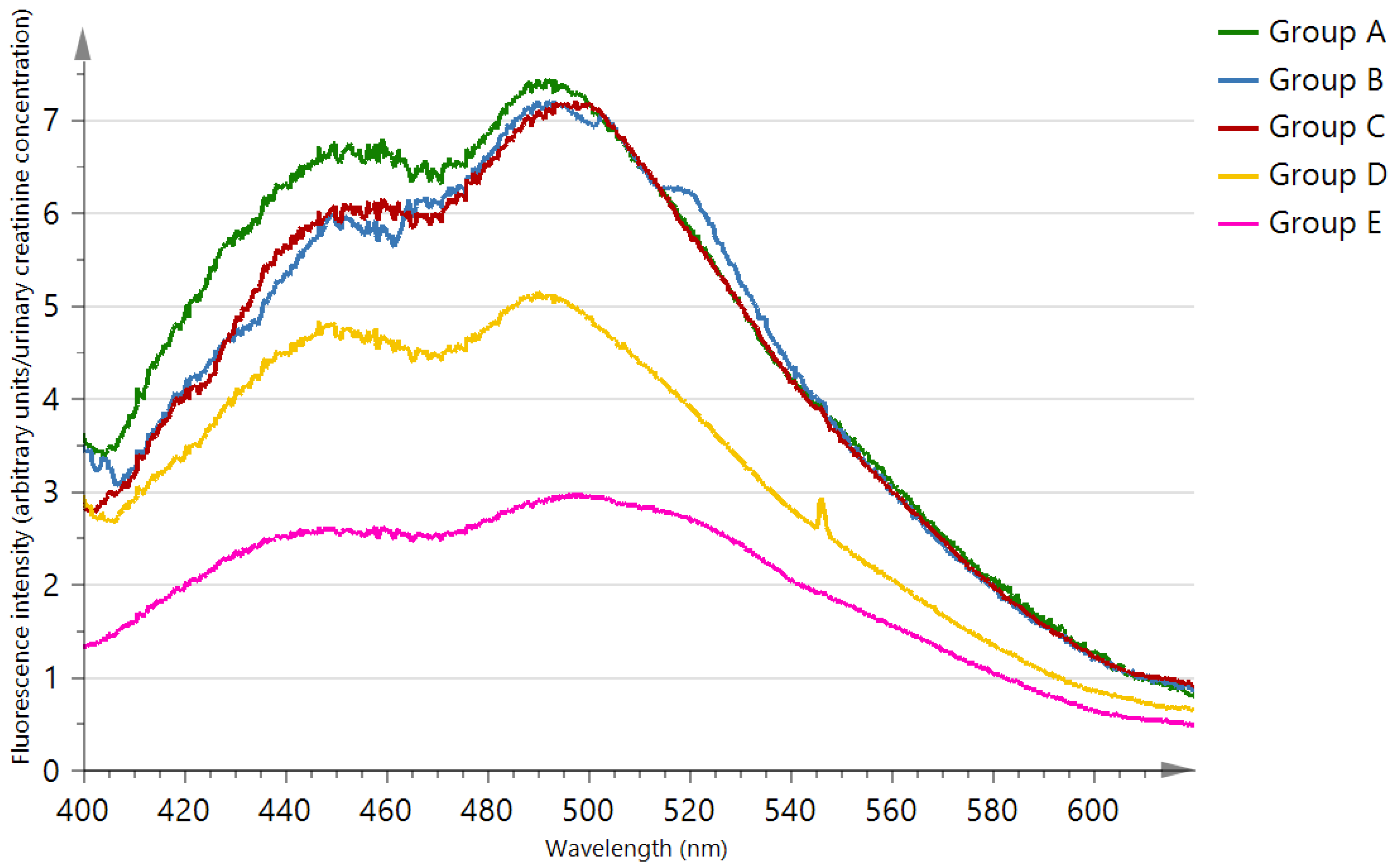
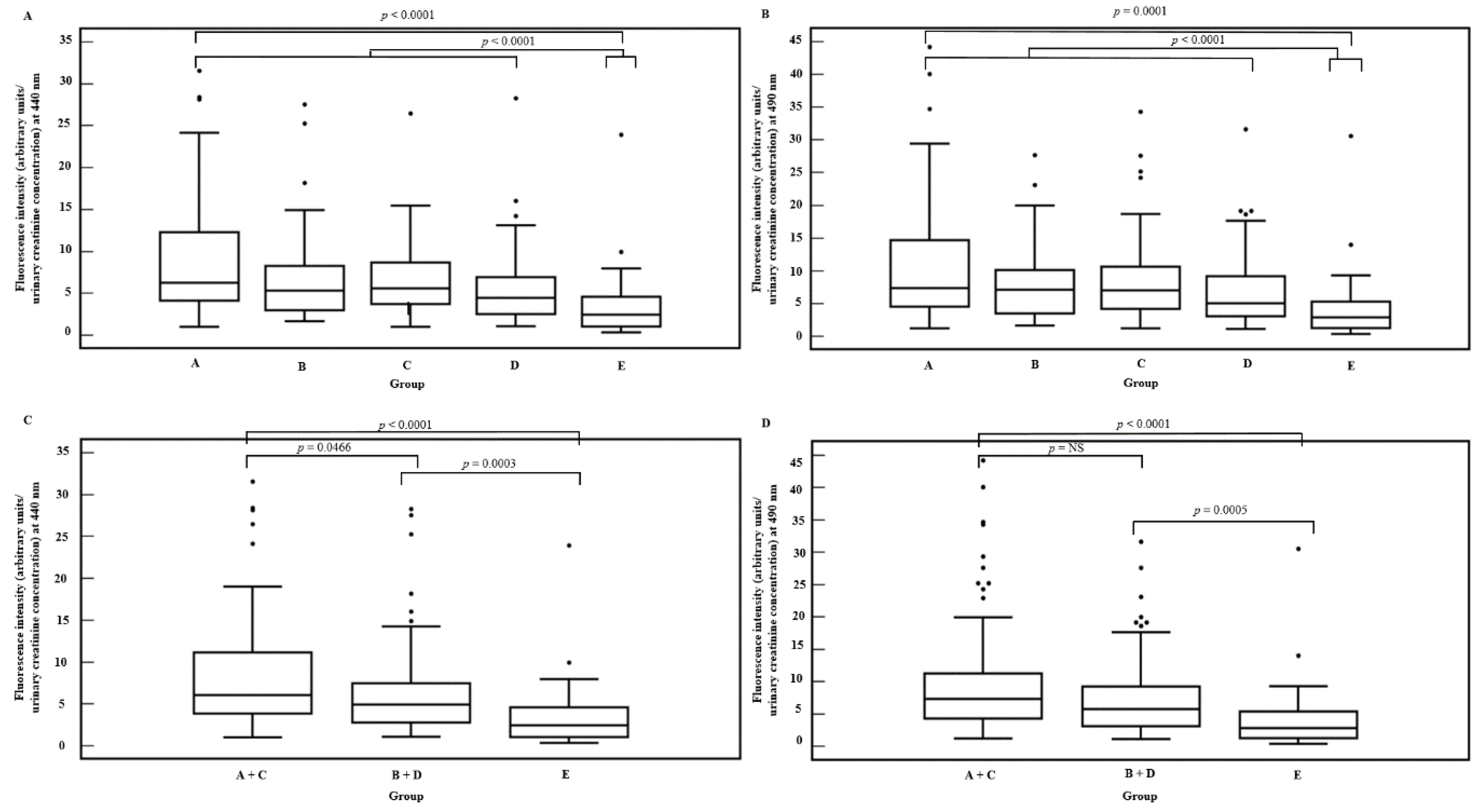
| Characteristics | Chronic Kidney Disease Patients | Healthy Subjects | p-Value | |||
|---|---|---|---|---|---|---|
| Group A | Group B | Group C | Group D | |||
| N | 46 | 27 | 45 | 46 | 31 | |
| Male/Female | 28/18 | 16/11 | 29/16 | 27/19 | 13/18 | |
| Age (years) | 68.5 (63–78) | 72 (66.5–79.8) | 68 (62–77.3) | 70 (64–77) | 30 (25–53.5) | <0.0001 |
| Diabetes mellitus | + | + | - | - | - | |
| Proteinuria | + | - | + | - | - | |
| eGFR (mL/min/1.73 m2) | 37.4 (29.4–42.3) | 33.0 (26.0–39.9) | 36.2 (29.0–46.7) | 41.2 (28.7–51.7) | >90 | <0.0001 |
| BMI (kg/m2) | 30.7 ± 4.8 | 30.8 ± 4.8 | 28.3 ± 4.2 | 27.4 ± 4.1 | 22.4 ± 3.3 | <0.0001 |
| Dependent Variable:Fluorescence Intensity | ||||
|---|---|---|---|---|
| Ln(440 nm) | Ln(490 nm) | |||
| Parameter | Correlation Coefficient | p-Value | Correlation Coefficient | p-Value |
| Age | 0.3930 | <0.0001 | 0.3806 | <0.0001 |
| BMI | 0.1418 | 0.0480 | 0.1181 | N.S. |
| Smoking | 0.1728 | 0.0171 | 0.1938 | 0.0074 |
| Ln(eGFR) | −0.3767 | <0.0001 | −0.3472 | <0.0001 |
| Ln(Urea) | 0.1822 | 0.0199 | 0.1453 | N.S. |
| Ln(CRP) | 0.2416 | 0.0008 | 0.2210 | 0.0023 |
| Hemoglobin | −0.1617 | 0.0392 | −0.1489 | N.S. |
| Treatment | ||||
| Aspirin | 0.1840 | 0.0110 | 0.1582 | 0.0293 |
| Peroral anticoagulant | 0.1711 | 0.0183 | 0.1464 | 0.0439 |
| ACE inhibitor or ARB | 0.1541 | 0.0337 | 0.1401 | N.S. |
| Beta blocker | 0.2023 | 0.0051 | 0.1724 | 0.0174 |
| Nondihydropyridine calcium | 0.1834 | 0.0113 | 0.1524 | 0.0358 |
| channel blocker | ||||
| Loop diuretic | 0.2570 | 0.0003 | 0.2243 | 0.0019 |
| Statin | 0.2053 | 0.0045 | 0.1990 | 0.0059 |
| Insulin | 0.2793 | 0.0001 | 0.2386 | 0.0009 |
| Vitamin D | 0.1505 | 0.0382 | 0.1219 | N.S. |
| Dependent Variable | Independent Variable | β (SE) | p-Value |
|---|---|---|---|
| Ln(Fluorescence intensity at emission wavelength 440 nm) R2 = 0.1970, p < 0.001 | Age (years) | 0.0107 (0.0046) | 0.0206 |
| Ln(eGFR) (mL/min/1.73 m2) | −0.2565 (0.1429) | 0.0743 | |
| Ln(CRP) (mg/L) | 0.1346 (0.0593) | 0.0245 | |
| Insulin treatment | 0.2798 (0.0844) | 0.0011 | |
| Ln(Fluorescence intensity at emission wavelength 490 nm) R2 = 0.1467, p < 0.001 | Age (years) | 0.0155 (0.0040) | 0.0001 |
| Ln(CRP) (mg/L) | 0.1166 (0.0632) | 0.0667 | |
| Insulin treatment | 0.2664 (0.0880) | 0.0028 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steenbeke, M.; De Bruyne, S.; Van Aken, E.; Glorieux, G.; Van Biesen, W.; Himpe, J.; De Meester, G.; Speeckaert, M.; Delanghe, J. UV Fluorescence-Based Determination of Urinary Advanced Glycation End Products in Patients with Chronic Kidney Disease. Diagnostics 2020, 10, 34. https://doi.org/10.3390/diagnostics10010034
Steenbeke M, De Bruyne S, Van Aken E, Glorieux G, Van Biesen W, Himpe J, De Meester G, Speeckaert M, Delanghe J. UV Fluorescence-Based Determination of Urinary Advanced Glycation End Products in Patients with Chronic Kidney Disease. Diagnostics. 2020; 10(1):34. https://doi.org/10.3390/diagnostics10010034
Chicago/Turabian StyleSteenbeke, Mieke, Sander De Bruyne, Elisabeth Van Aken, Griet Glorieux, Wim Van Biesen, Jonas Himpe, Gilles De Meester, Marijn Speeckaert, and Joris Delanghe. 2020. "UV Fluorescence-Based Determination of Urinary Advanced Glycation End Products in Patients with Chronic Kidney Disease" Diagnostics 10, no. 1: 34. https://doi.org/10.3390/diagnostics10010034
APA StyleSteenbeke, M., De Bruyne, S., Van Aken, E., Glorieux, G., Van Biesen, W., Himpe, J., De Meester, G., Speeckaert, M., & Delanghe, J. (2020). UV Fluorescence-Based Determination of Urinary Advanced Glycation End Products in Patients with Chronic Kidney Disease. Diagnostics, 10(1), 34. https://doi.org/10.3390/diagnostics10010034








