Cultivar-Specific Performance and Qualitative Descriptors for Butterhead Salanova Lettuce Produced in Closed Soilless Cultivation as a Candidate Salad Crop for Human Life Support in Space
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Chamber Conditions, Experimental Design and Harvesting Schedule
2.2. Sampling, Growth Analysis, and SPAD Index Measurement
2.3. Water Uptake, Water Use Efficiency and Relative Growth Rate
2.4. Water Potential and Relative Water Content
2.5. Dry Matter, Total Nitrogen and Mineral Content Analysis
2.6. Analysis of Lipophilic Antioxidant Activity
2.7. Analysis of Total Ascorbic Acid and Total Phenols
2.8. Statistical Analysis
3. Results
3.1. Growth Response, Water Uptake and Water Use Efficiency
3.2. Leaf Macro-Mineral Composition
3.3. Relative Water Content, Leaf Water Potential and SPAD Index.
3.4. Qualitative Parameters
3.5. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wheeler, R.M. Horticulture for mars. In Proceedings of the XXVI International horticultural congress: Horticulture, art and science for life- Colloquia Presentations, Toronto, ON, Canada, 11–17 August 2002; Volume 642, pp. 201–215. [Google Scholar] [CrossRef]
- Walker, J.; Granjou, C. MELiSSA the minimal biosphere: Human life, waste and refuge in deep space. Futures 2017, 92, 59–69. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; De Pascale, S.; Kyratzis, A.; Rouphael, Y. Microgreens as a component of space life support systems: A cornucopia of functional food. Front. Plant Sci. 2017, 8, 1587. [Google Scholar] [CrossRef]
- Dueck, T.; Kempkes, F.; Esther, M.; Cecilia, S. Choosing crops for cultivation in space. In Proceedings of the 46th International Conference on Environmental Systems, Vienna, Austria, 10–14 July 2016. [Google Scholar]
- Zabel, P.; Bamsey, M.; Schubert, D.; Tajmar, M. Review and analysis of over 40 years of space plant growth systems. Life Sci. Space Res. 2016, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W. Health effects of vegetables and fruit: Assessing mechanisms of action in human experimental studies. Am. J. Clin. Nutr. 1999, 70, 475–490. [Google Scholar] [CrossRef]
- Wolff, S.; Coelho, L.; Karoliussen, I.; Jost, A.I. Effects of the extraterrestrial environment on plants: Recommendations for future space experiments for the MELiSSA higher plant compartment. Life 2014, 4, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, R.M. Agriculture for space: People and places paving the way. Open Agric. 2017, 2, 14–32. [Google Scholar] [CrossRef]
- Chunxiao, X.; Hong, L. Crop candidates for the bioregenerative life support systems in China. Acta Astronaut. 2008, 63, 1076–1080. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Comp. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Putra, P.A.; Yuliando, H. Soilless culture system to support water use efficiency and product quality: A review. Agric. Agric. Sci. Procedia 2015, 3, 283–288. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Tullio, M.; Rivera, C.M.; Rea, E. Alleviation of salt stress by arbuscular mycorrhizal in zucchini plants grown at low and high phosphorus concentration. Biol. Fertil. Soils 2008, 44, 501–509. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis; Black, C.A., Evans, D.D., White, I.L., Ensminger, L.E., Clark, F.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Pellegrini, N.; Ke, R.; Yang, M.; Rice-Evans, C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2, 2′-azinobis (3-ethylenebenzothiazoline-6-sulfonic acid radical cation decolorization assay. Methods. Enzym. 1999, 299, 379–389. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods. Enzym. 1999, 299, 152–178, Academic press. [Google Scholar] [CrossRef]
- Carillo, P.; Cirillo, C.; De Micco, V.; Arena, C.; De Pascale, S.; Rouphael, Y. Morpho-anatomical, physiological and biochemical adaptive responses to saline water of Bougainvillea spectabilis Willd. trained to different canopy shapes. Agric. Water Manag. 2019, 212, 12–22. [Google Scholar] [CrossRef]
- Carillo, P.; Raimondi, G.; Kyriacou, M.C.; Pannico, A.; El-Nakhel, C.; Cirillo, V.; Colla, G.; De Pascale, S.; Rouphael, Y. Morpho-physiological and homeostatic adaptive responses triggered by omeprazole enhance lettuce tolerance to salt stress. Sci. Hortic. 2019, 49, 22–30. [Google Scholar] [CrossRef]
- González, L.; González-Vilar, M. Determination of relative water content. In Handbook Plant Ecophysiology Techniques; Springer: Dordrecht, The Netherlands, 2001; pp. 207–212. [Google Scholar] [CrossRef]
- Galindo, F.G.; Herppich, W.; Gekas, V.; Sjöholm, I. Factors affecting quality and postharvest properties of vegetables: Integration of water relations and metabolism. Crit. Rev. Food Sci. Nutr. 2004, 44, 139–154. [Google Scholar] [CrossRef]
- Burns, I.G.; Zhang, K.; Turner, M.K.; Meacham, M.; Al-Redhiman, K.; Lynn, J.; Broadley, M.R.; Hand, P.; Pink, D. Screening for genotype and environment effects on nitrate accumulation in 24 species of young lettuce. J. Sci. Food Agric. 2011, 91, 553–562. [Google Scholar] [CrossRef]
- Barbosa, G.; Gadelha, F.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.; Halden, R. Comparison of land, water, and energy requirements of lettuce grown using hydroponic vs. conventional agricultural methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Growth, yield, fruit quality and nutrient uptake of hydroponically cultivated zucchini squash as affected by irrigation systems and growing seasons. Sci. Hortic. 2005, 105, 177–195. [Google Scholar] [CrossRef]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2019, 17, 364. [Google Scholar] [CrossRef]
- Brechner, M.; Both, A.J.; Staff, C.E.A. Hydroponic lettuce handbook. Cornell Control. Environ. Agric. 1996, 1, 504–509. [Google Scholar]
- Albornoz, F.; Lieth, J.H. Daily macronutrient uptake patterns in relation to plant age in hydroponic lettuce. J. Plant Nutr. 2016, 39, 1357–1364. [Google Scholar] [CrossRef]
- Hsiao, T.C.; Acevedo, E.; Fereres, E.; Henderson, D.W. Water stress, growth and osmotic adjustment. Philos. Trans. R. Soc. Lond. Biol. Sci. 1976, 273, 479–500. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Water uptake by growing cells: An assessment of the controlling roles of wall relaxation, solute uptake, and hydraulic conductance. Intern. J. Plant Sci. 1993, 154, 10–21. [Google Scholar] [CrossRef]
- Barg, M.; Agüero, M.V.; Yommi, A.; Roura, S.I. Evolution of plant water statusindices during butterhead lettuce growth and its impact on post-storage quality. J. Sci. Food Agric. 2009, 89, 422–429. [Google Scholar] [CrossRef]
- Wilson, J.W.; Rose, C.W. The components of leaf water potential I. osmotic and matric potentials. Aust. J. Biol. Sci. 1967, 20, 329–348. [Google Scholar] [CrossRef]
- Carillo, P. GABA shunt in durum wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef]
- Mengel, K.; Arneke, W.W. Effect of potassium on the water potential, the pressure potential, the osmotic potential and cell elongation in leaves of Phaseolus vulgaris. Physiol. Plant. 1982, 54, 402–408. [Google Scholar] [CrossRef]
- Albornoz, F.; Lieth, J.H. N, P, K and S uptake response to various levels of CO2 assimilation and growth rate in lettuce. J. Plant Nut. 2017, 40, 773–783. [Google Scholar] [CrossRef]
- Broadley, M.R.; Seginer, I.; Burns, A.; Escobar-Gutiérrez, A.J.; Burns, I.G.; White, P.J. The nitrogen and nitrate economy of butterhead lettuce (Lactuca sativa var. capitata L.). J. Exp. Bot. 2003, 54, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Burns, I.G.; Zhang, K.; Turner, M.K.; Edmondson, R. Iso osmotic regulation of nitrate accumulation in lettuce. J. Plant Nutr. 2010, 34, 283–313. [Google Scholar] [CrossRef]
- Weiguo, F.; Pingping, L.; Yanyou, W.; Jianjian, T. Effects of different light intensities on anti-oxidative enzyme activity, quality and biomass in lettuce. Hortic. Sci. 2012, 39, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Sublett, W.L.; Barickman, T.C.; Sams, C.E. The effect of environment and nutrients on hydroponic lettuce yield, quality, and phytonutrients. Horticulturae 2018, 4, 48. [Google Scholar] [CrossRef]
- Chutichudet, B.; Chutichudet, P.; Kaewsit, S. Influence of developmental stage on activities of polyphenol oxidase, internal characteristics and colour of lettuce cv. grand rapids. Am. J. Food Technol. 2011, 6, 215–225. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Lee, K.P.; Kim, C.; Landgraf, F.; Apel, K. Executor-1 and Executor-2 dependent transfer of stress related signals from the plastid to the nucleus of Arabidopsis Thaliana. Environ. Poll. 2007, 166, 136–143. [Google Scholar] [CrossRef]
- Nobel, P.S. Internal leaf area and cellular CO2 resistance: Photosynthetic implications of variations with growth conditions and plant species. Physiol. Plant. 1977, 40, 137–144. [Google Scholar] [CrossRef]
- Tzvetkova-Chevolleau, T.; Franck, F.; Alawady, A.E.; Dall’Osto, L.; Carrière, F.; Bassi, R.; Grimm, B.; Nussaume, L.; Havaux, M. The light stress-induced protein ELIP2 is a regulator of chlorophyll synthesis in Arabidopsis thaliana. Plant J. 2007, 50, 795–809. [Google Scholar] [CrossRef]
- Varvara, M.; Bozzo, G.; Celano, G.; Disanto, C.; Pagliarone, C.N.; Celano, G.V. The use of ascorbic acid as a food additive: Technical-legal issues. Ital. J. Food Saf. 2016, 5. [Google Scholar] [CrossRef]
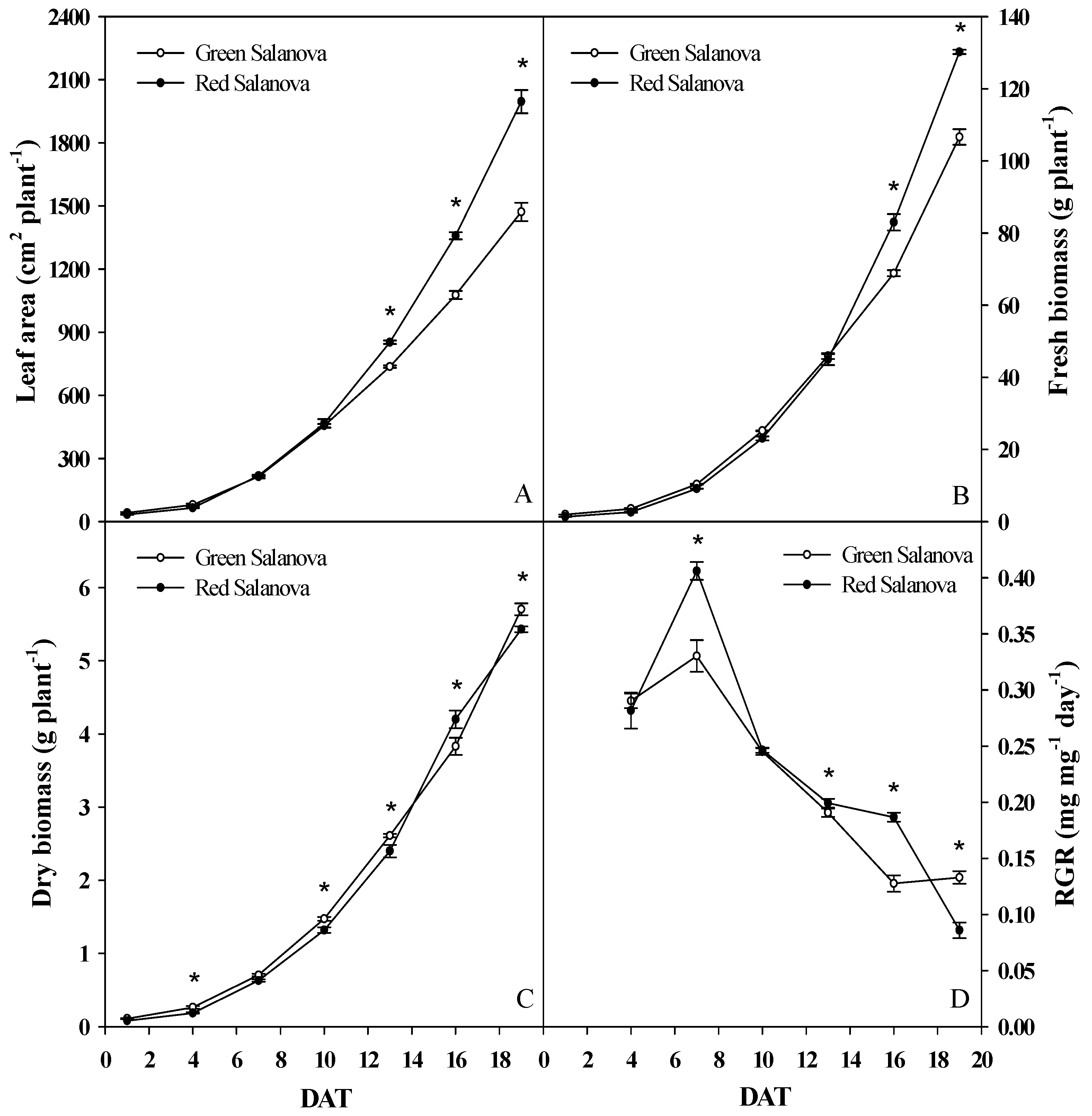
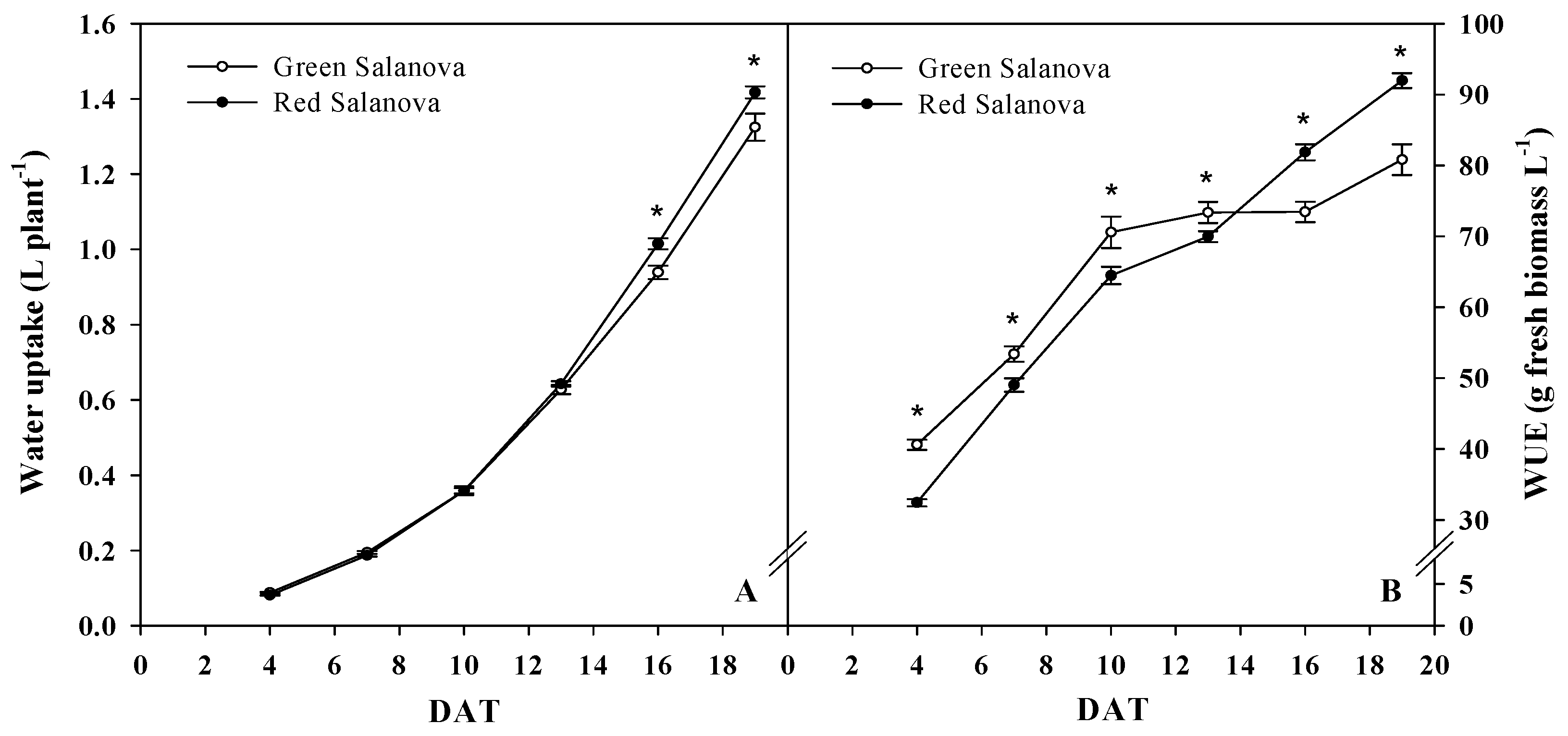
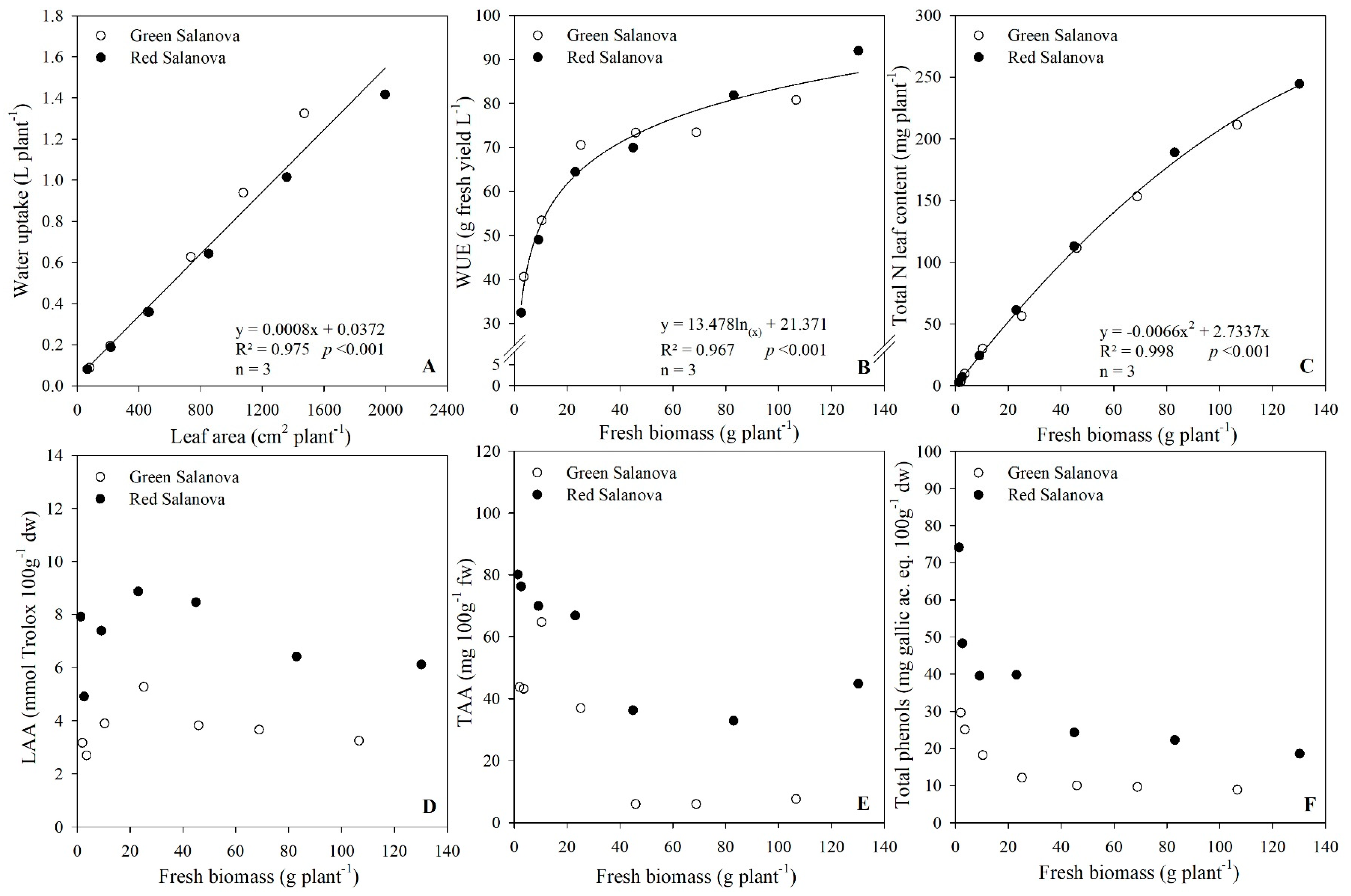
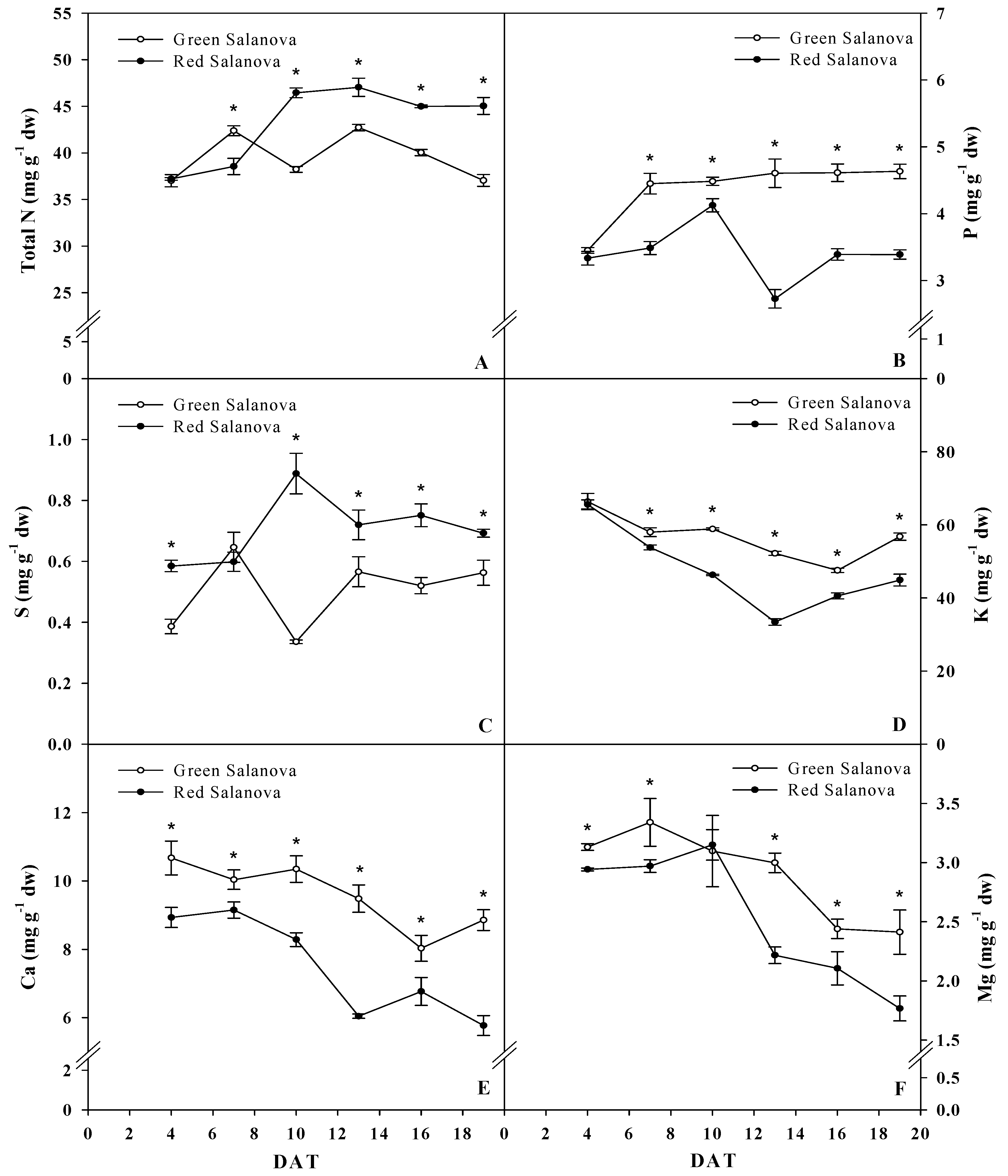

| Daily Variables | Cultivar | 4 DAT | 7 DAT | 10 DAT | 13 DAT | 16 DAT | 19 DAT | Significance | Mean |
|---|---|---|---|---|---|---|---|---|---|
| Leaf Area | Green Salanova | 12.81 f | 44.66 e | 80.80 d | 93.36 c | 113.49 b | 131.72 a | *** | 79.47 |
| (cm2 plant−1 day−1) | Red Salanova | 10.38 f | 50.86 e | 82.82 d | 128.79 c | 168.53 b | 212.70 a | *** | 109.01 |
| t-test | 0.008 | 0.002 | 0.772 | 0.002 | 0.001 | 0.006 | 0.001 | ||
| Fresh biomass | Green Salanova | 0.53 e | 2.26 d | 4.94 c | 6.91 b | 7.64 b | 12.59 a | *** | 5.81 |
| (g plant−1 day−1) | Red Salanova | 0.42 f | 2.18 e | 4.64 d | 7.29 c | 12.68 b | 15.74 a | *** | 7.16 |
| t-test | 0.232 | 0.217 | 0.141 | 0.454 | 0.000 | 0.020 | 0.000 | ||
| Dry biomass | Green Salanova | 0.05 e | 0.15 d | 0.26 c | 0.38 b | 0.41 b | 0.62 a | *** | 0.31 |
| (g plant−1 day−1) | Red Salanova | 0.04 f | 0.15 e | 0.23 d | 0.36 c | 0.60 b | 0.41 a | *** | 0.30 |
| t-test | 0.035 | 1.000 | 0.036 | 0.338 | 0.005 | 0.002 | 0.045 | ||
| Water uptake | Green Salanova | 29.47 d | 35.43 cd | 54.71 c | 89.50 b | 104.00 b | 128.45 a | *** | 73.59 |
| (mL plant−1 day−1) | Red Salanova | 26.97 f | 35.47 e | 57.13 d | 94.76 c | 123.98 b | 134.04 a | *** | 78.72 |
| t-test | 0.055 | 0.976 | 0.477 | 0.068 | 0.000 | 0.743 | 0.145 | ||
| Total N | Green Salanova | 2.32 e | 6.73 d | 8.79 c | 18.39 a | 13.97 b | 19.31 a | *** | 11.58 |
| (mg plant−1 day−1) | Red Salanova | 1.41 e | 5.79 d | 12.35 c | 17.23 b | 25.31 a | 18.54 b | *** | 13.44 |
| t-test | 0.013 | 0.026 | 0.002 | 0.407 | 0.002 | 0.322 | 0.020 | ||
| P | Green Salanova | 0.15 e | 0.75 d | 1.15 c | 1.81 b | 1.89 b | 2.91 a | *** | 1.44 |
| (mg plant−1 day−1) | Red Salanova | 0.05 e | 0.53 d | 1.08 c | 0.37 d | 2.56 a | 1.38 b | *** | 1.00 |
| t-test | 0.004 | 0.009 | 0.333 | 0.002 | 0.017 | 0.000 | 0.002 | ||
| S | Green Salanova | 0.02 d | 0.12 c | 0.02 d | 0.32 b | 0.17 c | 0.41 a | *** | 0.18 |
| (mg plant−1 day−1) | Red Salanova | 0.02 c | 0.09 c | 0.27 b | 0.19 b | 0.48 a | 0.20 b | *** | 0.21 |
| t-test | 0.741 | 0.082 | 0.001 | 0.039 | 0.001 | 0.041 | 0.139 | ||
| K | Green Salanova | 3.73 d | 7.82 c | 15.24 b | 16.52 b | 15.31 b | 47.24 a | *** | 17.65 |
| (mg plant−1 day−1) | Red Salanova | 1.89 d | 7.22 c | 9.07 c | 6.42 c | 30.05 a | 24.44 b | *** | 13.18 |
| t-test | 0.019 | 0.019 | 0.000 | 0.001 | 0.002 | 0.000 | 0.005 | ||
| Ca | Green Salanova | 0.59 e | 1.43 d | 2.72 b | 3.17 b | 2.02 c | 6.57 a | *** | 2.75 |
| (mg plant−1 day−1) | Red Salanova | 0.26 c | 1.37 bc | 1.73 b | 1.19 bc | 4.67 a | 0.94 bc | *** | 1.69 |
| t-test | 0.020 | 0.389 | 0.005 | 0.000 | 0.020 | 0.000 | 0.003 | ||
| Mg | Green Salanova | 0.18 d | 0.51 cd | 0.74 c | 1.08 b | 0.51 cd | 1.47 a | *** | 0.75 |
| (mg plant−1 day−1) | Red Salanova | 0.10 d | 0.44 c | 0.76 b | 0.39 c | 1.18 a | 0.24 cd | *** | 0.52 |
| t-test | 0.004 | 0.210 | 0.832 | 0.002 | 0.022 | 0.007 | 0.035 |
| Physiological Parameters | Cultivar | 4 DAT | 7 DAT | 10 DAT | 13 DAT | 16 DAT | 19 DAT | Significance |
|---|---|---|---|---|---|---|---|---|
| RWC | Green Salanova | 68.41 d | 78.45 c | 88.85 a | 84.97 ab | 83.81 b | 83.71 b | *** |
| (%) | Red Salanova | 61.77 f | 69.94 e | 75.25 d | 82.40 c | 94.25 b | 97.13 a | *** |
| t-test | 0.000 | 0.002 | 0.000 | 0.277 | 0.000 | 0.000 | ||
| Leaf total potential | Green Salanova | −1.15 d | −0.78 c | −0.59 a | −0.66 b | −0.78 c | −0.78 c | *** |
| (MPa) | Red Salanova | −1.30 e | −0.85 cd | −0.98 d | −0.72 bc | −0.61 ab | −0.52 a | *** |
| t-test | 0.002 | 0.246 | 0.003 | 0.441 | 0.001 | 0.000 | ||
| Leaf osmotic potential | Green Salanova | −0.65 c | −0.55 bc | −0.24 a | −0.46 b | −0.47 b | −0.50 b | *** |
| (MPa) | Red Salanova | −0.66 d | −0.25 ab | −0.37 c | −0.26 ab | −0.30 bc | −0.21 a | *** |
| t-test | 0.635 | 0.000 | 0.019 | 0.015 | 0.005 | 0.000 | ||
| Leaf turgor pressure | Green Salanova | 0.50 a | 0.23 cd | 0.35 b | 0.20 d | 0.31 bc | 0.28 bcd | *** |
| (MPa) | Red Salanova | 0.64 a | 0.60 a | 0.61 a | 0.46 b | 0.31 c | 0.32 c | *** |
| t-test | 0.008 | 0.000 | 0.002 | 0.001 | 0.964 | 0.264 | ||
| SPAD index | Green Salanova | 23.81 c | 24.75 c | 25.71 bc | 25.78 bc | 28.54 b | 31.76 a | *** |
| Red Salanova | 39.42 d | 39.39 d | 41.83 c | 43.86 b | 44.98 b | 46.90 a | *** | |
| t-test | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Qualitative Parameters | Cultivar | 4 DAT | 7 DAT | 10 DAT | 13 DAT | 16 DAT | 19 DAT | Significance |
|---|---|---|---|---|---|---|---|---|
| Nitrate | Green Salanova | 1323 b | 1727 a | 1927 a | 1478 b | 1319 b | 1871 a | *** |
| (mg kg−1 fw) | Red Salanova | 786 c | 1079 b | 772 c | 536 d | 1109 ab | 1175 a | *** |
| t-test | 0.000 | 0.008 | 0.000 | 0.000 | 0.007 | 0.000 | ||
| Dry matter | Green Salanova | 7.35 a | 6.81 b | 5.85 c | 5.68 cd | 5.56 cd | 5.35 d | *** |
| (%) | Red Salanova | 7.12 a | 6.87 b | 5.72 c | 5.34 d | 5.06 e | 4.17 f | *** |
| t-test | 0.339 | 0.742 | 0.221 | 0.014 | 0.015 | 0.000 | ||
| LAA | Green Salanova | 2.70 c | 3.90 b | 5.27 a | 3.83 b | 3.66 b | 3.25 bc | *** |
| (mmol Trolox 100 g−1 dw) | Red Salanova | 4.91 c | 7.39 ab | 8.87 a | 8.47 a | 6.42 bc | 6.12 bc | ** |
| t-test | 0.005 | 0.024 | 0.002 | 0.003 | 0.007 | 0.017 | ||
| Total phenols | Green Salanova | 25.10 a | 18.22 b | 12.15 c | 10.06 d | 9.66 d | 8.90 d | *** |
| (mg gallic ac. eq. 100 g−1 dw) | Red Salanova | 48.27 a | 39.55 b | 39.84 b | 24.33 c | 22.29 c | 18.57 c | *** |
| t-test | 0.005 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Total ascorbic acid | Green Salanova | 43.20 b | 64.76 a | 36.95 b | 5.99 c | 5.98 c | 7.61 c | *** |
| (mg AA 100 g−1 fw) | Red Salanova | 76.26 a | 69.95 ab | 66.83 b | 36.28 d | 32.91 d | 44.86 c | *** |
| t-test | 0.001 | 0.263 | 0.005 | 0.000 | 0.000 | 0.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Nakhel, C.; Giordano, M.; Pannico, A.; Carillo, P.; Fusco, G.M.; De Pascale, S.; Rouphael, Y. Cultivar-Specific Performance and Qualitative Descriptors for Butterhead Salanova Lettuce Produced in Closed Soilless Cultivation as a Candidate Salad Crop for Human Life Support in Space. Life 2019, 9, 61. https://doi.org/10.3390/life9030061
El-Nakhel C, Giordano M, Pannico A, Carillo P, Fusco GM, De Pascale S, Rouphael Y. Cultivar-Specific Performance and Qualitative Descriptors for Butterhead Salanova Lettuce Produced in Closed Soilless Cultivation as a Candidate Salad Crop for Human Life Support in Space. Life. 2019; 9(3):61. https://doi.org/10.3390/life9030061
Chicago/Turabian StyleEl-Nakhel, Christophe, Maria Giordano, Antonio Pannico, Petronia Carillo, Giovanna Marta Fusco, Stefania De Pascale, and Youssef Rouphael. 2019. "Cultivar-Specific Performance and Qualitative Descriptors for Butterhead Salanova Lettuce Produced in Closed Soilless Cultivation as a Candidate Salad Crop for Human Life Support in Space" Life 9, no. 3: 61. https://doi.org/10.3390/life9030061











