Exploring the Emergence of RNA Nucleosides and Nucleotides on the Early Earth
Abstract
1. Introduction
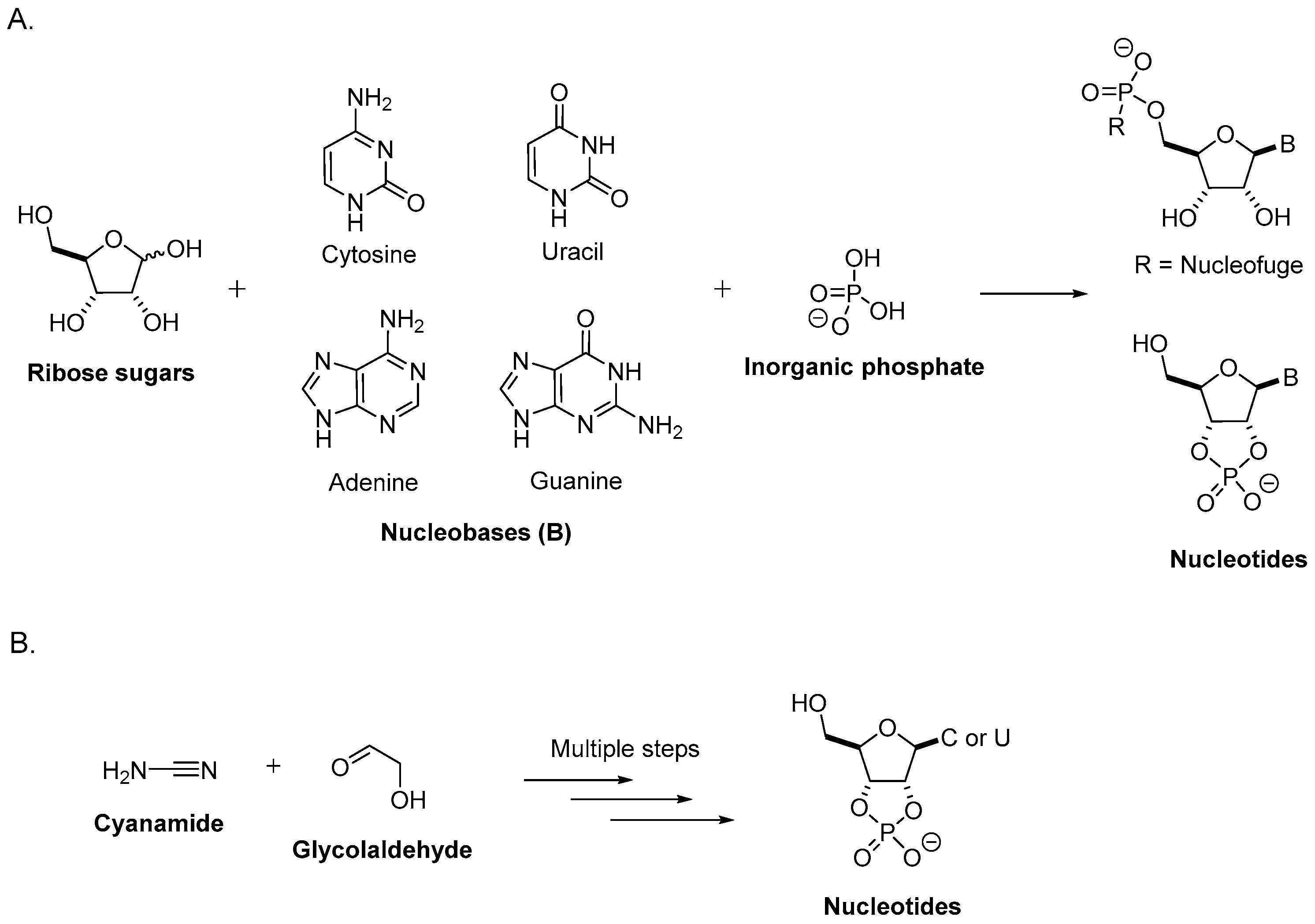
2. Prebiotic Synthesis of Nucleotides from the Assembly of a Nucleobase, a Ribose, and a Phosphate
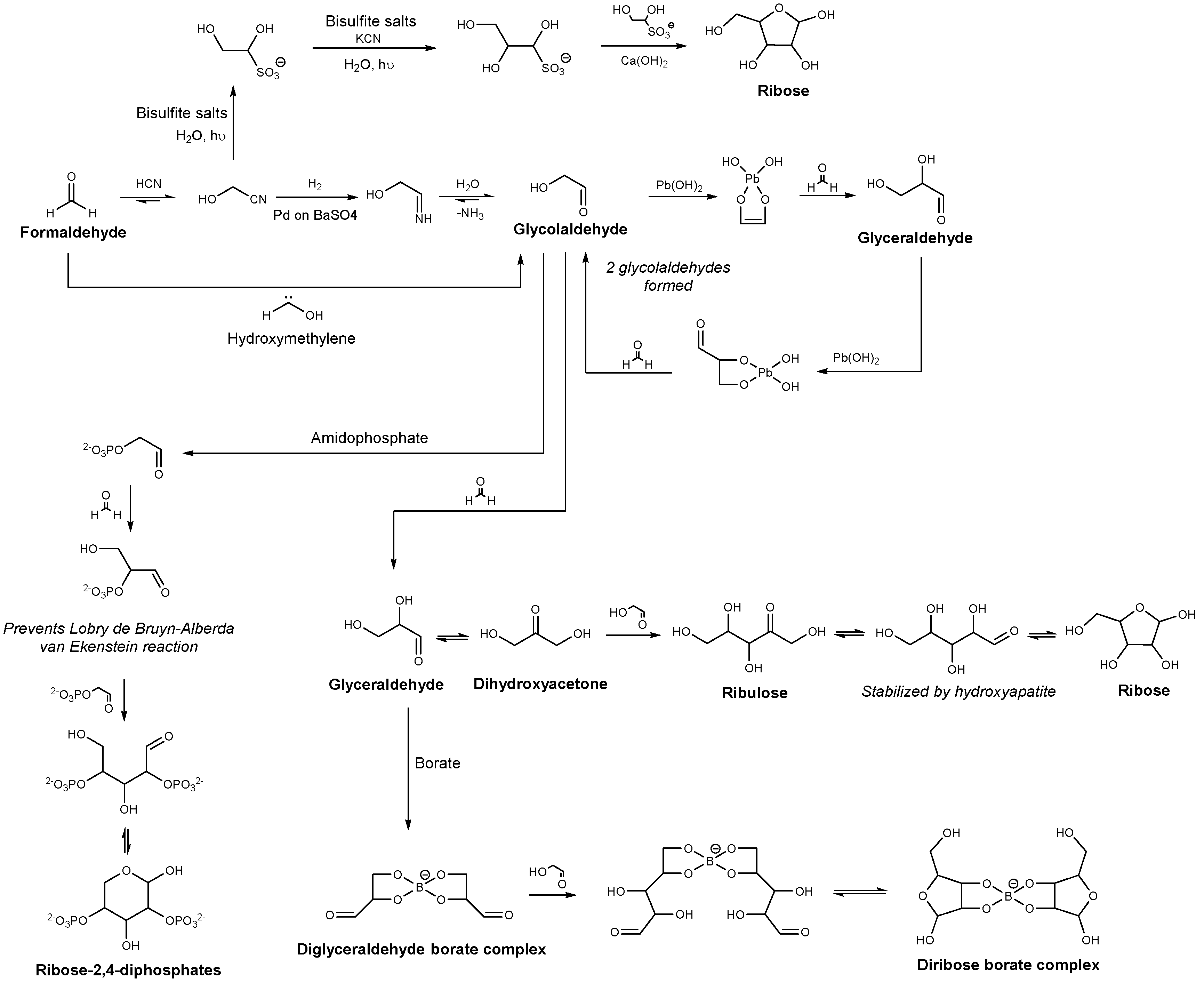
2.1. Sugar Synthesis
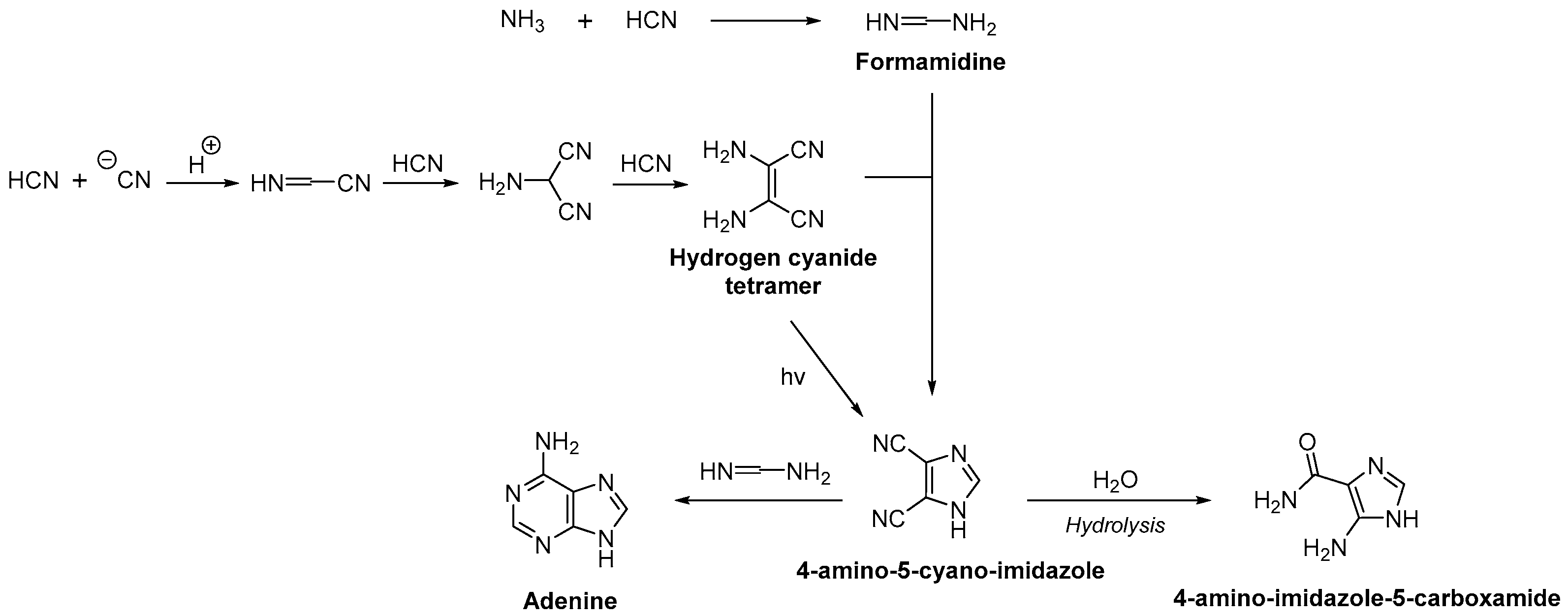
2.2. Nucleobase Synthesis
2.3. Nucleoside Synthesis from Sugars and Nucleobases
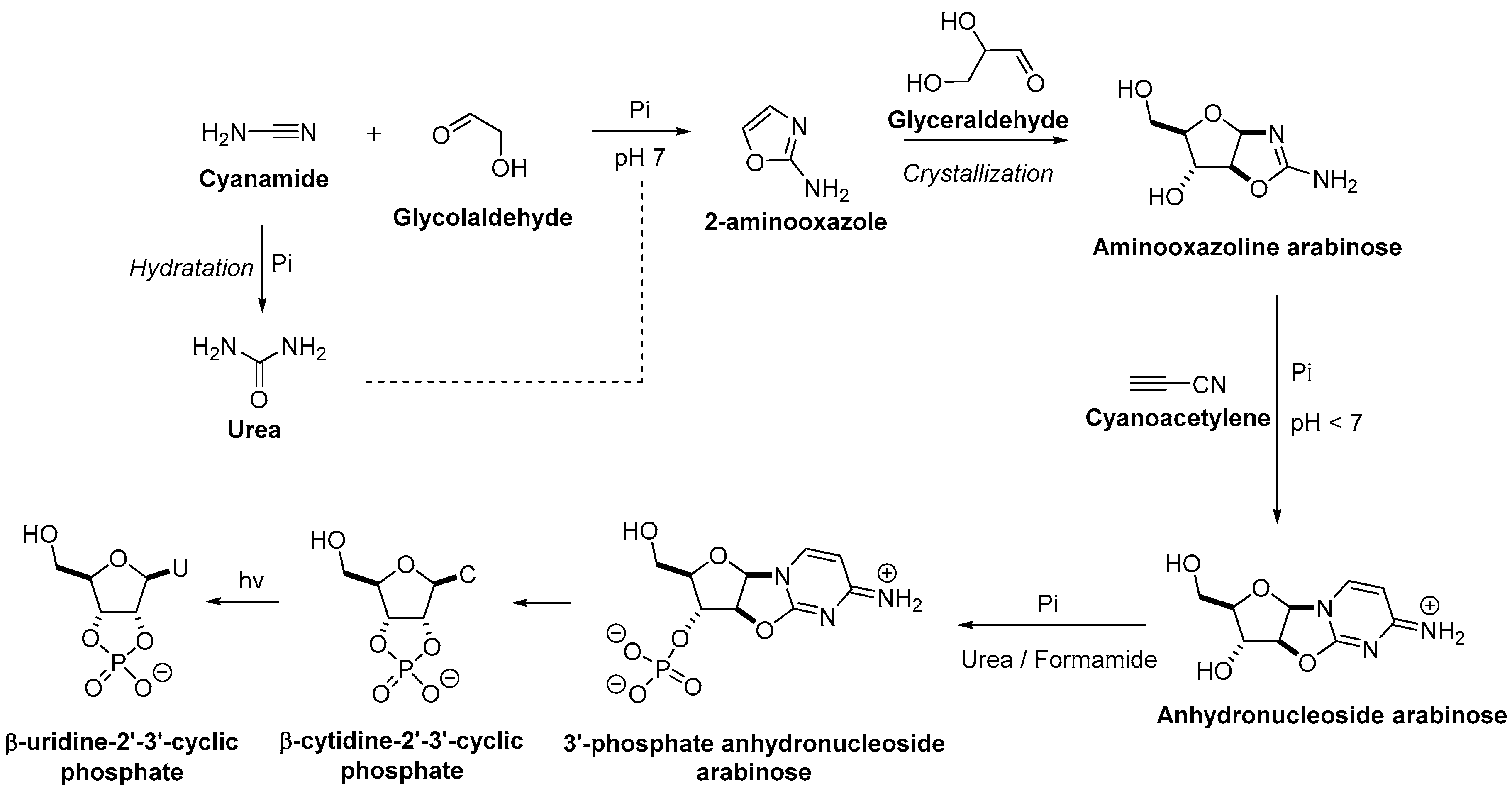
3. The Revisited Approach for the Synthesis of Nucleosides
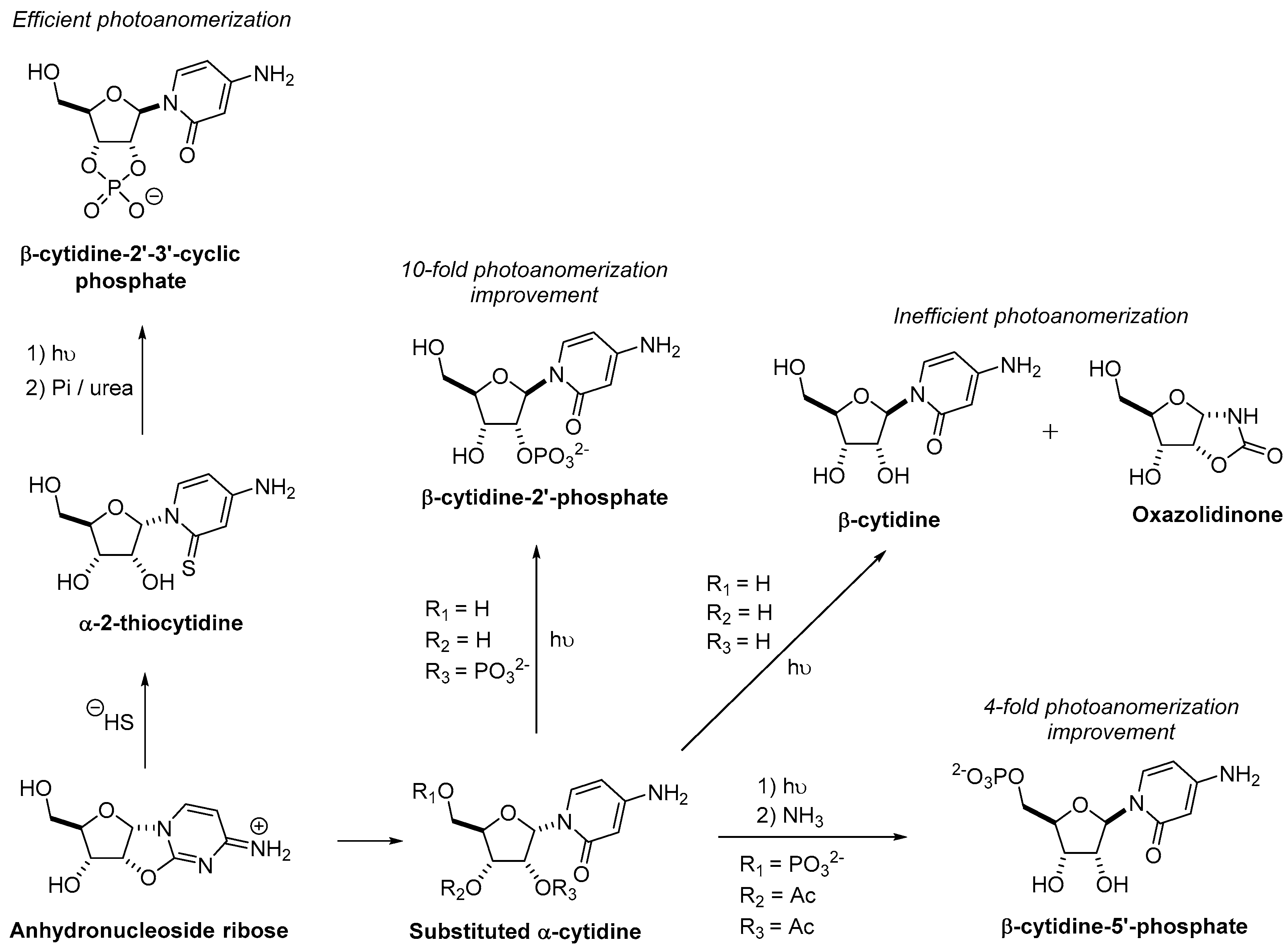
3.1. Pyrimidine Nucleoside Synthesis
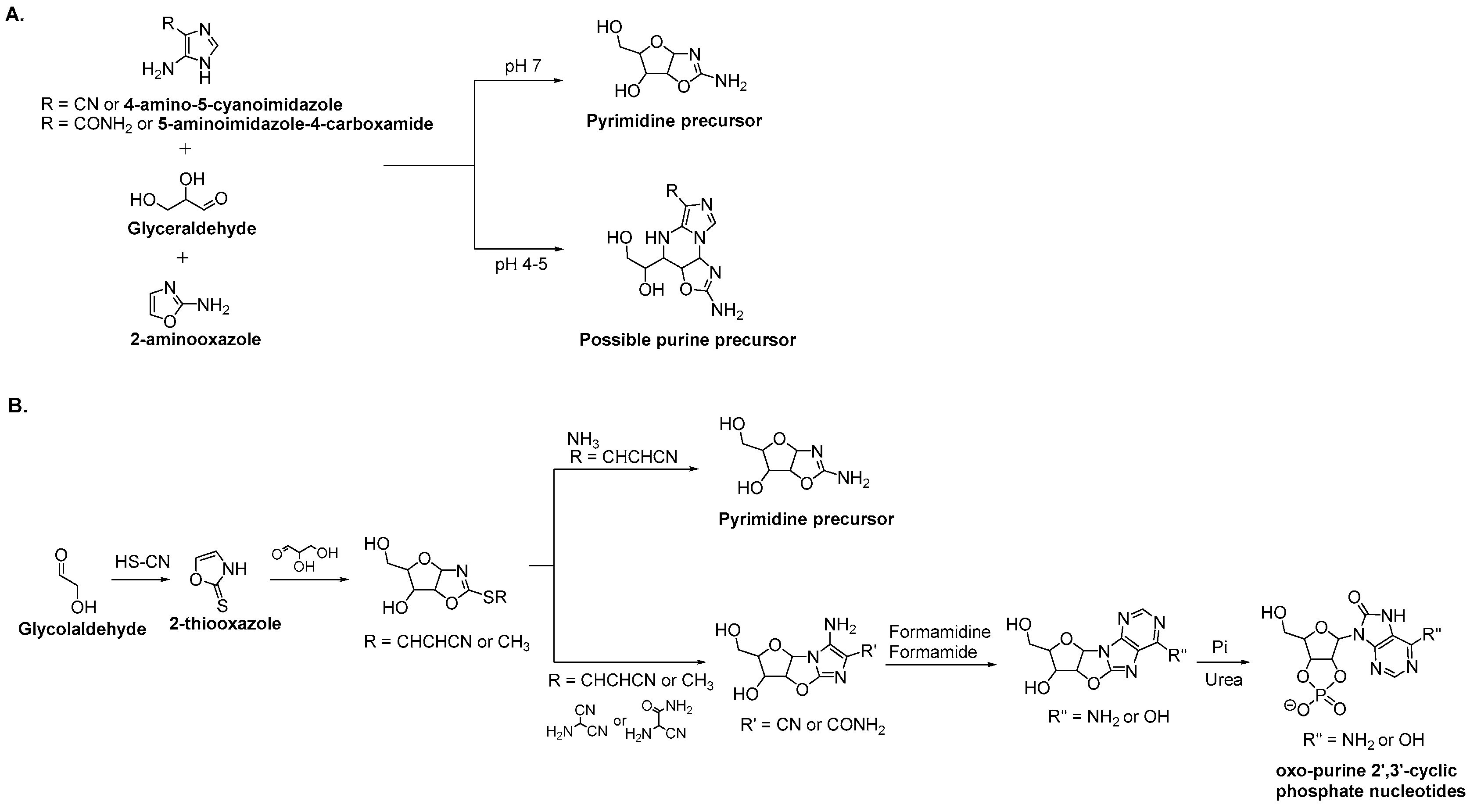
3.2. Purine Nucleoside Synthesis
4. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Eschenmoser, A. Etiology of potentially primordial biomolecular structures: From vitamin B12 to the nucleic acids and an inquiry into the chemistry of life’s origin: A retrospective. Angew. Chem. Int. Ed. 2011, 50, 12412–12472. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.Y.; Cordiner, M.A.; Nixon, C.A.; Charnley, S.B.; Teanby, N.A.; Kisiel, Z.; Irwin, P.G.J.; Mumma, M.J. Alma detection and astrobiological potential of vinyl cyanide on titan. Sci. Adv. 2017, 3, e1700022. [Google Scholar] [CrossRef] [PubMed]
- Boehnhardt, H.; Bibring, J.-P.; Apathy, I.; Auster, H.U.; Finzi, A.E.; Goesmann, F.; Klingelhöfer, G.; Knapmeyer, M.; Kofman, W.; Krüger, H.; et al. The philae lander mission and science overview. Philos. Trans. A Math. Phys. Eng. Sci. 2017, 375, 20160248. [Google Scholar] [CrossRef] [PubMed]
- Altwegg, K.; Balsiger, H.; Bar-Nun, A.; Berthelier, J.-J.; Bieler, A.; Bochsler, P.; Briois, C.; Calmonte, U.; Combi, M.R.; Cottin, H.; et al. Prebiotic chemicals—Amino acid and phosphorus—In the coma of comet 67p/churyumov-gerasimenko. Sci. Adv. 2016, 2, e1600285. [Google Scholar] [CrossRef] [PubMed]
- Powner, M.W.; Sutherland, J.D. Prebiotic chemistry: A new modus operandi. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J.W.; Bartel, D.P.; Luisi, P.L. Synthesizing life. Nature 2001, 409, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Pace, N.R. The universal nature of biochemistry. Proc. Natl. Acad. Sci. USA 2001, 98, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.P.; Joyce, G.F. The origins of the RNA world. Cold Spring Harb. Perspect. Biol. 2012, 4, a003608. [Google Scholar] [CrossRef] [PubMed]
- Kruger, K.; Grabowski, P.J.; Zaug, A.J.; Sands, J.; Gottschling, D.E.; Cech, T.R. Self-splicing RNA: Autoexcision and autocyclization of the ribosomal RNA intervening sequence of tetrahymena. Cell 1982, 31, 147–157. [Google Scholar] [CrossRef]
- Guerrier-Takada, C.; Gardiner, K.; Marsh, T.; Pace, N.; Altman, S. The RNA moiety of ribonuclease p is the catalytic subunit of the enzyme. Cell 1983, 35, 849–857. [Google Scholar] [CrossRef]
- Miller, S.L. A production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Oro, J.; Kimball, A. Synthesis of adenine from ammonium cyanide. Biochem. Biophys. Res. Commun. 1960, 2, 407–412. [Google Scholar] [CrossRef]
- Zubay, G.; Mui, T. Prebiotic synthesis of nucleotides. Orig. Life Evol. Biosph. 2001, 31, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Butlerow, A. Bildung einer zuckerartigen substanz durch synthese. Liebigs Ann. Chem. 1861, 120, 295–298. [Google Scholar] [CrossRef]
- Breslow, R. On the mechanism of the formose reaction. Tetrahedron Lett. 1959, 1, 22–26. [Google Scholar] [CrossRef]
- Eckhardt, A.K.; Linden, M.M.; Wende, R.C.; Bernhardt, B.; Schreiner, P.R. Gas-phase sugar formation using hydroxymethylene as the reactive formaldehyde isomer. Nat. Chem. 2018, 10, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Weiss, A.H. Synthesis and utilization of formose sugars. Adv. Carbohydr. Chem. Biochem. 1974, 29, 173–227. [Google Scholar]
- Decker, P.; Schweer, H.; Pohlamnn, R. Bioids: X. Identification of formose sugars, presumable prebiotic metabolites, using capillary gas chromatography/gas chromatography—MAS spectrometry of n-butoxime trifluoroacetates on ov-225. J. Chromatogr. 1982, 244, 281–291. [Google Scholar] [CrossRef]
- Zubay, G. Studies on the lead-catalyzed synthesis of aldopentoses. Orig. Life Evol. Biosph. 1998, 28, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Usami, K.; Okamoto, A. Hydroxyapatite: Catalyst for a one-pot pentose formation. Org. Biomol. Chem. 2017, 15, 8888–8893. [Google Scholar] [CrossRef] [PubMed]
- Larralde, R.; Robertson, M.P.; Miller, S.L. Rates of decomposition of ribose and other sugars: Implications for chemical evolution. Proc. Natl. Acad. Sci. USA 1995, 92, 8158–8160. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, A.; Carrigan, M.A.; Olcott, A.N.; Benner, S.A. Borate minerals stabilize ribose. Science 2004, 303, 196. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Horiuchi, M.; Kakegawa, T. Selective stabilization of ribose by borate. Orig. Life Evol. Biosph. 2013, 43, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Ricardo, A.; Illangkoon, H.I.; Kim, M.J.; Carrigan, M.A.; Frye, F.; Benner, S.A. Synthesis of carbohydrates in mineral-guided prebiotic cycles. J. Am. Chem. Soc. 2011, 133, 9457–9468. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Pitsch, S.; Kittaka, A.; Wagner, E.; Wintner, C.E.; Eschenmoser, A.; Ohlofjgewidmet, G. Chemistry of α-aminonitriles. Aldomerisation of glycolaldehyde phosphate to RAC-hexose 2,4,6-triphosphates and (in presence of formaldehyde) RAC-pentose 2,4-diphosphates: RAC-allose 2,4,6-triphosphate and RAC-ribose 2,4-diphosphate are the main reaction products. Helv. Chim. Acta 1990, 73, 1410–1468. [Google Scholar]
- Ritson, D.; Sutherland, J.D. Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nat. Chem. 2012, 4, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, S.; Todd, Z.R.; Sutherland, J.D.; Sasselov, D.D. Sulfidic anion concentrations on early earth for surficial origins-of-life chemistry. Astrobiology 2018, 18, 1023–1040. [Google Scholar] [CrossRef] [PubMed]
- Ritson, D.; Battilocchio, C.; Ley, S.V.; Sutherland, J.D. Mimicking the surface and prebiotic chemistry of early earth using flow chemistry. Nat. Commun. 2018, 9, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ritson, D.J.; Ranjan, S.; Todd, Z.R.; Sasselov, D.D.; Sutherland, J.D. Photochemical reductive homologation of hydrogen cyanide using sulfite and ferrocyanide. ChemComm 2018, 54, 5566–5569. [Google Scholar] [CrossRef] [PubMed]
- Meiner, C.; Myrgorodska, I.; de Marcellus, P.; Buhse, T.; Nahon, L.; Hoffmann, S.V.; d’Hendecourt, L.S.; Meierhenrich, U.J. Ribose and related sugars from ultraviolet irradiation of interstellar ice analogs. Science 2016, 352, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.W.; Joosten, H.; Voet, A.B. Prebiotic adenine synthesis via HCN oligomerization in ice. Biosystems 1982, 15, 191–193. [Google Scholar] [CrossRef]
- Ferris, J.P.; Orgel, L.E. An unusual photochemical rearrangement in the synthesis of adenine from hydrogen cyanide. J. Am. Chem. Soc. 1966, 88, 1074. [Google Scholar] [CrossRef]
- Ferris, J.P.; Orgel, L.E. Aminomalononitrile and 4-amino-5-cyanoimidazole in hydrogen cyanide polymerization and adenine synthesis. J. Am. Chem. Soc. 1965, 87, 4976–4977. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Orgel, L.E. Studies in prebiotic synthesis. I. Aminomalononitrile and 4-amino-5-cyanoimidazole. J. Am. Chem. Soc. 1966, 88, 3829–3831. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.A.; Ferris, J.P.; Orgel, L.E. Studies in prebiotic synthesis. Iv. Conversion of 4-aminoimidazole-5-carbonitrile derivatives to purines. J. Mol. Biol. 1968, 38, 121–128. [Google Scholar] [CrossRef]
- Sanchez, R.; Ferris, J.; Orgel, L.E. Conditions for purine synthesis: Did prebiotic synthesis occur at low temperatures? Science 1966, 153, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, S.; Murasawa, K.-I.; Kobayashi, K.; Sawaoka, A.B. Abiotic synthesis of guanine with high-temperature plasma. Orig. Life Evol. Biosph. 2000, 30, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Sanchez, R.A.; Orgel, L.E. Studies in prebiotic synthesis: Iii. Synthesis of pyrimidines from cyanoacetylene and cyanate. J. Mol. Biol. 1968, 33, 693–704. [Google Scholar] [CrossRef]
- Ferris, J.P.; Zamek, O.S.; Altbuch, A.M.; Freiman, H. Chemical evolution. 18. Synthesis of pyrimidines from guanidine and cyanoacetaldehyde. J. Mol. Biol. 1974, 3, 301–309. [Google Scholar]
- Robertson, M.P.; Miller, S.L. An efficient prebiotic synthesis of cytosine and uracil. Nature 1995, 375, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.E.; Robertson, M.P.; Levy, M.; Miller, S.L. Concentration by evaporation and the prebiotic synthesis of cytosine. Orig. Life Evol. Biosph. 2001, 31, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.A.; Ferris, J.P.; Orgel, L.E. Cyanoacetylene in prebiotic synthesis. Science 1966, 154, 784–785. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E. Is cyanoacetylene prebiotic? Orig. Life Evol. Biosph. 2002, 32, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Menor-Salván, C.; Ruiz-Bermejo, D.M.; Guzmán, M.I.; Osuna-Esteban, S.; Veintemillas-Verdaguer, S. Synthesis of pyrimidines and triazines in ice: Implications for the prebiotic chemistry of nucleobases. Chemistry 2009, 15, 4411–4418. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.D. Ribonucleotides. Cold Spring Harb. Perspect. Biol. 2010, 2, a005439. [Google Scholar] [CrossRef] [PubMed]
- Fuller, W.D.; Sanchez, R.A.; Orgel, L.E. Studies in prebiotic synthesis. Vii solid-state synthesis of purine nucleosides. J. Mol. Evol. 1972, 1, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Thoma, I.; Deutsch, A.; Gehrke, T.; Mayer, P.; Zipse, H.; Carell, T. A high-yielding, strictly regioselective prebiotic purine nucleoside formation pathway. Science 2016, 352, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Schneider, C.; Okamura, H.; Crisp, A.; Amatov, T.; Dejmek, M.; Carell, T. Wet-dry cycles enable the parallel origin of canonical and non-canonical nucleosides by continuous synthesis. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Benner, S.A. Prebiotic stereoselective synthesis of purine and noncanonical pyrimidine nucleotide from nucleobases and phosphorylated carbohydrates. Proc. Natl. Acad. Sci. USA 2017, 114, 11315–11320. [Google Scholar] [CrossRef] [PubMed]
- Nam, I.; Nam, H.G.; Zareb, R.N. Abiotic synthesis of purine and pyrimidine ribonucleosides in aqueous microdroplets. Proc. Natl. Acad. Sci. USA 2018, 115, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, D.J.; Vaida, V. The influence of organic films at the air−aqueous boundary on atmospheric processes. Chem. Rev. 2006, 106, 1445–1461. [Google Scholar] [CrossRef] [PubMed]
- Kathmann, S.M.; Kuo, I.F.; Mundy, C.J. Electronic effects on the surface potential at the vapor-liquid interface of water. J. Am. Chem. Soc. 2008, 130, 16556–16561. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Strazewski, P. Bringing prebiotic nucleosides and nucleotides down to earth. Angew. Chem. Int. Ed. 2016, 55, 13930–13933. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.A.; Orgel, L.E. Studies in prebiotic synthesis. V. Synthesis and photoanomerization of pyrimidine nucleosides. J. Mol. Biol. 1970, 47, 531–543. [Google Scholar] [CrossRef]
- Powner, M.W.; Anastasi, C.; Crowe, M.A.; Parkes, A.L.; Raftery, J.; Sutherland, J.D. On the prebiotic synthesis of ribonucleotides: Photoanomerisation of cytosine nucleosides and nucleotides revisited. ChemBioChem 2007, 8, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-T.; Yang, N.C. Photochemistry of cytosine derivatives. 2. Photohydration of cytosine derivatives. Proton magnetic resonance study on the chemical structure and property of photohydrates. Biochemistry 1978, 17, 4877–4885. [Google Scholar] [CrossRef] [PubMed]
- Powner, M.W.; Gerland, B.; Sutherland, J.D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 2009, 459, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, C.M.; Nagyvary, J. Prebiotic formation of cytidine nucleotides. Nature 1971, 231, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, C.; Crowe, M.A.; Powner, M.W.; Sutherland, J.D. Direct assembly of nucleoside precursors from two- and three-carbon units. Angew. Chem. Int. Ed. 2006, 45, 6176–6179. [Google Scholar] [CrossRef] [PubMed]
- Springsteen, G.; Joyce, G.F. Selective derivatization and sequestration of ribose from a prebiotic mix. J. Am. Chem. Soc. 2004, 126, 9578–9583. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, A.F.; Deacon, A.; Harrison, R.G.; Osborne, D.J.; Prime, D.M.; Ross, W.J.; Todd, A.; Verge, J.P. An improved synthesis of 2-amino-1,3-oxazoles under basic catalysis. Synthesis 1976, 1976, 591–593. [Google Scholar] [CrossRef]
- Lohrmann, R.; Orgel, L.E. Prebiotic synthesis: Phosphorylation in aqueous solution. Science 1968, 161, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Powner, M.W.; Sutherland, J.D. Potentially prebiotic synthesis of pyrimidine β-d-ribonucleotides by photoanomerization/hydrolysis of α-d-cytidine-2′-phosphate. ChemBioChem 2008, 9, 2386–2387. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, C.; Grefenstette, N.M.; Powner, M.W. Selective aqueous acetylation controls the photoanomerization of α-cytidine-5′-phosphate. Chem. Comm. 2018, 54, 4850–4853. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tsanakopoulou, M.; Magnani, C.J.; Szabla, R.; Šponer, J.E.; Šponer, J.; Góra, R.W.; Sutherland, J.D. A prebiotically plausible synthesis of pyrimidine β-ribonucleosides and their phosphate derivatives involving photoanomerization. Nat. Chem. 2017, 9, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Powner, M.W.; Sutherland, J.D.; Szostak, J.W. Chemoselective multicomponent one-pot assembly of purine precursors in water. J. Am. Chem. Soc. 2010, 132, 16677–16688. [Google Scholar] [CrossRef] [PubMed]
- Stairs, S.; Nikmal, A.; Bučar, D.-K.; Zheng, S.-L.; Szostak, J.W.; Powner, M.W. Divergent prebiotic synthesis of pyrimidine and 8-oxo-purine ribonucleotides. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, H.; Miura, H.; Murata-Kamiya, N.; Ishikawa, H.; Sakaguchi, T.; Inoue, H.; Sasaki, T.; Masutanl, C.; Hanaoka, F.; Nishimura, S.; et al. 8-hydroxyadenine (7, 8-dihydro-8-oxoadenine) induces misincorporation in in vitro DNA synthesis and mutations in NIH 3t3 cells. Nucleic Acids Res. 1995, 23, 2893–2899. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L. Prebiotic chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Boil. 2004, 39, 99–123. [Google Scholar]
- Li, L.; Prywes, N.; Tam, C.P.; O’Flaherty, D.K.; Lelyveld, V.S.; Izgu, E.C.; Pal, A.; Szostak, J.W. Enhanced nonenzymatic RNA copying with 2-aminoimidazole activated nucleotides. J. Am. Chem. Soc. 2017, 139, 1810–1813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tam, C.P.; Zhou, L.; Soo Oh, S.; Wang, J.; Szostak, J.W. A structural rationale for the enhanced catalysis of nonenzymatic RNA primer extension by a downstream oligonucleotide. J. Am. Chem. Soc. 2018, 140, 2829–2840. [Google Scholar] [CrossRef] [PubMed]
- Jheeta, S.; Joshi, P.C. Prebiotic RNA synthesis by montmorillonite catalysis. Life 2014, 4, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Burcar, B.T.; Jawed, M.; Shah, H.; McGown, L.B. In situ imidazole activation of ribonucleotides for abiotic RNA oligomerization reactions. Orig. Life Evol. Biosph. 2014, 45, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Russell, D.A.; Javelle, T.; Sutherland, J.D. A light-releasable potentially prebiotic nucleotide activating agent. J. Am. Chem. Soc. 2018, 140, 8657–8661. [Google Scholar] [CrossRef] [PubMed]
- Joyce, G.F.; Szostak, J.W. Protocells and RNA self-replication. Cold Spring Harb. Perspect. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J.W. The eightfold path to non-enzymatic RNA replication. J. Syst. Chem. 2012, 3, 2. [Google Scholar] [CrossRef]
- Carter, C.W. What RNA world? Why a peptide/RNA partnership merits renewed experimental attention. Life 2015, 5, 294–320. [Google Scholar] [CrossRef] [PubMed]
- Wills, P.R.; Carter, C.W. Insuperable problems of the genetic code initially emerging in an RNA world. Biosystems 2018, 164, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Powner, M.W. Prebiotic systems chemistry: Complexity overcoming clutter. Chem 2017, 2, 470–501. [Google Scholar] [CrossRef]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biscans, A. Exploring the Emergence of RNA Nucleosides and Nucleotides on the Early Earth. Life 2018, 8, 57. https://doi.org/10.3390/life8040057
Biscans A. Exploring the Emergence of RNA Nucleosides and Nucleotides on the Early Earth. Life. 2018; 8(4):57. https://doi.org/10.3390/life8040057
Chicago/Turabian StyleBiscans, Annabelle. 2018. "Exploring the Emergence of RNA Nucleosides and Nucleotides on the Early Earth" Life 8, no. 4: 57. https://doi.org/10.3390/life8040057
APA StyleBiscans, A. (2018). Exploring the Emergence of RNA Nucleosides and Nucleotides on the Early Earth. Life, 8(4), 57. https://doi.org/10.3390/life8040057




