Ultraviolet Irradiation on a Pyrite Surface Improves Triglycine Adsorption
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
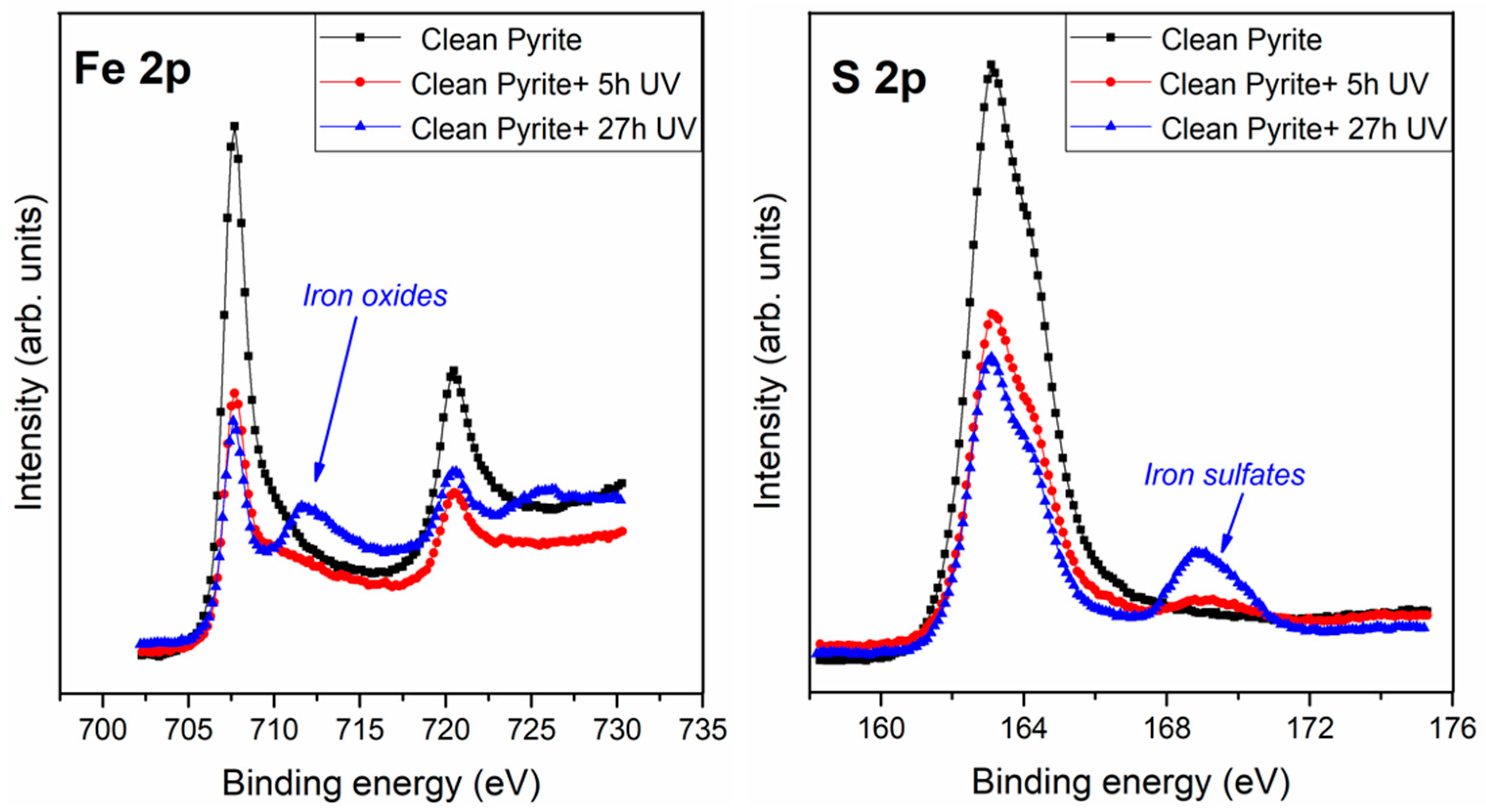
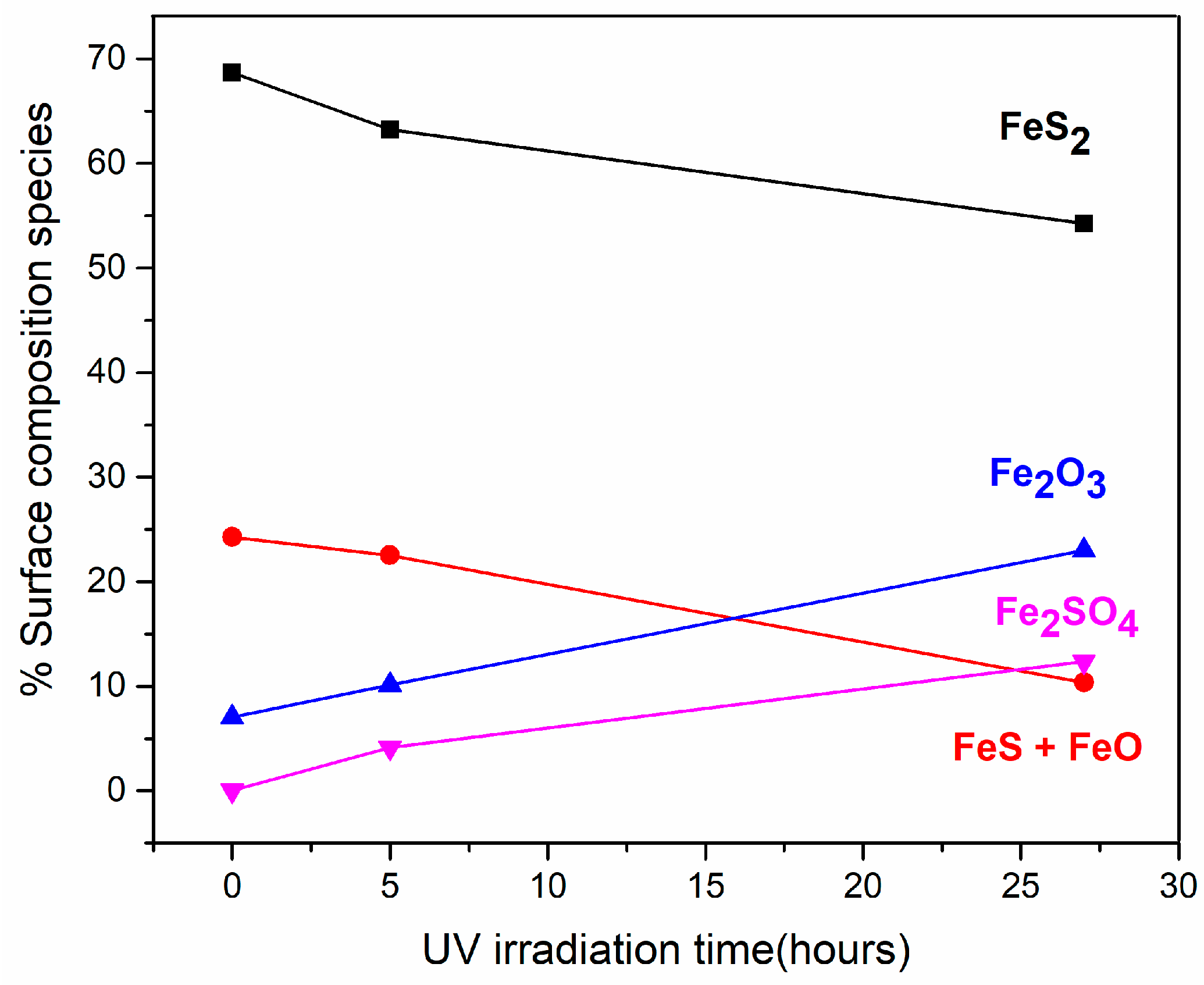
3.1. Effect of Ultraviolet (UV) Radiation on the Pyrite Surface
3.2. Interaction of Triglycine with Pyrite Surfaces under Several Conditions
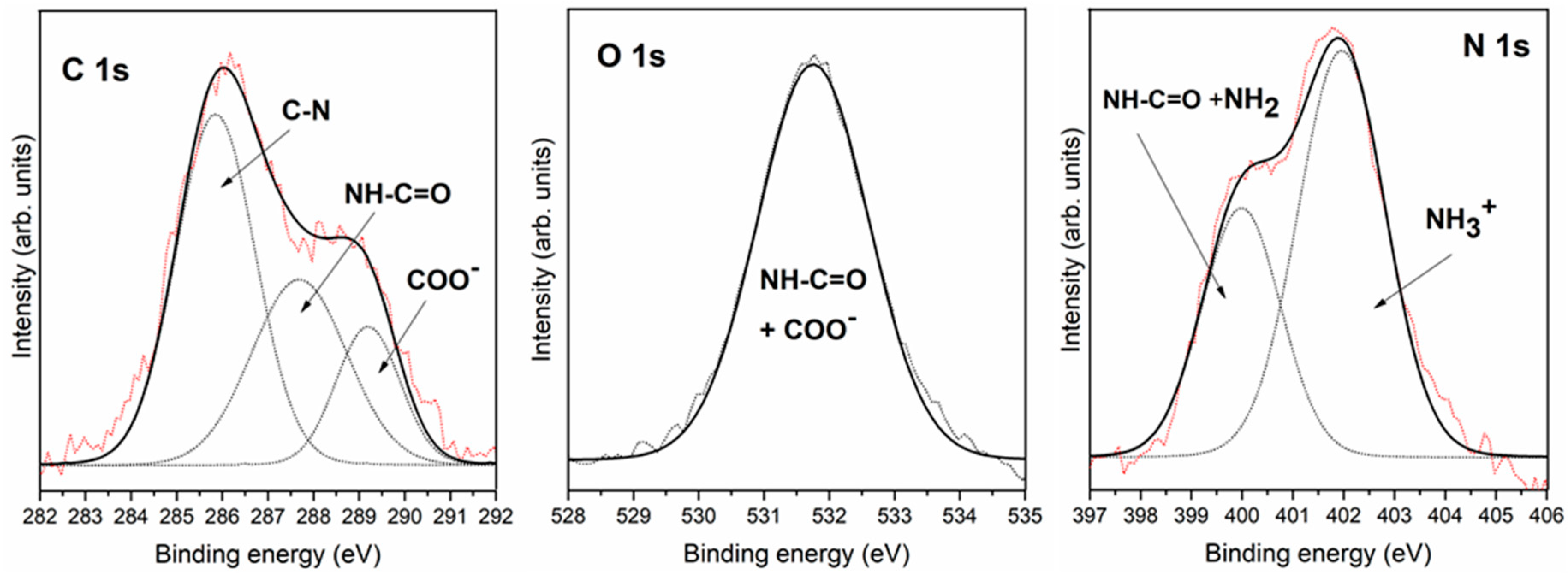
3.2.1. Interaction of Triglycine with Pyrite Surfaces by Molecular Evaporation Deposition under Ultra-High Vacuum Conditions
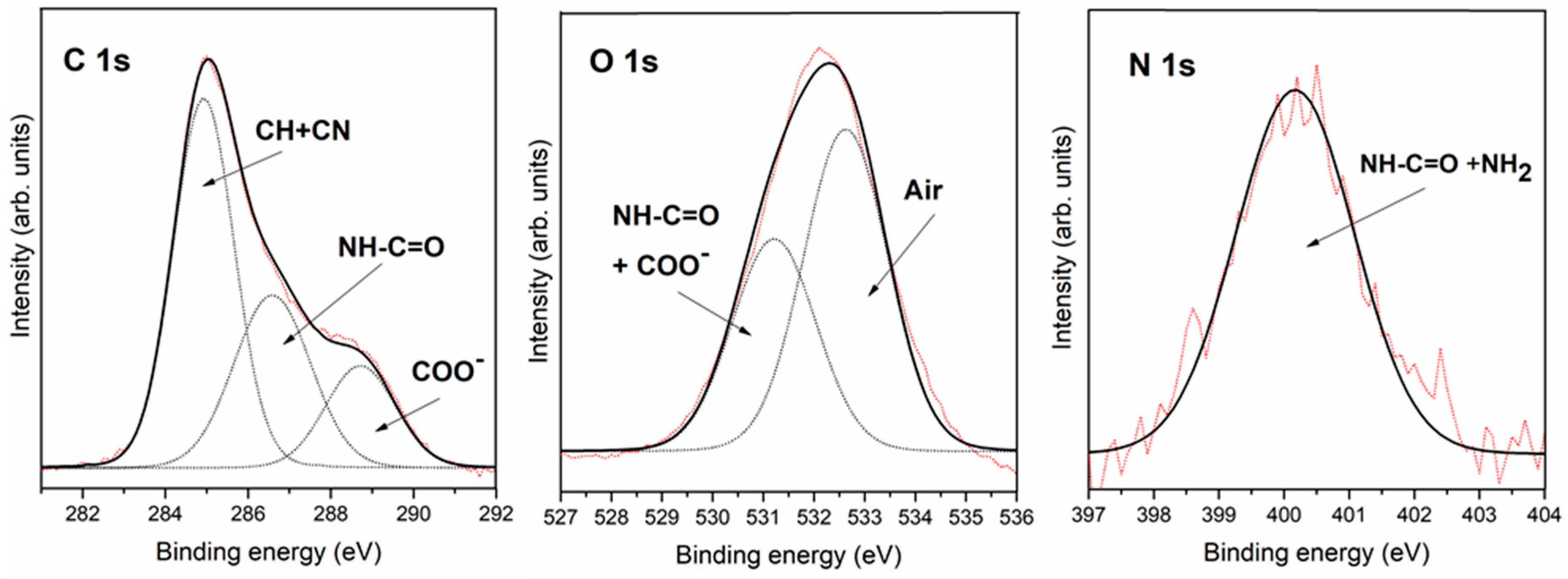
3.2.2. Interaction of Triglycine Solution Adsorption on the Pyrite Surface
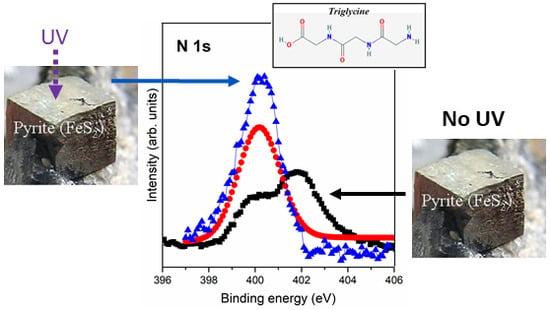
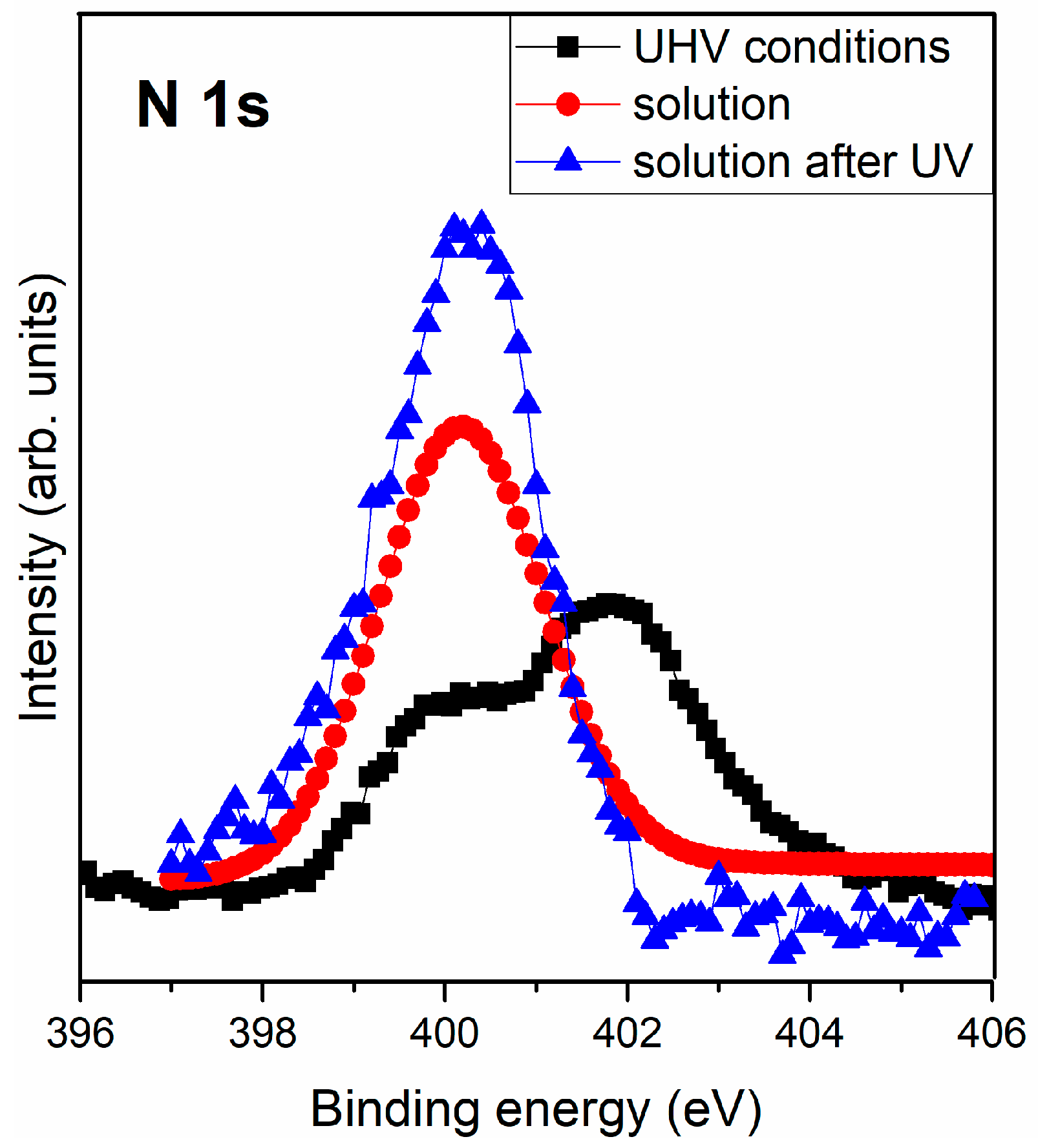
3.2.3. Interaction of Triglycine Solution Adsorption on the UV Oxidized Pyrite Surface
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cleaves, H.J., II; Scott, A.M.; Hill, F.C.; Leszczynski, J.; Sahai, N.; Hazen, R. Mineral-organic interfacial processes: Potential roles in the origins of life. Chem. Soc. Rev. 2012, 41, 5502–5525. [Google Scholar] [CrossRef] [PubMed]
- Colin-Garcia, M.; Heredia, A.; Negron-Mendoza, A.; Ramos-Bernal, S. Organics-Minerals Interactions and the Origin of Life; LPI Contribution: Houston, TX, USA, 2012. [Google Scholar]
- Bernal, J.D. The Physical Basis of Life; Routledge and Paul: London, UK, 1951. [Google Scholar]
- Schoonen, M.; Smirnov, A.; Cohn, C. A perspective on the Role of Minerals in Prebiotic Synthesis. Ambio 2004, 33, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.F. Adsorption and polymerization of amino acids on mineral surfaces: A review. Orig. Life Evol. Biospheres 2008, 38, 211–242. [Google Scholar] [CrossRef] [PubMed]
- Rosso, K.M.; Becker, U.; Hochella, M.F., Jr. Atomically resolved electronic structure of pyrite {100} surfaces: An experimental and theoretical investigation with implications for reactivity. Am. Mineral. 1999, 84, 1535–1548. [Google Scholar] [CrossRef]
- Liu, T.; Temprano, I.; Jenkins, S.J.; King, D.A.; Driver, S.M. Nitrogen adsorption and desorption at iron pyrite FeS2 100 surfaces. Phys. Chem. Chem. Phys. 2012, 14, 11491–11499. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Temprano, I.; Jenkins, S.J.; King, D.A.; Driver, S.M. Low Temperature Synthesis of NH3 from Atomic N and H at the surfaces of FeS2{100} Crystals. J. Phys. Chem. 2013, 117, 10990–10998. [Google Scholar] [CrossRef]
- Saladino, R.; Neri, V.; Crestini, C.; Costanzo, G.; Graciotti, M.; Di Mauro, E. Synthesis and degradation of nucleic acid components by formamide and iron sulfur minerals. J. Am. Chem. Soc. 2008, 130, 15512–15518. [Google Scholar] [CrossRef] [PubMed]
- Lahav, N.; Chang, S. The Possible Role of Solid Surface Area in Condensation Reactions during Chemical Evolution: Reevaluation. J. Mol. Evol. 1976, 8, 357–380. [Google Scholar] [CrossRef] [PubMed]
- Huber, C.; Wächtershäuser, G. Peptides by Activation of Amino Acids with CO on (Ni,Fe)S Surfaces: Implications for the Origin of Life. Science 1998, 281, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Hazen, R.M.; Sverjensky, D.A. Mineral surfaces, geochemical complexities, and the origins of life. Cold Spring Harb. Perspect. Boil. 2010, 2, a002162. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Hill, A.R., Jr.; Liu, R.; Orgel, L.E. Synthesis of long prebiotic oligomers on mineral surfaces. Nature 1996, 381, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Zaia, D.A.M. A review of adsorption of amino acids on minerals: Was it important for origin of life? Amino Acids 2004, 27, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Rode, B.M.; Son, H.L.; Suwannachot, Y.; Bujdak, J. The combination of salt induced peptide formation reaction and clay catalysis: A way to higher peptides under primitive Earth conditions. Orig. Life Evol. Biospheres 1999, 29, 273–286. [Google Scholar] [CrossRef]
- Fornaro, T.; Brucato, J.R.; Feuillie, C.; Sverjensky, D.A.; Hazen, R.M.; Brunetto, R.; D’Amore, M.; Barone, V. Binding of Nucleic Acid Components to the Serpentinite-hosted Hydrothermal Mineral Brucite. Astrobiology 2018, 18, 989–1007. [Google Scholar] [CrossRef] [PubMed]
- Powner, M.W.; Gerland, B.; Sutherland, J.D. Synthesis of activated pyrimide ribonucleotides in prebiotically plausible conditions. Nature 2009, 459, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Scappini, F.; Casadei, F.; Zamboni, R.; Franchi, M.; Gallori, E.; Monti, S. Protective effect of clay minerals on adsorbed nucleic acid against UV radiation: Possible role in the origin of life. Int. J. Astrobiol. 2004, 3, 17–19. [Google Scholar] [CrossRef]
- Degens, E.T. Perspectives on Biogeochemistry; Springer-Verlag: Berlin, Germany, 1989. [Google Scholar]
- Patel, M.R.; Bérces, A.; Kerékgyárto, T.; Rontó, Gy.; Lammer, H.; Zarnecki, J.C. Annual solar UV exposure and biological effective dose rates on the Martian surface. Adv. Space Res. 2004, 33, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.S. Ultraviolet radiation and the photobiology of earth’s oceans. Orig. Life Evol. Biospheres 2000, 30, 467–499. [Google Scholar] [CrossRef]
- Horneck, G. Complete Course in Astrobiology; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Dos Santos, R.; Patel, M.; Cuadros, J.; Martins, Z. Influence of mineralogy on the preservation of amino acids under simulated Mars conditions. Icarus 2016, 277, 342–353. [Google Scholar] [CrossRef]
- Fornaro, T.; Boosman, A.; Brucato, J.R.; ten Kate, I.L.; Siljeström, S.; Poggiali, G.; Steele, A.; Hazen, R.M. UV Irradiation of Biomarkers Adsorbed on Minerals under Martian-like Conditions: Hints for Life Detection on Mars. Icarus 2018, 313, 38–60. [Google Scholar] [CrossRef]
- Poch, O.; Jaber, M.; Stalport, F.; Nowak, S.; Georgelin, T.; Lambert, J.-F.; Szopa, C.; Coll, P. Effect of Nontronite Smectite Clay on the Chemical Evolution of Several Organic Molecules under Simulated Martian Surface Ultraviolet Radiation Conditions. Astrobiology 2015, 15, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Cleaves II, H.J.; Lazcano, A.; Ledesma Mateos, I.; Negrón-Mendoza, A.; Peretó, J.; Silva, E. Herrera’s Plasmogenia and Other Collected Works. Early Writting on the Experimental Study of the Origin of Life; Springer: Berlin, Germany, 2014. [Google Scholar]
- Eggleston, C.M.; Ehrhardt, J.J.; Stumm, W. Surface structural controls on pyrite oxidation kinetics: An XPS-UPS, STM, and modeling study. Am. Mineral. 1996, 81, 1036–1056. [Google Scholar] [CrossRef]
- Karthe, S.; Szargan, R.; Suoninen, E. Oxidation of pyrite surfaces: A photoelectron spectroscopic study. Appl. Surface Sci. 1993, 72, 157–170. [Google Scholar] [CrossRef]
- De la Cruz-López, A.; Del Ángel-Meraz, E.; Colín-García, M.; Ramos-Bernal, S.; Negrón-Mendoza, A.; Heredia, A. Ultraviolet irradiation of glycine in presence of pyrite as a model of chemical evolution: An experimental and molecular modelling approach. Int. J. Astrobiol. 2017, 16, 237–24321. [Google Scholar] [CrossRef]
- Sanchez-Arenillas, M.; Mateo-Marti, E. Spectroscopic study of cystine adsorption on pyrite surface: From vacuum to solution conditions. Chem. Phys. 2015, 458, 92–98. [Google Scholar] [CrossRef]
- Uvdal, K.; Bodö, P.; Ihs, A.; Lieberg, B.; Salaneck, W.R. X-ray photoelectron and infrared spectroscopy of clycine adsorbed upon copper. J. Colloid Interface Sci. 1990, 140, 207–216. [Google Scholar] [CrossRef]
- Uvdal, K.; Bodö, P.; Lieberg, B. L-cysteine adsorbed on gold and copper: An X-ray photoelectron spectroscopy study. J. Colloid Interface Sci. 1992, 149, 162–173. [Google Scholar] [CrossRef]
- Mateo-Marti, E.; Rogero, C.; Briones, C.; Martín-Gago, J.A. Does peptides nucleic acids on pyrite surface form self-assembled monolayers? Surf. Sci. 2007, 601, 4195–4199. [Google Scholar] [CrossRef]
- Sanchez-Arenillas, M.; Mateo-Marti, E. Pyrite surface environment drives molecular adsorption: Cystine on pyrite (100) investigated by X-ray photoemission spectroscopy and low energy electron diffraction. Phys. Chem. Chem. Phys. 2016, 18, 27219–27225. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Arenillas, M.; Galvez-Martinez, S.; Mateo-Marti, E. Sulfur amino acids and alanine on pyrite (100) by X-ray photoemission spectroscopy: Surface or molecular role? Appl. Surf. Sci. 2017, 414, 303–312. [Google Scholar] [CrossRef]
- Cavalleri, O.; Gonella, G.; Terreni, S.; Vignolo, M.; Floreano, L.; Morgante, A.; Canepa, M.; Rolandi, R. High resolution X-ray photoelectron spectroscopy of L-cysteine self-assembled films. Phys. Chem. Chem. Phys. 2004, 6, 4042–4046. [Google Scholar] [CrossRef]
- Gonella, G.; Terreni, S.; Cvetko, D.; Cossaro, A.; Mattera, L.; Cavalleri, O.; Rolandi, R.; Morgante, A.; Floreano, L.; Canepa, M. Ultrahigh vacuum deposition of L-cysteine on Au (110) studied by high-resolution X-ray photoemission: From early stages of adsorption to molecular organization. J. Phys. Chem. B 2005, 109, 18003–18009. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Papageorgiou, A.C.; Marschall, M.; Reichert, J.; Diller, K.; Klappenberger, F.; Allegretti, F.; Nefedov, A.; Wöll, C.; Barth, J.V. L-Cysteine on Ag (111): A combined STM and X-ray spectroscopy study of anchorage and deprotonation. J. Phys. Chem. C 2012, 116, 20356–20362. [Google Scholar] [CrossRef]
- Clark, D.T.; Peeling, J.; Colling, L. An experimental and theoretical investigation of the core level spectra of a series of amino acids, dipeptides and polypeptides. Biochim. Biophys. Acta 1976, 453, 533–545. [Google Scholar] [CrossRef]
- Andersson, K.; Nyberg, M.; Ogasawara, H.; Nordlund, D.; Kendelewicz, T.; Doyle, C.S.; Brown, G.E.; Pettersson, L.G.M., Jr.; Nilsson, A. Experimental and theoretical characterization of the structure of defects at the pyrite FeS2 (100) surface. Phys. Rev. B 2004, 70, 195404. [Google Scholar] [CrossRef]
- Andersson, K.J.; Ogasawara, H.; Nordlund, D.; Brown, G.E., Jr.; Nilsson, A. Preparation, Structure, and Orientation of Pyrite FeS2 {100} Surfaces: Anisotropy, Sulfur Monomers, Dimer Vacancies, and a Possible FeS Surface Phase. J. Phys. Chem. C 2014, 118, 21896–21903. [Google Scholar] [CrossRef]
| Clean Pyrite | Clean Pyrite + 5 h UV | Clean Pyrite + 27 h UV | Assignment | ||||
|---|---|---|---|---|---|---|---|
| BE (eV) | % | BE (eV) | % | BE (eV) | % | ||
| Fe 2p | 707.3 | 69 | 707.3 | 63 | 707.3 | 54 | Fe2+ (FeS2) |
| 708.9 | 24 | 709.1 | 23 | 708.9 | 10 | Fe2+ (FeS + FeO) | |
| 711.1 | 7 | 711.1 | 10 | 711.1 | 23 | Fe3+ (Fe2O3) | |
| 712.9 | 4 | 712.9 | 12 | Fe3+ (Fe2SO4) | |||
| S 2p | 161.8 | 11 | 161.9 | 10 | 161.9 | 6 | S2− (FeS) |
| 162.8 | 77 | 162.8 | 71 | 162.8 | 60 | S22− (FeS2) | |
| 164.7 | 12 | 164.6 | 13 | 164.6 | 12 | Sn2− (2 ≤ n ≤ 8) | |
| 168.5 | 6 | 168.5 | 22 | SO24− (sulfate) | |||
| UHV Conditions | Solution | Solution after UV | ||||
|---|---|---|---|---|---|---|
| BE (eV) | Assignment | BE (eV) | Assignment | BE (eV) | Assignment | |
| C 1s | 285.8 | C-N | 284.9 | C-H + air | 284.9 | C-H + air |
| 287.7 | -NH-CO- | 286.6 | C-N + -NH-CO- | 286.5 | C-N + -NH-CO- | |
| 289.2 | COOH or COO− | 288.7 | COOH or COO− | 288.5 | COOH or COO− | |
| O 1s | 531.8 | COO− + -NH-CO- | 531.2 | COO− + -NH-CO- | 531.4 | COO− + -NH-CO- |
| 532.2 | air | 532.5 | air | |||
| N 1s | 400.0 | NH2 + -NH-CO- | 400.2 | NH2 + -NH-CO- | 399.8 | NH2 + -NH-CO- |
| 402.0 | NH3+ | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvez-Martinez, S.; Mateo-Marti, E. Ultraviolet Irradiation on a Pyrite Surface Improves Triglycine Adsorption. Life 2018, 8, 50. https://doi.org/10.3390/life8040050
Galvez-Martinez S, Mateo-Marti E. Ultraviolet Irradiation on a Pyrite Surface Improves Triglycine Adsorption. Life. 2018; 8(4):50. https://doi.org/10.3390/life8040050
Chicago/Turabian StyleGalvez-Martinez, Santos, and Eva Mateo-Marti. 2018. "Ultraviolet Irradiation on a Pyrite Surface Improves Triglycine Adsorption" Life 8, no. 4: 50. https://doi.org/10.3390/life8040050
APA StyleGalvez-Martinez, S., & Mateo-Marti, E. (2018). Ultraviolet Irradiation on a Pyrite Surface Improves Triglycine Adsorption. Life, 8(4), 50. https://doi.org/10.3390/life8040050