Testing New Concepts for Crop Cultivation in Space: Effects of Rooting Volume and Nitrogen Availability
Abstract
1. Introduction
- (i)
- The effect of a restricted rooting volume was studied by comparing a small (0.6 L) and a large (3.5 L) root container. We hypothesize that as longs as the conditions in both containers are similar there will be no effect of the root container.
- (ii)
- The effects of a limited amount of nutrient solution were tested by comparing a 3.4 L nutrient solution for the cultivation of two lettuce heads to plants which receive an unlimited supply of fresh nutrient solution. We hypothesize that plants in both systems will be similar.
- (iii)
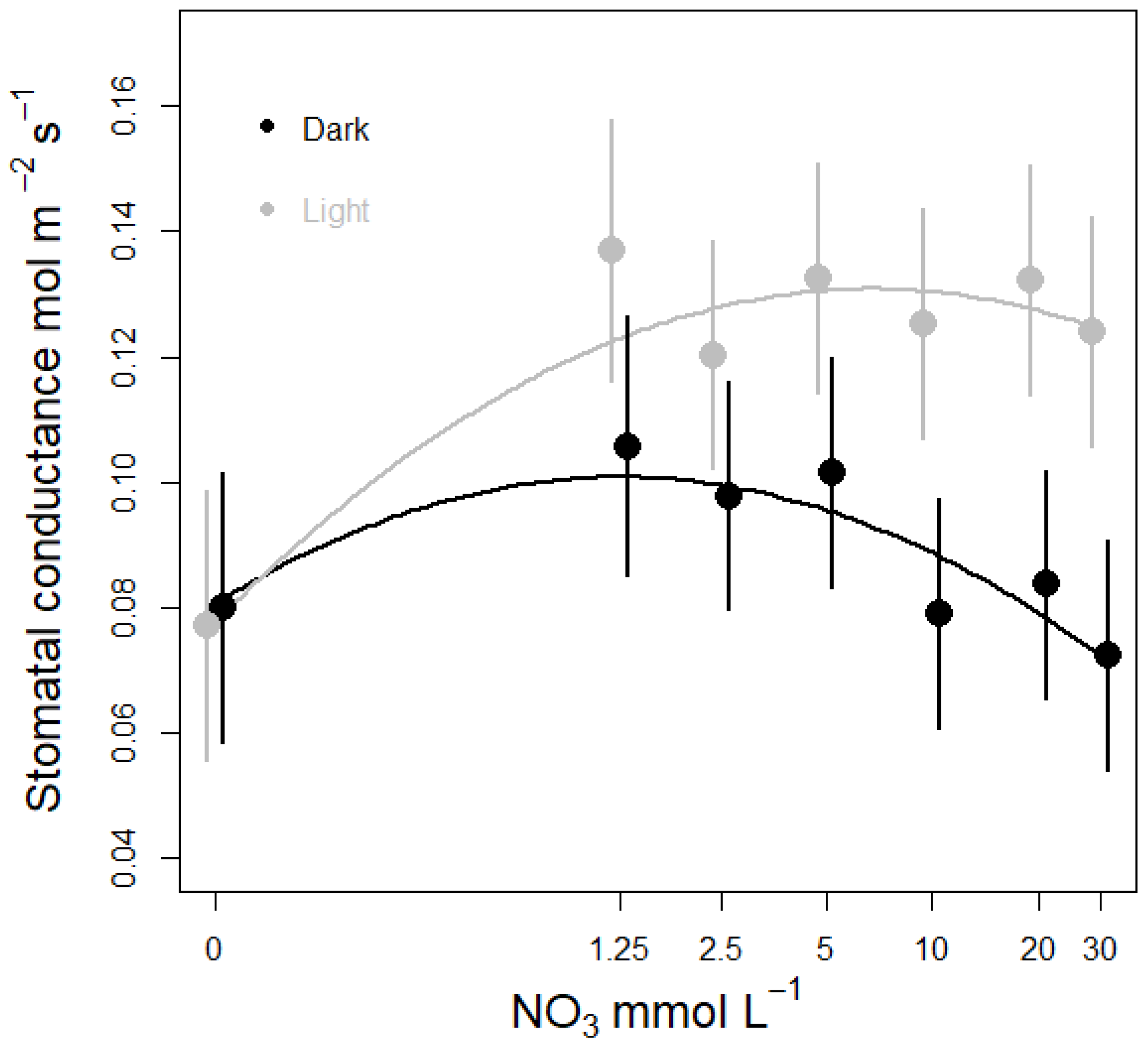
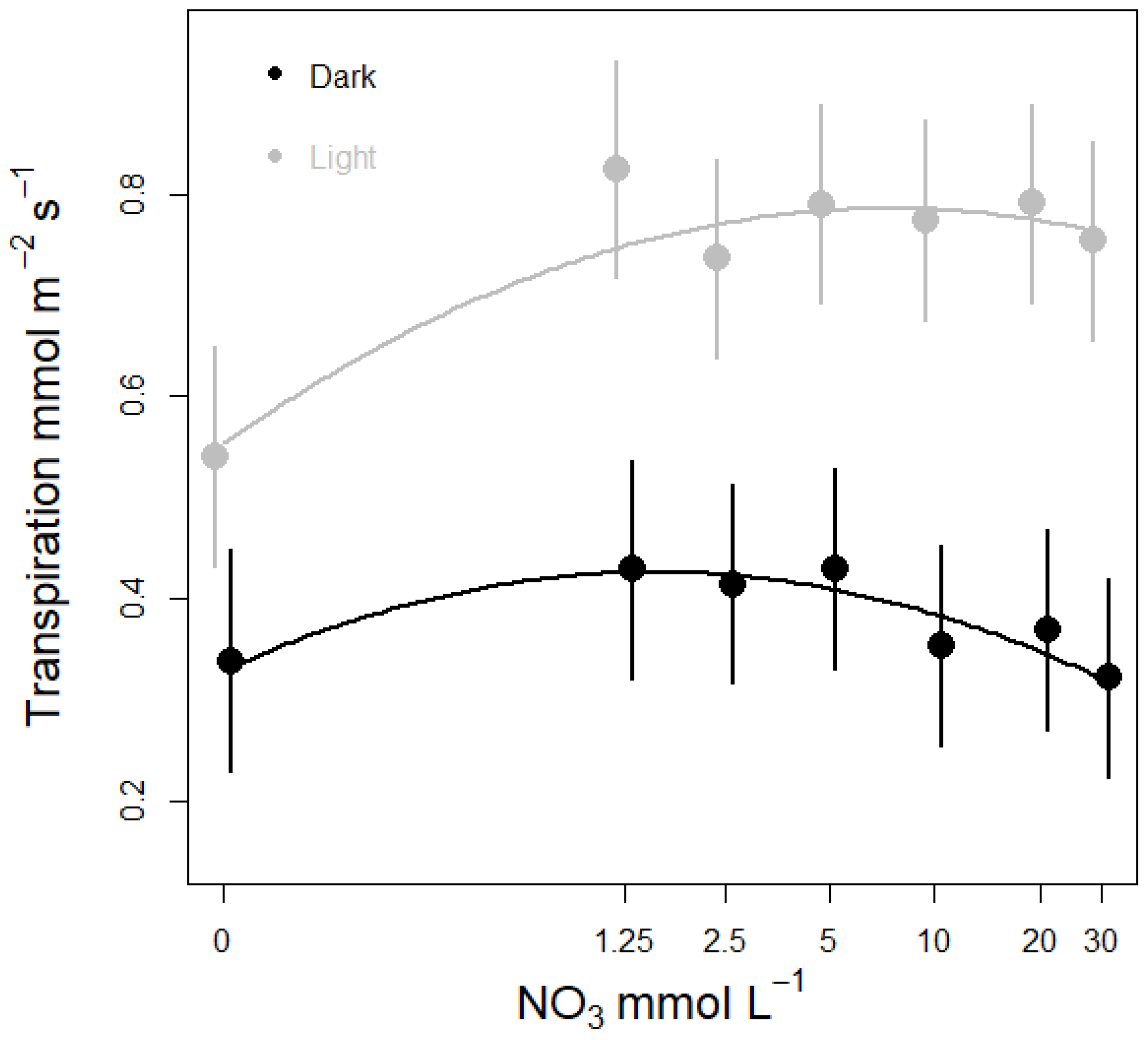
- The effects of nitrate concentration on stomatal conductance, transpiration and nitrate uptake in intact lettuce was studied by growing plants on different nitrate concentrations; causing growth limitation but no morphological deficiency symptoms. To look for variations throughout the diel cycle, conductance and transpiration was measured during both dark and light conditions. We hypothesize that nitrate concentration has a regulating effect on plant water fluxes and that the relation between nitrate concentration and transpiration can be represented by a “bell curve” as described by Wilkinson, Bacon and Davies [16]. That is, when nitrate is supplied in a concentration range between 0 and 30 mM plant responses will gradually increase until reaching an “optimum concentration” at which transpiration peaks and then declines as nitrate concentrations becomes supra optimal.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
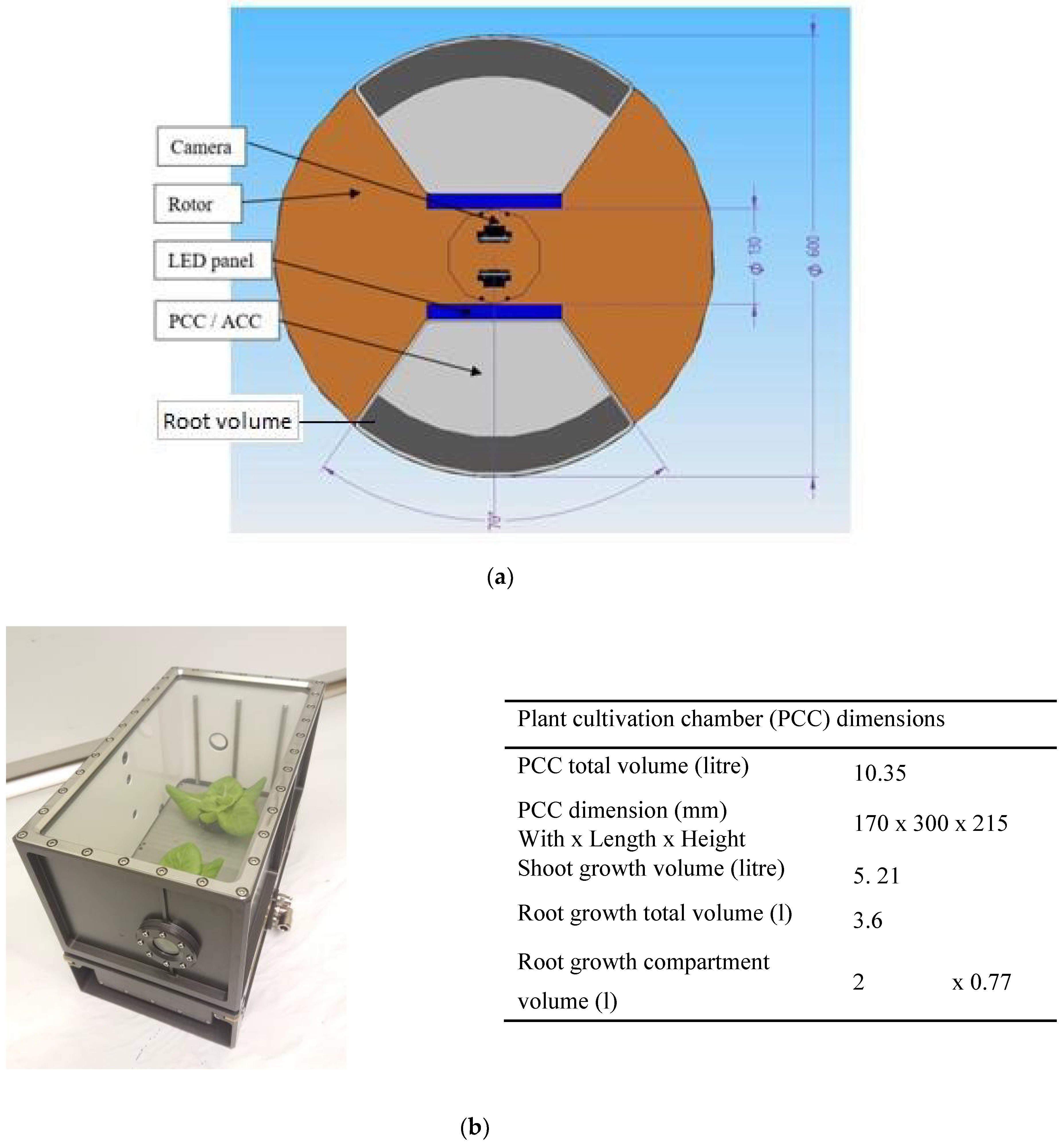
2.2. The Effects of a Restricted Rooting- and Nutrient Solution Volume
2.3. Plant Responses to Various Nitrate Concentrations
2.3.1. Nutrient solution formulation for nitrate treatments
2.3.2. Stomatal Conductance and Transpiration Rate Measurements
2.3.3. Statistical Set-Up and Analysis
3. Results and Discussion
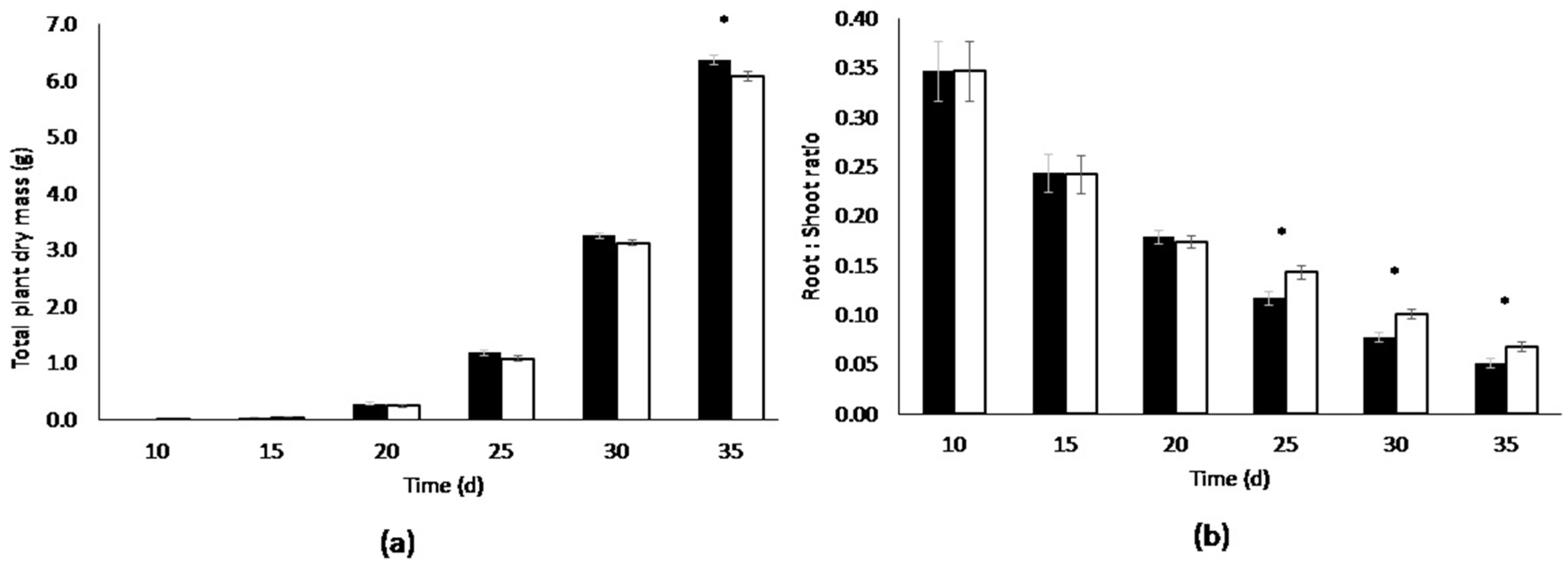
3.1. Root Volume Experiments
3.2. Nutrient Solution Volume Experiments
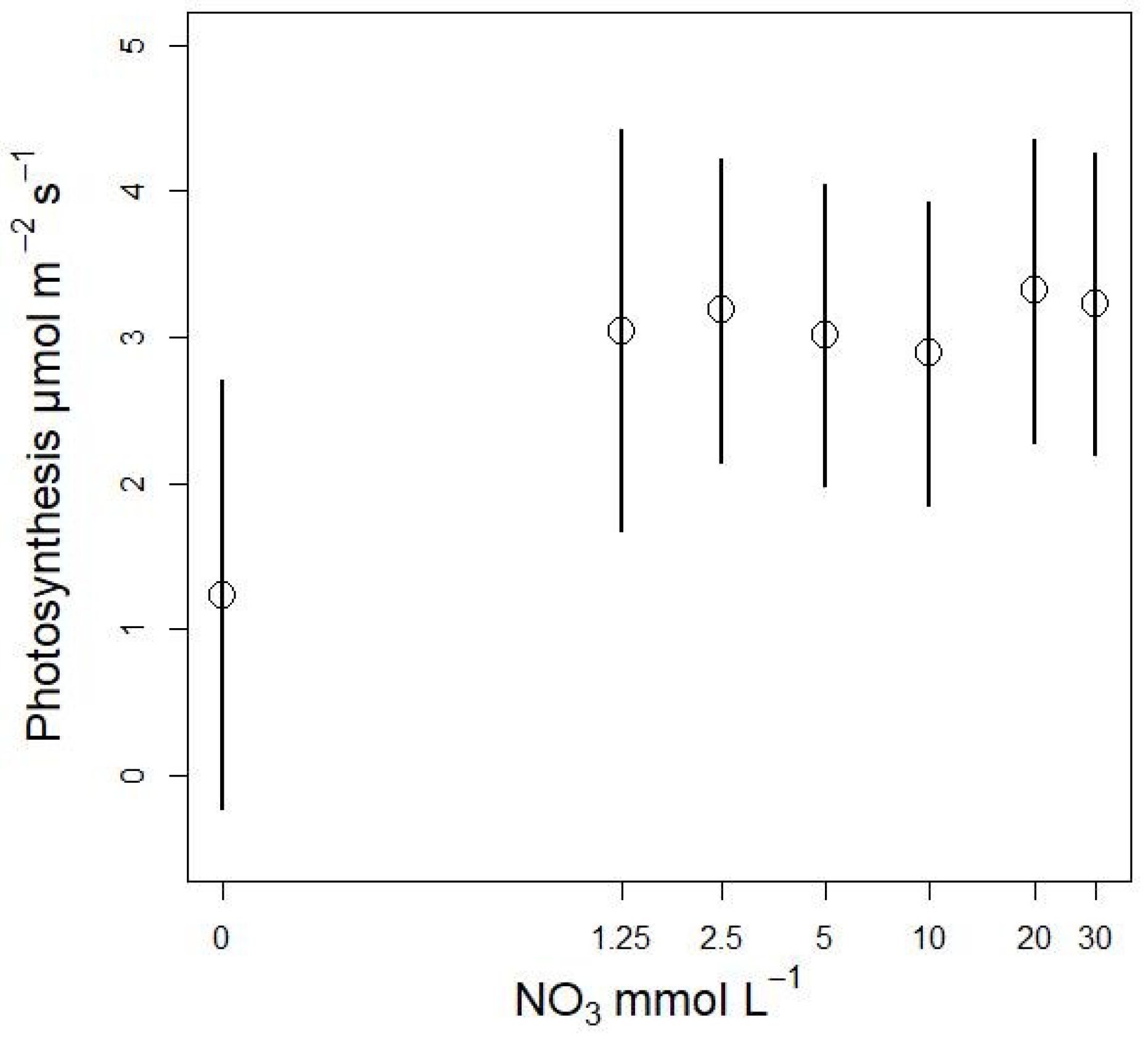
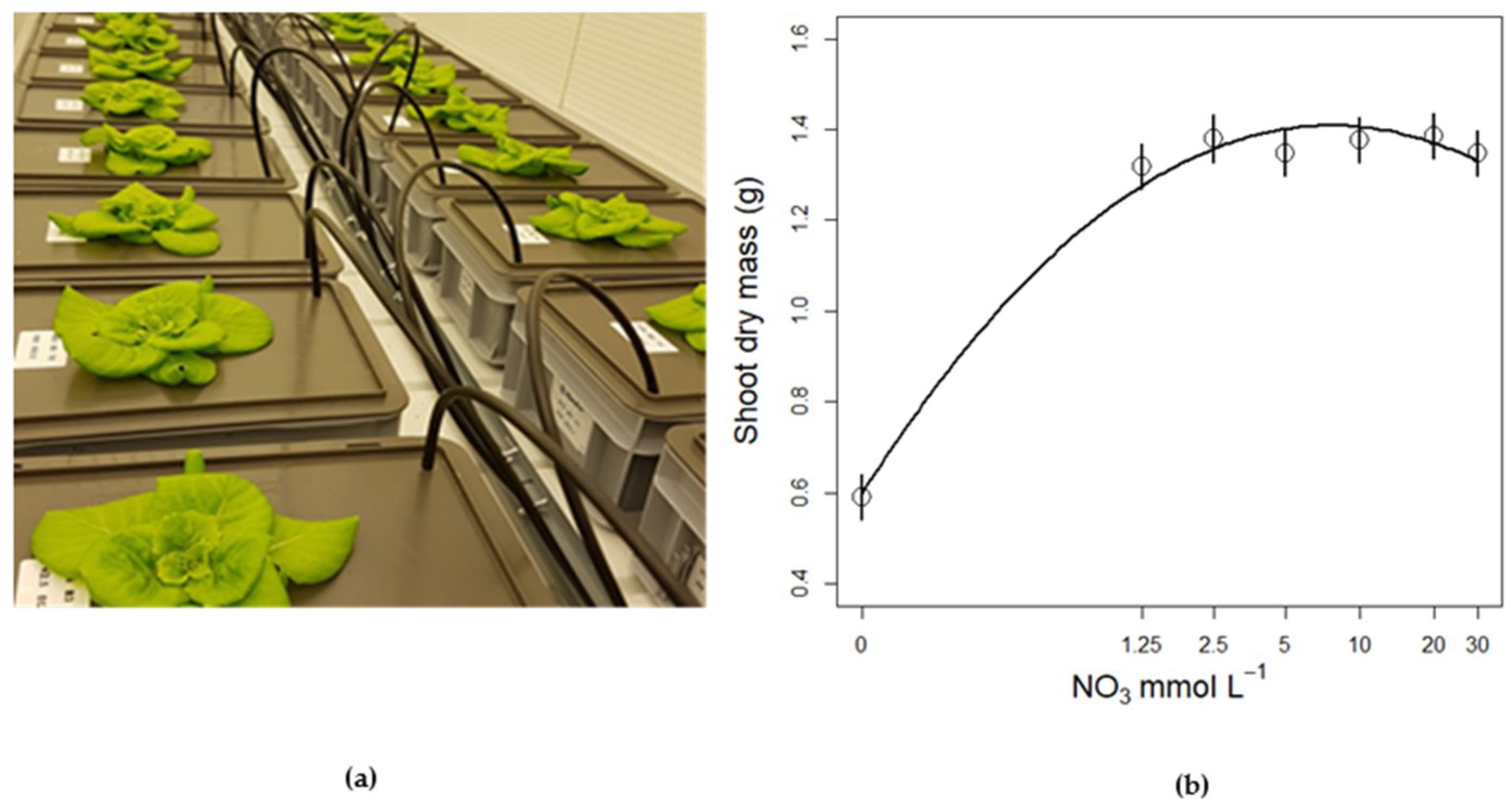
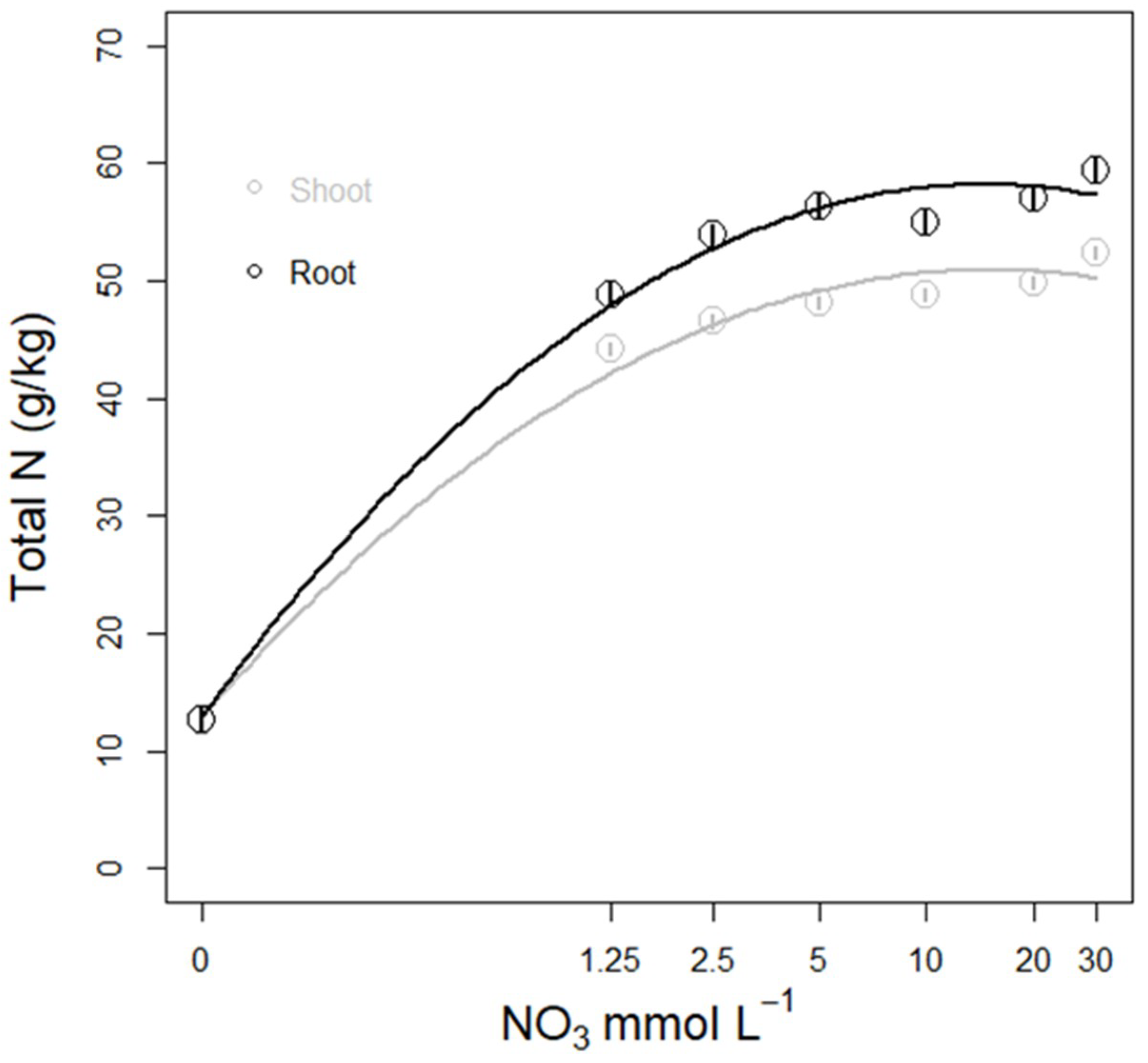
3.3. Nitrate Concentration Experiments
3.3.1. Effects on Stomatal Conductance and Transpiration
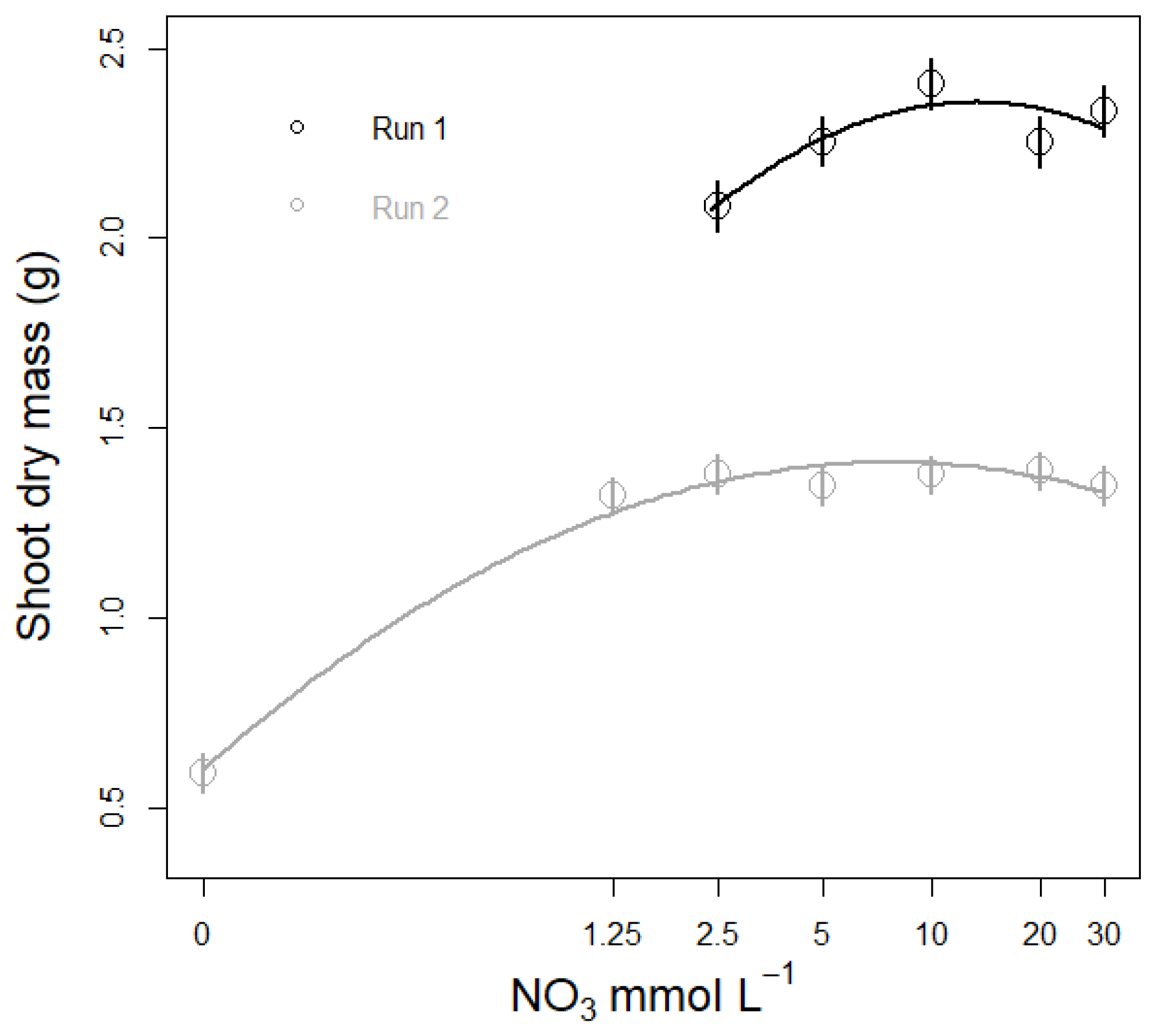
3.3.2. Plant Biomass Response to NO3 Concentration
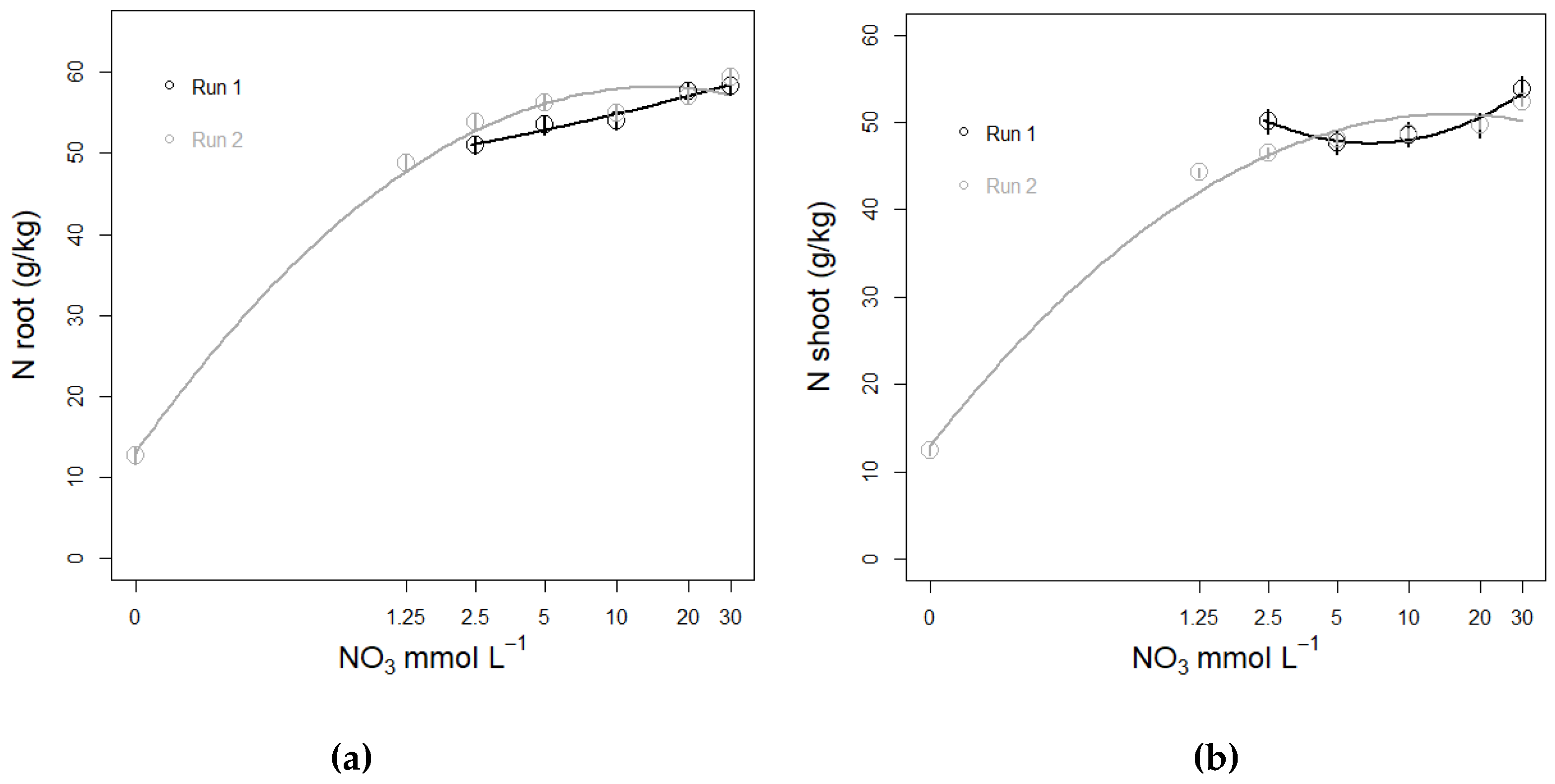
3.3.3. N Amount in Plant Tissue
3.4. Relevance for Future Crop Cultivation in Space
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| N-NO₃ Treatment | P-H₂PO₄ | K | Ca | Mg | S-SO₄ | Cl | Si |
| 30.00 | 1.50 | 13.90 | 7.00 | 2.30 | 0.50 | 0.00 | 0.50 |
| 20.00 | 1.50 | 13.90 | 7.00 | 2.30 | 3.50 | 4.00 | 0.50 |
| 10.00 | 1.50 | 13.90 | 7.00 | 2.30 | 7.00 | 7.00 | 0.50 |
| 5.00 | 1.50 | 13.90 | 7.00 | 2.30 | 8.63 | 8.75 | 0.50 |
| 2.50 | 1.50 | 13.90 | 7.00 | 2.30 | 9.50 | 9.50 | 0.50 |
| 1.25 | 1.50 | 13.90 | 7.00 | 2.30 | 9.88 | 10.00 | 0.50 |
| 0.00 | 1.50 | 13.90 | 7.00 | 2.30 | 10.33 | 10.35 | 0.50 |
| Model Rank | Run | NO3 | NO3^2 | Light | Run:NO3 | NO3:Light | NO3^2:Light | AIC | ΔAIC | Loglik |
|---|---|---|---|---|---|---|---|---|---|---|
| (a) Conductance | ||||||||||
| 1 | + | + | + | + | + | -622.4 | 0.00 | 320.76 | ||
| 2 | + | + | + | + | + | -620.3 | 2.08 | 320.85 | ||
| 3 | + | + | + | + | + | + | -620.2 | 2.19 | 320.79 | |
| 4 | + | + | + | + | + | + | -620.1 | 2.25 | 320.77 | |
| 5 | + | + | + | + | + | + | -618.7 | 3.70 | 321.18 | |
| (b) Transpiration | ||||||||||
| 1 | + | + | + | + | + | -119.5 | 0.00 | 69.32 | ||
| 2 | + | + | + | + | + | + | -117.5 | 2.05 | 69.42 | |
| 3 | + | + | + | + | + | -117.4 | 2.14 | 69.38 | ||
| 4 | + | + | + | + | + | + | -117.3 | 2.18 | 69.36 | |
| 5 | + | + | + | + | + | + | + | -116.0 | 3.54 | 69.82 |
| Days After Sowing (d) | Container.volume (L) | Root Dry Mass(g) [dry weight] | Shoot Dry Mass(g) [dry weight] | Total Dry Mass (g) [Plant dry weight] | Root:Shoot Ratio | Leaf Area (cm2) | Specific Leaf Area (cm2·g−1) | Biomass (%) # |
|---|---|---|---|---|---|---|---|---|
| 10 | 3.5 | 0.00218 ± 0.00 | 0.00670 ± 0.00 | 0.0089 ± 0.00 | 0.347 ± 0.01 | 3.08 ± 0.10 | 356 ± 10.7 | 8.30 ± 0.25 |
| 0.6 | 0.00218 ± 0.00 | 0.00670 ± 0.00 | 0.0089 ± 0.00 | 0.347 ± 0.01 | 3.08 ± 0.10 | 356 ± 10.7 | 8.30 ± 0.25 | |
| 15 | 3.5 | 0.00935 ± 0.00 | 0.0393 ± 0.00 | 0.0486 ± 0.00 | 0.243 ± 0.02 | 18.1 ± 0.27 | 373 ± 8.23 | 6.48 ± 0.15 |
| 0.6 | 0.00868 ± 0.00 | 0.0366 ± 0.00 | 0.0453 ± 0.00 | 0.242 ± 0.01 | 17.2 ± 0.28 | 385 ± 13.8 | 6.61 ± 0.32 | |
| 20 | 3.5 | 0.0424 ± 0.00 | 0.240 ± 0.02 | 0.283 ± 0.02 | 0.179 ± 0.00 | 113 ± 3.93 | 409 ± 12.3 | 5.91 ± 0.10 |
| 0.6 | 0.0363 ± 0.00 * | 0.214 ± 0.02 | 0.250 ± 0.02 | 0.174 ± 0.01 | 102 ± 5.52 | 414 ± 9.85 | 5.88 ± 0.07 | |
| 25 | 3.5 | 0.124 ± 0.00 | 1.07 ± 0.02 | 1.19 ± 0.02 | 0.117 ± 0.00 | 423 ± 6.88 | 356 ± 6.89 | 5.30 ± 0.11 |
| 0.6 | 0.133 ± 0.00 | 0.94 ± 0.04 | 1.07 ± 0.04 | 0.143 ± 0.00 * | 389 ± 6.00 | 367 ± 10.8 | 5.48 ± 0.09 | |
| 30 | 3.5 | 0.233 ± 0.00 | 3.02 ± 0.02 | 3.25 ± 0.01 | 0.0774 ± 0.00 | 984 ± 12.9 | 302 ± 3.83 | 5.12 ± 0.11 |
| 0.6 | 0.284 ± 0.00 | 2.84 ± 0.05 | 3.13 ± 0.05 | 0.1001 ± 0.00 * | 982 ± 15.3 | 314 ± 2.89 | 4.98 ± 0.03 | |
| 35 | 3.5 | 0.310 ± 0.02 | 6.06 ± 0.05 | 6.37 ± 0.03 | 0.0514 ± 0.00 | 1857 ± 35.5 | 291 ± 4.53 | 4.58 ± 0.03 |
| 0.6 | 0.386 ± 0.01 * | 5.69 ± 0.06 * | 6.07 ± 0.06 | 0.0680 ± 0.00 * | 1698 ± 40.0 * | 279 ± 4.04 | 4.86 ± 0.05 |
| Model rank | Run | [NO3] | [NO3]^2 | Run:[NO3] | Run:[NO3]^2 | AIC | ΔAIC | Loglik |
|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | + | + | −93.9 | 0.00 | 55.89 |
| 2 | + | + | + | + | −93.3 | 0.61 | 54.36 | |
| 3 | + | + | + | + | −90.8 | 3.06 | 53.13 | |
| 4 | + | + | + | −85.1 | 8.76 | 49.09 |
| Shoot Dry Mass (g) | ||
|---|---|---|
| Fixed effects: | β±SE | p-value |
| Intercept | 1.23±0.043 | <0.001 |
| Run 1 | 0.45±016 | 0.005 |
| N concentration | 0.17±0.001 | <0.001 |
| N concentration ^2 | −0.043±0.005 | <0.001 |
| Run 1: N conc. | 0.35±0.15 | 0.023 |
| Run 1: N conc.^2 | −006±0.034 | 0.009 |
| Random effects: | SD | n |
| Block | 0.010 | 14 |
| Residual | 0.11 | |
| N Shoot (g) | N Root (g) | |||
|---|---|---|---|---|
| Fixed effects: | β ± SE | p-value | β ± SE | p-value |
| Intercept | 39.79 ± 0.66 | <0.001 | 45.10 ± 0.69 | <0.001 |
| Run 1 | 17.69 ± 3.17 | <0.001 | 3.93 ± 3.81 | 0.305 |
| N concentration | 8.18 ± 0.22 | <0.001 | 9.74 ± 0.27 | <0.001 |
| N concentration ∧2 | −1.50 ± 0.097 | <0.001 | −1.81 ± 0.12 | <0.001 |
| Run 1: N conc. | −18.43 ± 3.15 | <0.001 | −7.79 ± 3.83 | 0.046 |
| Run 1 : N conc. ^2 | 4.16 ± 0.71 | <0.001 | 2.053 ± 0.87 | 0.021 |
| Random effects: | SD | n | SD | n |
| Block | 1.156 | 14 | 0.823 | 14 |
| Residual | 2.367 | 2.874 |

References
- Clauwaert, P.; Muys, M.; Alloul, A.; De Paepe, J.; Luther, A.; Sun, X.; Ilgrande, C.; Christiaens, M.E.R.; Hu, X.; Zhang, D.; et al. Nitrogen cycling in bioregenerative life support systems: Challenges for waste refinery and food production processes. Prog. Aerosp. Sci. 2017, 91, 87–98. [Google Scholar] [CrossRef]
- Paradiso, R.; De Micco, V.; Buonomo, R.; Aronne, G.; Barbieri, G.; De Pascale, S. Soilless cultivation of soybean for bioregenerative life-support systems: A literature review and the experience of the melissa project - food characterisation phase i. Plant Biol. 2014, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.A.; Coelho, L.H.; Zabrodina, M.; Brinckmann, E.; Kittang, A.I. Plant mineral nutrition, gas exchange and photosynthesis in space: A review. Adv. Space Res. 2013, 51, 465–475. [Google Scholar] [CrossRef]
- Wheeler, R.M.; Stutte, G.W.; Sobarrao, G.V.; Yorio, N.C. Plant growth and human life support for space travel. In Handbook of Plant and Crop Physiology; Pessarakli, M., Ed.; Marcel Dekker: New York, NY, USA, 2001; pp. 925–941. [Google Scholar]
- Wheeler, R.M. Agriculture for space: People and places paving the way. Open Agric. 2017, 2, 14–32. [Google Scholar] [CrossRef]
- Wolff, S.A.; Coelho, L.; Karoliussen, I.; Jost, A.I.K. Effects of the extraterrestrial environment on plants: Recommendations for future space experiments for the melissa higher plant compartment. Life 2014, 4, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.A.; Coelho, L.; Hauan, T.M.; Kittang Jost, A.I.; Aronne, G. Water Management in Space-Examples of Hydration Systems for Cultivation in Microgravity and Future Prospects; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2018; pp. 45–52. [Google Scholar]
- Kittang, A.I.; Iversen, T.H.; Fossum, K.R.; Mazars, C.; Carnero-Diaz, E.; Boucheron-Dubuisson, E.; Le Disquet, I.; Legué, V.; Herranz, R.; Pereda-Loth, V.; et al. Exploration of plant growth and development using the european modular cultivation system facility on the international space station. Plant Biol. 2014, 16, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Zabel, P.; Bamsey, M.; Schubert, D.; Tajmar, M. Review and analysis of over 40 years of space plant growth systems. Life Sci. Space Res. 2016, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gisela, D.; Belz, S.; Bretschneider, J.; Ewald, R.; Fasoulas, S. A hybrid life support system for a moon base. In 68th International Astronautical Congress (IAC); International Astronautical Congress: Adelaide, Australia, 2017. [Google Scholar]
- Gisela, D.; Belz, S.; Bretschneider, J.; Jost, A.I.K.; Jakobsen, Ø.M. Design of a test platform for algae cultivation research at different gravitation levels. In Proceedings of the 48th International Conference on Environmental Systems, Albuquerque, New Mexico, 8–12 July 2018. [Google Scholar]
- Graham, T.; Wheeler, R. Root restriction: A tool for improving volume utilization efficiency in bioregenerative life-support systems. Life Sci. Space Res. 2016, 9, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Forde, B.; Lorenzo, H. The nutritional control of root development. Plant Soil 2001, 232, 51–68. [Google Scholar] [CrossRef]
- Cramer, M.D.; Hawkins, H.J.; Verboom, G.A. The importance of nutritional regulation of plant water flux. Oecologia 2009, 161, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Matimati, I.; Verboom, G.A.; Cramer, M.D. Nitrogen regulation of transpiration controls mass-flow acquisition of nutrients. J. Exp. Bot. 2014, 65, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Bacon, M.A.; Davies, W.J. Nitrate signalling to stomata and growing leaves: Interactions with soil drying, aba, and xylem sap ph in maize. J. Exp. Bot. 2007, 58, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Kupper, P.; Rohula, G.; Saksing, L.; Sellin, A.; Lohmus, K.; Ostonen, I.; Helmisaari, H.S.; Sober, A. Does soil nutrient availability influence night-time water flux of aspen saplings? Environ. Exp. Bot. 2012, 82, 37–42. [Google Scholar] [CrossRef]
- Porterfield, D.M. The biophysical limitations in physiological transport and exchange in plants grown in microgravity. J. Plant Growth Regul. 2002, 21, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Tibbitts, T.W. Humidity and plants. Bioscience 1979, 29, 358–363. [Google Scholar] [CrossRef]
- Conroy, J.; Hocking, P. Nitrogen nutrition of C-3 plants at elevated atmospheric CO2 concentrations. Physiol. Plantarum. 1993, 89, 570–576. [Google Scholar] [CrossRef]
- McGrath, J.M.; Lobell, D.B. Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant Cell Environ. 2013, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Kudoyarova, G.R.; Dodd, I.C.; Veselov, D.S.; Rothwell, S.A.; Veselov, S.Y. Common and specific responses to availability of mineral nutrients and water. J. Exp. Bot. 2015, 66, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.; Barros, R.; Bright, J.; Desikan, R.; Hancock, J.; Harrison, J.; Morris, P.; Ribeiro, D.; Wilson, I. Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 2008, 59, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Transpiration: How many functions? New Phytol. 2008, 179, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Cramer, M.D.; Hoffmann, V.; Verboom, G.A. Nutrient availability moderates transpiration in ehrharta calycina. New Phytol. 2008, 179, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Peterson, T.A.; Reinsel, M.D.; Krizek, D.T. Tomato (lycopersicon-esculentum mill, cv better bush) plant-response to root restriction.1. Alteration of plant morphology. J. Exp. Bot. 1991, 42, 1233–1240. [Google Scholar] [CrossRef]
- Chun, C.; Takakura, T. Rate of root respiration of lettuce under various dissolved oxygen concentrations in hydroponics. Environ. Control Biol. 1994, 32, 125–135. [Google Scholar] [CrossRef]
- Goto, E.; Both, A.J.; Albright, L.D.; Langhans, R.W.; Leed, A.R. Effect of Dissolved Oxygen Concentration on Lettuce Growth in Floating Hydroponics; International Society for Horticultural Science (ISHS): Leuven, Belgium, 1996; pp. 205–210. [Google Scholar]
- Poorter, H.; Buhler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef]
- Drew, M.C. Comparison of effects of a localized supply of phosphate, nitrate, ammonium and potassium on growth of seminal root system, and shoot, in barley. New Phytol. 1975, 75, 479–490. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Young, I.M.; Montagu, K.; Conroy, J.; Bengough, A.G. Mechanical impedance of root growth directly reduces leaf elongation rates of cereals. New Phytol. 1997, 135, 613–619. [Google Scholar] [CrossRef]
- Caird, M.A.; Richards, J.H.; Donovan, L.A. Nighttime stomatal conductance and transpiration in C-3 and C-4 plants. Plant Physiol. 2007, 143, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Senbayram, M.; Trankner, M.; Dittert, K.; Bruck, H. Daytime leaf water use efficiency does not explain the relationship between plant n status and biomass water-use efficiency of tobacco under non-limiting water supply. J. Plant Nutr. Soil Sci. 2015, 178, 682–692. [Google Scholar] [CrossRef]
- Hoson, T.; Soga, K.; Mori, R.; Saiki, M.; Nakamura, Y.; Wakabayashi, K.; Kamisaka, S. Cell wall changes involved in the automorphic curvature of rice coleoptiles under microgravity conditions in space. J. Plant Res. 2004, 117, 449–455. [Google Scholar] [CrossRef] [PubMed]
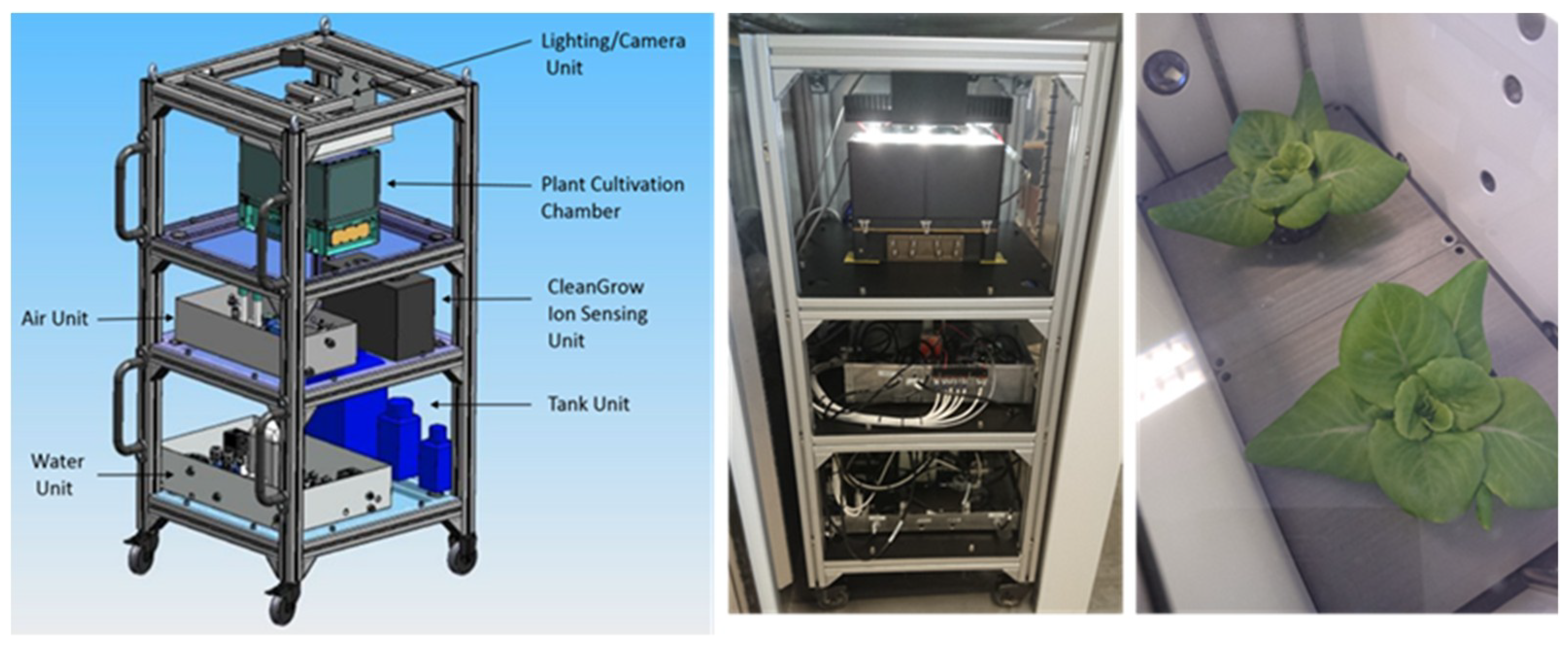
| Run 1 | Run 2 | |
|---|---|---|
| Nutrient Solution | NO3 (mmol L−1) | NO3 (mmol L−1) |
| 1 | 30 | 30 |
| 2 | 20 | 20 |
| 3 | 10 | 10 |
| 4 | 5 | 5 |
| 5 | 2.5 | 2.5 |
| 6 | 1.25 | |
| 7 | 0 |
| Conductance (gs) | Transpiration (E) | |||
|---|---|---|---|---|
| Fixed effects: | β ± SE | P-value | β ± SE | P-value |
| Intercept (i.e., Run 2, Dark) | 0.12 ± 0.0078 | <0.001 | 0.5 1± 0.034 | <0.001 |
| Run 1 | 0.032 ± 0.0091 | <0.001 | 0.18 ± 0.040 | <0.001 |
| Light | 0.019 ± 0.0067 | 0.005 | 0.31 ± 0.030 | <0.001 |
| NO3 concentration | 0.0020 ± 0.0033 | 0.56 | 0.013 ± 0.014 | 0.37 |
| NO3 concentration ^ 2 | −0.0030 ± 0.0011 | 0.011 | −0.012 ± 0.005 | 0.011 |
| NO3 concentration: Light | 0.0096 ± 0.0029 | 0.001 | 0.038 ± 0.013 | 0.005 |
| Random effects: | SD | N | SD | n |
| Plant ID | 0.025 | 85 | 0.001 | 85 |
| Block | 0.009 | 14 | 0.042 | 14 |
| Residual | 0.029 | 0.134 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolff, S.A.; Palma, C.F.; Marcelis, L.; Kittang Jost, A.-I.; Van Delden, S.H. Testing New Concepts for Crop Cultivation in Space: Effects of Rooting Volume and Nitrogen Availability. Life 2018, 8, 45. https://doi.org/10.3390/life8040045
Wolff SA, Palma CF, Marcelis L, Kittang Jost A-I, Van Delden SH. Testing New Concepts for Crop Cultivation in Space: Effects of Rooting Volume and Nitrogen Availability. Life. 2018; 8(4):45. https://doi.org/10.3390/life8040045
Chicago/Turabian StyleWolff, Silje A., Carolina F. Palma, Leo Marcelis, Ann-Iren Kittang Jost, and Sander H. Van Delden. 2018. "Testing New Concepts for Crop Cultivation in Space: Effects of Rooting Volume and Nitrogen Availability" Life 8, no. 4: 45. https://doi.org/10.3390/life8040045
APA StyleWolff, S. A., Palma, C. F., Marcelis, L., Kittang Jost, A.-I., & Van Delden, S. H. (2018). Testing New Concepts for Crop Cultivation in Space: Effects of Rooting Volume and Nitrogen Availability. Life, 8(4), 45. https://doi.org/10.3390/life8040045





