Tracing the Repertoire of Promiscuous Enzymes along the Metabolic Pathways in Archaeal Organisms
Abstract
1. Introduction
2. Material and Methods
2.1. Genomes and Proteomes Analyzed
2.2. Identification of Enzymes
Accuracy of the Promiscuous Enzymes
2.3. Statistical Analysis
3. Results and Discussion
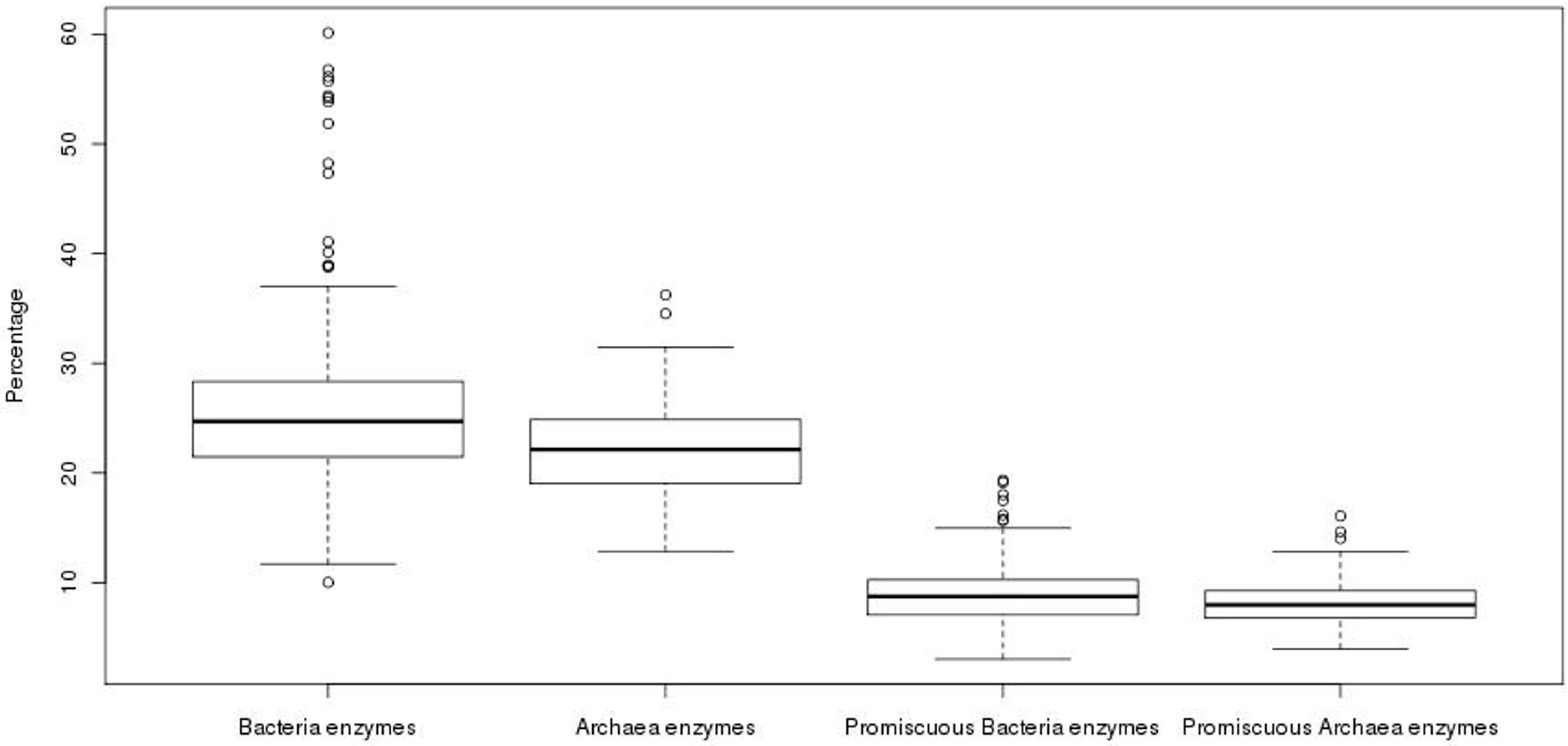
3.1. Archaea Exhibit a Lower Promiscuous Enzyme Fraction than Bacterial Genomes
3.2. Structural Diversity in Promiscuous Enzymes is Higher than Specialist Enzymes
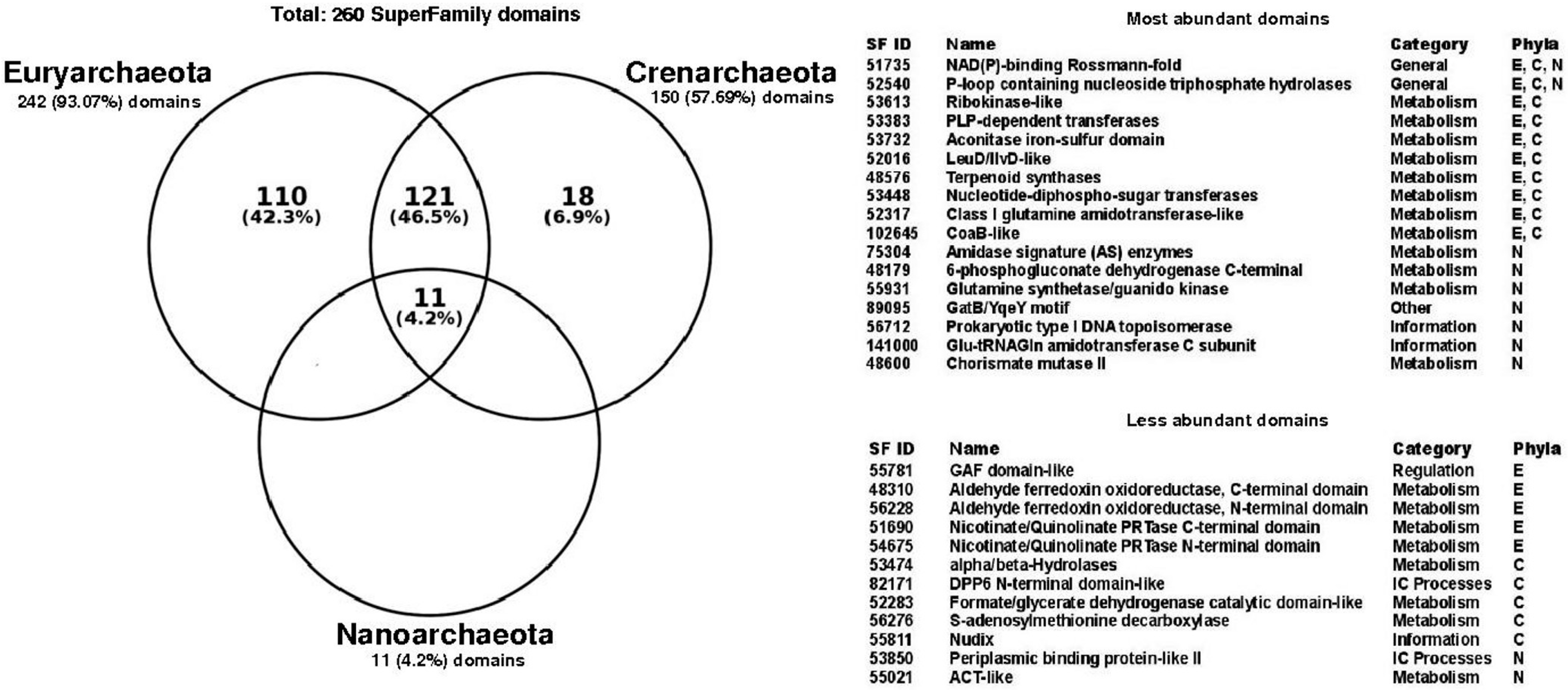
3.3. Functional Structural Diversity in Promiscuous Enzymes Exhibits a Non-Homogeneous Distribution
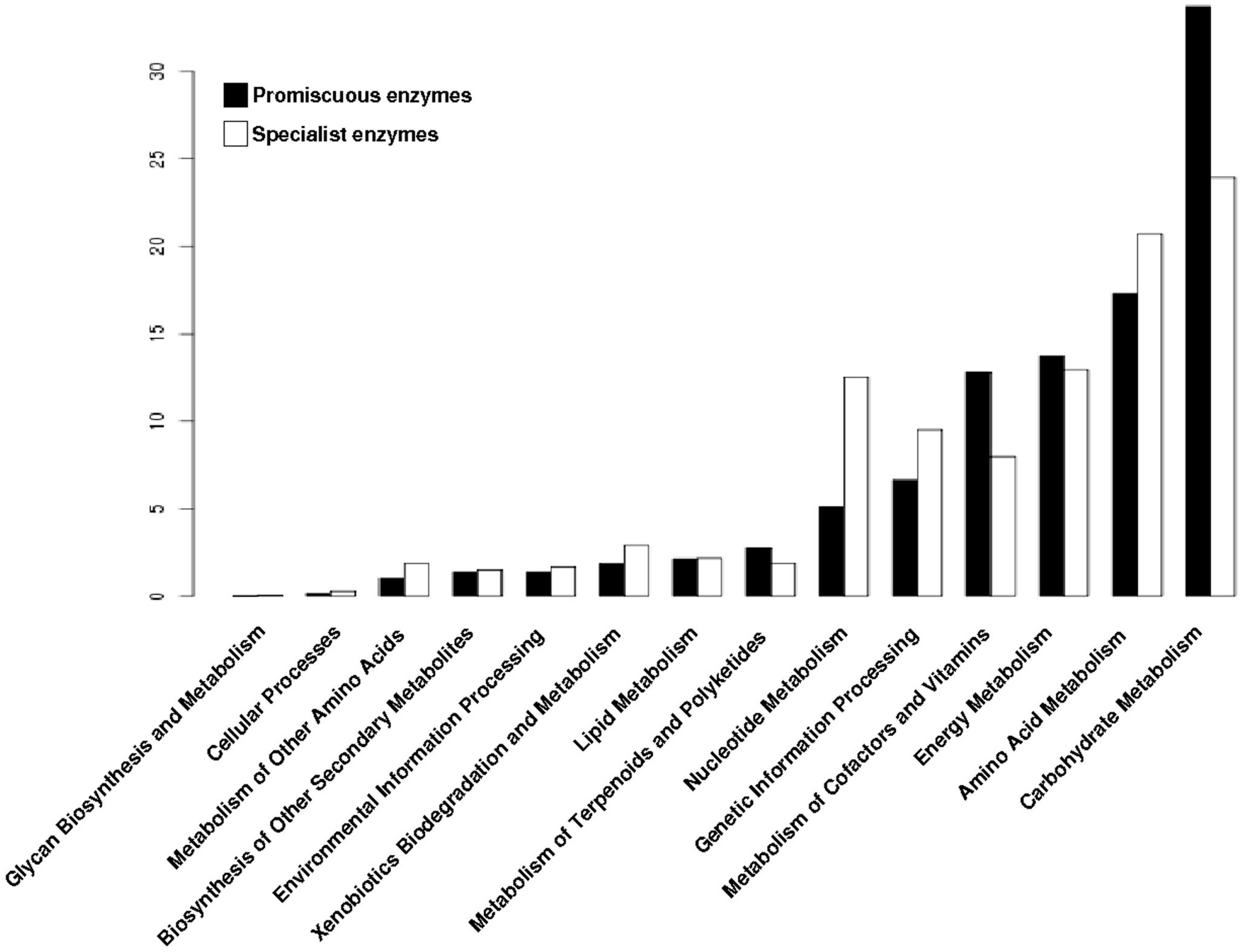
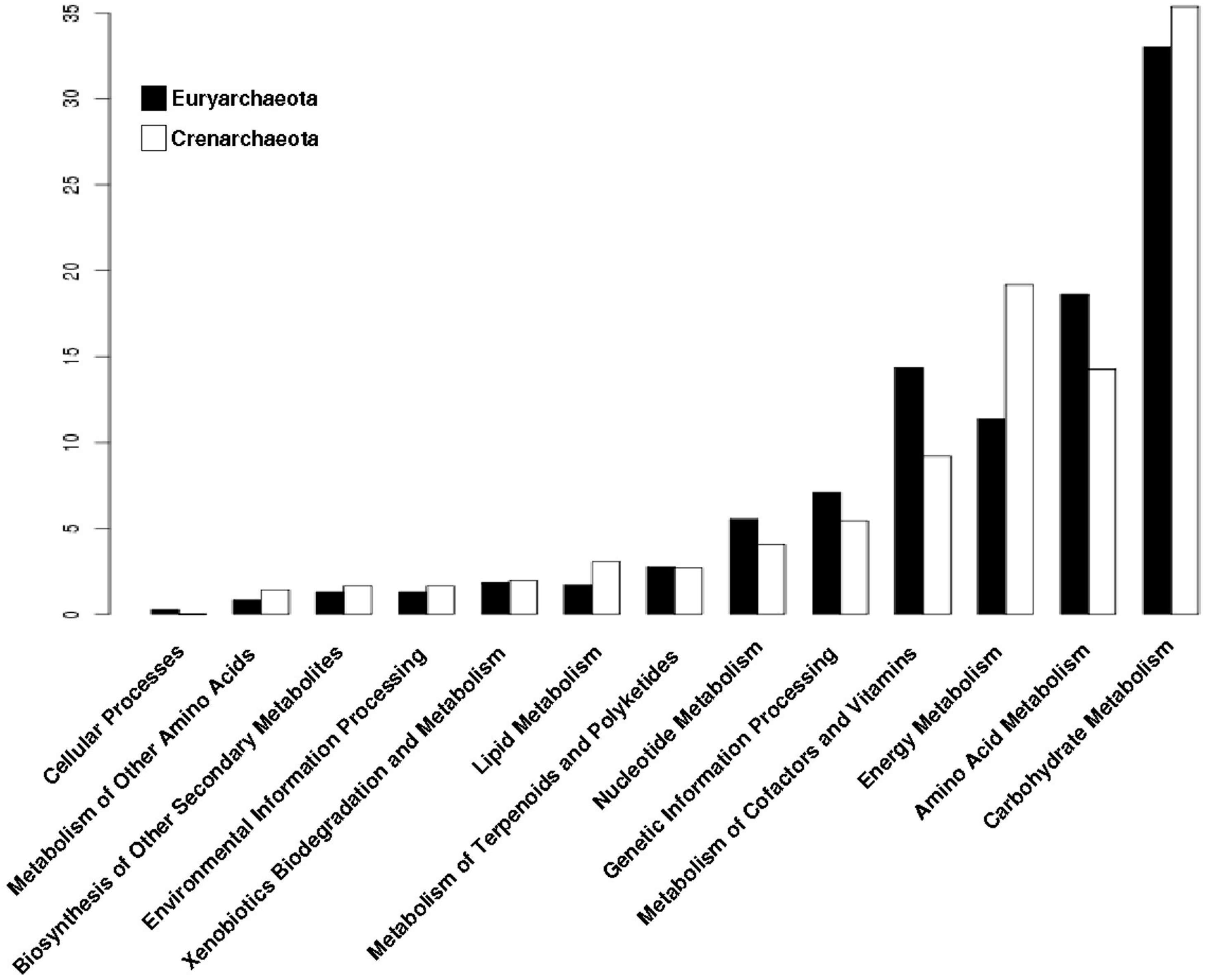
3.4. The Distribution of Promiscuous Enzymes between Functional Metabolic Categories is Variable
3.5. Promiscuous Enzymes Exhibit a High Enrichment of Substrate Promiscuity rather than Catalytic Promiscuity
3.6. Substrate Promiscuity is Represented to a Greater Extent in the Functional Metabolic Categories
4. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Nam, H.; Lewis, N.E.; Lerman, J.A.; Lee, D.H.; Chang, R.L.; Kim, D.; Palsson, B.O. Network context and selection in the evolution to enzyme specificity. Science 2012, 337, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Cheong, G.W.; Zhang, S. Multifunctional enzymes in archaea: Promiscuity and moonlight. Extremophiles 2013, 17, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Ghosh, S.; Nagaraja, V. Moonlighting function of glutamate racemase from mycobacterium tuberculosis: Racemization and DNA gyrase inhibition are two independent activities of the enzyme. Microbiology 2008, 154, 2796–2803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, X.Y.; Huang, W.J.; Hu, S.C.; Zhang, H.L.; Wang, H.; Zhang, J.X.; Lin, H.H.; Chen, Y.Z.; Zou, Q.; Ji, Z.L. A global characterization and identification of multifunctional enzymes. PLoS ONE 2012, 7, e38979. [Google Scholar] [CrossRef] [PubMed]
- Huberts, D.H.; van der Klei, I.J. Moonlighting proteins: An intriguing mode of multitasking. Biochim. Biophys. Acta 2010, 1803, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Hult, K.; Berglund, P. Enzyme promiscuity: Mechanism and applications. Trends Biotechnol. 2007, 25, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R. Recent advances in enzyme promiscuity. Sustain. Chem. Process. 2016, 4, 2. [Google Scholar] [CrossRef]
- Khersonsky, O.; Roodveldt, C.; Tawfik, D.S. Enzyme promiscuity: Evolutionary and mechanistic aspects. Curr. Opin. Chem. Biol. 2006, 10, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Khersonsky, O.; Tawfik, D.S. The histidine 115-histidine 134 dyad mediates the lactonase activity of mammalian serum paraoxonases. J. Biol. Chem. 2006, 281, 7649–7656. [Google Scholar] [CrossRef] [PubMed]
- Suen, S.; Lu, H.H.; Yeang, C.H. Evolution of domain architectures and catalytic functions of enzymes in metabolic systems. Genome Biol. Evol. 2012, 4, 976–993. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A. Metabolic networks and their evolution. Adv. Exp. Med. Biol. 2012, 751, 29–52. [Google Scholar] [PubMed]
- Martinez-Nunez, M.A.; Poot-Hernandez, A.C.; Rodriguez-Vazquez, K.; Perez-Rueda, E. Increments and duplication events of enzymes and transcription factors influence metabolic and regulatory diversity in prokaryotes. PLoS ONE 2013, 8, e69707. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.; Hohn, M.J.; Ahel, I.; Graham, D.E.; Adams, M.D.; Barnstead, M.; Beeson, K.Y.; Bibbs, L.; Bolanos, R.; Keller, M.; et al. The genome of nanoarchaeum equitans: Insights into early archaeal evolution and derived parasitism. Proc. Natl. Acad. Sci. USA 2003, 100, 12984–12988. [Google Scholar] [CrossRef] [PubMed]
- Maeder, D.L.; Anderson, I.; Brettin, T.S.; Bruce, D.C.; Gilna, P.; Han, C.S.; Lapidus, A.; Metcalf, W.W.; Saunders, E.; Tapia, R.; et al. The methanosarcina barkeri genome: Comparative analysis with methanosarcina acetivorans and methanosarcina mazei reveals extensive rearrangement within methanosarcinal genomes. J. Bacteriol. 2006, 188, 7922–7931. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Douady, C.J.; Italia, M.J.; Marshall, W.E.; Stanhope, M.J. Universal trees based on large combined protein sequence data sets. Nat. Genet. 2001, 28, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Yamada, T.; Hamajima, M.; Itoh, M.; Katayama, T.; Bork, P.; Goto, S.; Kanehisa, M. KEGG atlas mapping for global analysis of metabolic pathways. Nucleic Acids Res. 2008, 36, W423–W426. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Pethica, R.; Zhou, Y.; Talbot, C.; Vogel, C.; Madera, M.; Chothia, C.; Gough, J. Superfamily--sophisticated comparative genomics, data mining, visualization and phylogeny. Nucleic Acids Res. 2009, 37, D380–D386. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, P.; Faulon, J.L. Molecular signatures-based prediction of enzyme promiscuity. Bioinformatics 2010, 26, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- R-programming. Development core team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Freilich, S.; Spriggs, R.V.; George, R.A.; Al-Lazikani, B.; Swindells, M.; Thornton, J.M. The complement of enzymatic sets in different species. J. Mol. Biol. 2005, 349, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Anolles, G.; Yafremava, L.S.; Gee, H.; Caetano-Anolles, D.; Kim, H.S.; Mittenthal, J.E. The origin and evolution of modern metabolism. Int. J. Biochem. Cell Biol. 2009, 41, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, S.A.; Rison, S.C.; Thornton, J.M.; Riley, M.; Gough, J.; Chothia, C. The evolution and structural anatomy of the small molecule metabolic pathways in Escherichia coli. J. Mol. Biol. 2001, 311, 693–708. [Google Scholar] [PubMed]
- Galperin, M.Y. Structural classification of bacterial response regulators: Diversity of output domains and domain combinations. J. Bacteriol. 2006, 188, 4169–4182. [Google Scholar] [CrossRef] [PubMed]
- Berquist, B.R.; Soneja, J.; DasSarma, S. Comparative genomic survey of information transfer systems in two diverse extremely halophilic archaea, Halobacterium sp. Strain NRC-1 and Haloarcula marismortui. In Adaptation to Life at High Salt Concentrations in Archaea, Bacteria, and Eukarya; Gunde-Cimerman, N., Oren, A., Plemenitas, A., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 148–182. [Google Scholar]
- Peregrin-Alvarez, J.M.; Tsoka, S.; Ouzounis, C.A. The phylogenetic extent of metabolic enzymes and pathways. Genome Res. 2003, 13, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, C.; Kawai, S.; Murata, K. Nadp (h) phosphatase activities of archaeal inositol monophosphatase and eubacterial 3′-phosphoadenosine 5′-phosphate phosphatase. Appl. Environ. Microbiol. 2007, 73, 5447–5452. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stieglitz, K.A.; Johnson, K.A.; Yang, H.; Roberts, M.F.; Seaton, B.A.; Head, J.F.; Stec, B. Crystal structure of a dual activity impase/fbpase (af2372) from Archaeoglobus fulgidus. The story of a mobile loop. J. Biol. Chem. 2002, 277, 22863–22874. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Urbanke, C.; Schonheit, P. Bifunctional phosphoglucose/phosphomannose isomerase from the hyperthermophilic archaeon Pyrobaculum aerophilum. Extremophiles 2004, 8, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Angelov, A.; Futterer, O.; Valerius, O.; Braus, G.H.; Liebl, W. Properties of the recombinant glucose/galactose dehydrogenase from the extreme thermoacidophile, Picrophilus torridus. FEBS J. 2005, 272, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Lamble, H.J.; Theodossis, A.; Milburn, C.C.; Taylor, G.L.; Bull, S.D.; Hough, D.W.; Danson, M.J. Promiscuity in the part-phosphorylative entner-doudoroff pathway of the archaeon Sulfolobus solfataricus. FEBS Lett. 2005, 579, 6865–6869. [Google Scholar] [CrossRef] [PubMed]
- Schonheit, P.; Schafer, T. Metabolism of hyperthermophiles. World J. Microbiol. Biotechnol. 1995, 11, 26–57. [Google Scholar] [CrossRef] [PubMed]
- Nunn, C.E.; Johnsen, U.; Schonheit, P.; Fuhrer, T.; Sauer, U.; Hough, D.W.; Danson, M.J. Metabolism of pentose sugars in the hyperthermophilic archaea Sulfolobus solfataricus and Sulfolobus acidocaldarius. J. Biol. Chem. 2010, 285, 33701–33709. [Google Scholar] [CrossRef] [PubMed]
- Blaut, M. Metabolism of methanogens. Antonie Van Leeuwenhoek 1994, 66, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Deppenmeier, U. The unique biochemistry of methanogenesis. Prog. Nucleic Acid Res. Mol. Biol. 2002, 71, 223–283. [Google Scholar] [PubMed]
- Papke, R.T.; Douady, C.J.; Doolittle, W.F.; Rodriguez-Valera, F. Diversity of bacteriorhodopsins in different hypersaline waters from a single spanish saltern. Environ. Microbiol. 2003, 5, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Arora, B.; Mukherjee, J.; Gupta, M.N. Enzyme promiscuity: Using the dark side of enzyme specificity in white biotechnology. Sustain. Chem. Proc. 2014, 2, 25. [Google Scholar] [CrossRef]
- Lamble, H.J.; Heyer, N.I.; Bull, S.D.; Hough, D.W.; Danson, M.J. Metabolic pathway promiscuity in the archaeon Sulfolobus solfataricus revealed by studies on glucose dehydrogenase and 2-keto-3-deoxygluconate aldolase. J. Biol. Chem. 2003, 278, 34066–34072. [Google Scholar] [CrossRef] [PubMed]

| Superfamily | Supfam ID | Functional Annotation | Phylum |
|---|---|---|---|
| Sulfolobus fructose-1,6-bisphosphatase-like superfamily (66) | 111249 | Metabolism | Euryarchaeota Crenarchaeota |
| Glu-tRNAGln amidotransferase C subunit superfamily (56) | 141000 | Information Translation | Euryarchaeota Crenarchaeota Nanoarchaeota |
| Siroheme synthase middle domains-like superfamily (51) | 75615 | Metabolism | Euryarchaeota Crenarchaeota |
| Heme-dependent peroxidases superfamily (18) | 48113 | Metabolism Redox | Euryarchaeota |
| Nitrous oxide reductase, N-terminal domain superfamily (4) | 50974 | Metabolism Redox | Euryarchaeota Crenarchaeota |
| Oxidoreductase molybdopterin-binding domain superfamily (3) | 56524 | Metabolism E- transfer | Euryarchaeota |
| Tropomyosin superfamily (2) | 57997 | Intra-Cellular processes Cell motility | Euryarchaeota |
| DNA breaking-rejoining enzymes superfamily (2) | 56349 | Information DNA replication/repair | Euryarchaeota Crenarchaeota |
| N-terminal domain of MutM-like DNA repair proteins superfamily (2) | 81624 | Information DNA replication/repair | Euryarchaeota |
| (Phosphotyrosine protein) phosphatases II superfamily (2) | 52799 | Regulation Kinases/phosphatases | Crenarchaeota |
| 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase, HPPK (7,8-dihydro-6-hydroxymethylpterin-pyrophosphokinase) superfamily (1) | 55083 | Metabolism Coenzyme metabolism and transport | Crenarchaeota |
| Eukaryotic DNA topoisomerase I, N-terminal DNA-binding fragment superfamily (1) | 56741 | Information DNA replication/repair | Crenarchaeota |
| Metabolic Categories (KEGG) | Substrate Promiscuity (%) | Catalytic Promiscuity (%) | ||
|---|---|---|---|---|
| Euryarchaeota | Crenarchaeota | Euryarchaeota | Crenarchaeota | |
| Carbohydrate metabolism | 33.21 | 36.12 | 32.26 | 34.79 |
| Amino acid metabolism | 21.06 | 14.69 | 10.36 | 9.94 |
| Energy metabolism | 11.87 | 18.81 | 10.04 | 19.59 |
| Metabolism of cofactors and vitamins | 10.94 | 7.34 | 25.96 | 17.83 |
| Genetic information processing | 7.04 | 5.9 | 6.94 | 2.63 |
| Nucleotide metabolism | 4.62 | 2.88 | 8.86 | 9.35 |
| Metabolism of terpenoids and polyketides | 3.53 | 3.36 | 0.21 | 0 |
| Lipid metabolism | 2.23 | 3.84 | 0 | 0 |
| Xenobiotics biodegradation and metabolism | 1.98 | 2.47 | 1.38 | 0 |
| Environmental information processing | 1.33 | 2.06 | 0.96 | 0 |
| Biosynthesis of other secondary metabolites | 1.61 | 1.23 | 0.21 | 3.5 |
| Metabolism of other amino acids | 0.4 | 1.23 | 2.02 | 2.33 |
| Cellular Processes | 0.12 | 0 | 0.74 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Núñez, M.A.; Rodríguez-Escamilla, Z.; Rodríguez-Vázquez, K.; Pérez-Rueda, E. Tracing the Repertoire of Promiscuous Enzymes along the Metabolic Pathways in Archaeal Organisms. Life 2017, 7, 30. https://doi.org/10.3390/life7030030
Martínez-Núñez MA, Rodríguez-Escamilla Z, Rodríguez-Vázquez K, Pérez-Rueda E. Tracing the Repertoire of Promiscuous Enzymes along the Metabolic Pathways in Archaeal Organisms. Life. 2017; 7(3):30. https://doi.org/10.3390/life7030030
Chicago/Turabian StyleMartínez-Núñez, Mario Alberto, Zuemy Rodríguez-Escamilla, Katya Rodríguez-Vázquez, and Ernesto Pérez-Rueda. 2017. "Tracing the Repertoire of Promiscuous Enzymes along the Metabolic Pathways in Archaeal Organisms" Life 7, no. 3: 30. https://doi.org/10.3390/life7030030
APA StyleMartínez-Núñez, M. A., Rodríguez-Escamilla, Z., Rodríguez-Vázquez, K., & Pérez-Rueda, E. (2017). Tracing the Repertoire of Promiscuous Enzymes along the Metabolic Pathways in Archaeal Organisms. Life, 7(3), 30. https://doi.org/10.3390/life7030030





